A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Plate-based Cytotoxicity Assay for the Assessment of Rat Placental Natural Killer Cell Cytolytic Function
In This Article
Summary
Here, we provide a detailed methodology to isolate and assess cytotoxic function of natural killer cells from placentas by a colorimetric plate assay. The reduced uterine perfusion pressure rat model of placental ischemia was chosen to demonstrate the antibody-mediated isolation and assessment of the cytotoxic function of natural killer cells.
Abstract
It is well known that decidual natural killer (NK) cells play a critical role in establishment and maintenance of normal pregnancy. Recent studies have demonstrated an altered population of circulating and decidual NK cells in women who suffer from adverse pregnancy complications such as recurrent miscarriage and preeclampsia. Studies from our group have shown that hypertension in pregnancy is associated with an increased population of activated NK cells in the placenta based on the expression of surface activation markers. This manuscript provides a detailed protocol to assess the cytotoxic function of NK cells isolated from placentas in a preeclampsia-like animal model of surgically induced placental ischemia. The following steps are described in detail: generation of single cell suspension, NK cell isolation, ex vivo stimulation, effector:target cell co-culture, and the cytotoxicity assay.
Introduction
Preeclampsia is a hypertensive disorder of pregnancy characterized by fetal growth restriction, end organ damage and chronic immune activation. Chronic immune activation in women with preeclampsia leads to increased circulating and placental inflammatory cytokines, an imbalance in CD4+ T Cells populations, and an increased population of activated Natural Killer (NK) cells1. Studies recently published by our lab demonstrate a role for NK cells in causing some of the pathophysiology associated with preeclampsia in the Reduced Uterine Perfusion Pressure (RUPP) rat model of preeclampsia. Using flow cytometry to measure surface expression of activation markers on NK cells, an increased population of activated NK cells in the circulation and placentas of RUPP rats compared to normal pregnant (NP) rats was observed2.
To confirm the flow cytometry observations, functional studies to assess the cytotoxic activity of NK cells isolated from the placentas of NP and RUPP rats were performed. There are several methods available for the assessment of cytotoxic function of cytotoxic CD8+ T cells and NK cells. The gold standard for functional cytotoxic analysis is the chromium release assay3. Other developed protocols utilized include flow cytometry4, image cytometry5, calcein release6, and most recently bioluminescence7. This video will provide a detailed protocol on using the well-established lactate dehydrogenase (LDH) release assay to measure cytotoxic function of NK cells using a commercially available LDH cytotoxicity assay kit.
Protocol
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. The care and handling of the animals were in accord with the National Institutes of Health guidelines for ethical animal treatment.
1. Lymphocyte Cell Isolation from Placentas
- Remove one placenta from the rat uterus (gestation day 19) and place in 10 mL of ice-cold PBS8.
- Place one placenta on a 100 µm filter and sit in a Petri dish containing 13.5 mL of RPMI and 1.5 mL of FBS (total volume is 15 mL). Use the flat side of a syringe plunger to push the placenta through the filter into the Petri dish.
- Prepare three 15 mL conical tubes for each tissue. Add 3 mL of density gradient medium (see Table of Materials) to each tube, then carefully overlay 5 mL of homogenized placenta into each tube.
- Centrifuge for 25 min at 300 x g at room temperature (RT) with no brake. Collect the thin white buffy layer with a transfer pipette.
NOTE: After centrifugation, 3 layers are visible, the red RPMI at the top, the white buffy layer in the middle, and the clear density gradient medium layer at the bottom. Use a transfer pipette to pull up the white buffy layer from the tube. Combine buffy layer from all tubes of the same placenta into 1 tube. - Add 10 mL of RPMI to combined buffy layers. Centrifuge for 10 min, 300 x g, at 4 °C and discard supernatant.
2. Isolation of Natural Killer Cells
- Resuspend cell pellet in 50 μL of ice-cold PBS
- Add biotin-labeled CD3 antibody to pelleted cells according to the manufacturer’s protocol and mix well with a pipette. Place the tube in a tube rotator and incubate 20 min at 4 °C.
- Add 1 mL of RPMI, centrifuge for 10 min at 400 x g and 4 °C, and discard supernatant.
- Resuspend pellet in 1 mL of RPMI and combine with 150 μL of magnetic beads in a 1.5 mL microcentrifuge tube. Place the microcentrifuge tube in a tube rotator and rotate while incubating for 30 min at 4 °C.
NOTE: Pull out release buffer at this time and allow to reach RT in the biosafety cabinet. - Place the tubes in the magnet for 1 min. Collect supernatant and save CD3- cell population in a 15 mL tube on ice.
NOTE: This is the CD3- population of cells. - Remove the tube from the magnet and add 1 mL of RPMI. Mix cells and beads 5 times with a pipette.
- Repeat step 2.5.
- Centrifuge the CD3- population of cells for 10 min at 400 x g and 4 °C and discard supernatant. Resuspend the cell pellet in 50 μL of ice-cold PBS
- Add biotin-labeled CD161a antibody to CD3- cells according to the manufacturer’s protocol and mix well. Place tube in a tube rotator and incubate for 20 min at 4 °C.
- Add 1 mL of RPMI, centrifuge for 10 min at 400 x g and 4 °C, and discard supernatant. Resuspend pellet in 1 mL of RPMI and combine with 150 μL of magnetic beads in a 1.5 mL microcentrifuge tube.
- Place microcentrifuge tube in a tube rotator and rotate while incubating for 30 min at 4 °C.
- Place the tubes in a magnet for 1 min. Collect supernatant and discard CD3-/CD161a- cells.
- Remove tube from magnet and add 1 mL of RPMI. Mix cells and beads 5 times with a pipette.
- Repeat step 2.12.
- Remove microcentrifuge tubes from the magnet and add 1 mL of RT Release buffer. Place tube on tube rotator and rotate while incubating for 15 min at RT.
- Place tubes in magnet for 1 min. Collect supernatant in a new 15 mL conical tube on ice.
NOTE: This is the population of NK cells. - Remove tube from magnet and add 1 mL of RT RPMI. Mix cells and beads 5 times with pipette.
- Repeat step 2.16, placing supernatant in the same tube. Mix well and take a 20 μL sample to count cells
NOTE: Keep tube on ice. - Centrifuge CD3-/CD161a+ cells for 10 min, 400 x g at 4 °C, then remove supernatant. Resuspend the cells in RPMI (10% FBS, 1% Pen/Strep, 2 ng/ mL IL-2) and seed at a concentration of 3 x 105 cells/well in a 6-well plate in 2.5 mL of NK Cell Activation Media. Incubate cells for 48 h at 37 °C, 5% CO2 in a humidified incubator.
3. Cytotoxicity Assay: Retrieving NK Cells or YAC1 from Culture or Passing Cells
NOTE: All steps must be conducted under the hood. All cell tubes must be kept on ice at all times.
- Use a glass serological pipet to collect YAC1 cells and media from flask, place in a 50 mL tube on ice, and mix well. Take 20 µL to count cells.
- Spin the YAC1 cells for 10 min at 300 x g and 4 °C. Count cells while these are spinning.
- Add trypsin/EDTA to each well in a NK cell 6-well plate. Tap the plate and place in incubator.
- After cells have incubated with trypsin/EDTA for ~5 min at 37 °C, scrape the plate/flask with a sterile plate scraper. Add 1 mL of NK Cell Media to each well.
- Collect cells and media with serological pipet and collect in a 15 mL centrifuge tube. Take 20 µL to count cells.
- Spin the NK cells for 10 min, 400 x g at 4 °C. Count the sample of cells from step 3.5 during this centrifugation.
NOTE: View the culture plates under a microscope before discarding them to make sure there are no more cells adhered to the bottom of the wells. Since the experiment uses NK cells and YAC1 cells, be sure to count each one separately. - Count cells and resuspend at the concentrations determined in optimization trials to test for the appropriate number of target:effector ratio. This will be achieved by re-suspending the pellet in their corresponding media to make the following cell concentrations: YAC1 at 4 x 105 cells/mL and NK cells at 2 x 107 cells/mL.
4. Cytotoxicity Assay: Assay Protocol
- Use a round bottom, culture treated 96-well plate to set up the plate as suggested in Table 1. This table shows the experimental controls and 3 sets of NK experimental columns. This can be expanded to a total of 10 NK experimental columns in a 96-well plate.
- Centrifuge the assay plate at 250 x g for 4 min to be certain that the effector and target cells are in contact. Incubate the 96-well plate for 5 hours in a humidified chamber incubator at 37 °C, 5% CO2 to achieve ample contact between target and effector cells and target cell lysis by effector cells.
NOTE: The protocol can be paused here. - 45 min prior to harvesting supernatants, add 10 µL of 10x Lysis Solution to the Target Cell Maximum LDH Release wells (Wells 1E, 1F, 1G, and 1H) and place the plate back in the humidified chamber.
- After the incubation time is completed, centrifuge the plate at 250 x g for 4 min. Using a multichannel pipettor, transfer 50 µL aliquots from all wells to a fresh 96-well flat-bottom assay plate.
NOTE: Do not touch the bottom of the wells so that cells are not transferred into the fresh assay plate. Do not use a cultured treated plate as the fresh assay plate. - Make Assay Reagent.
- After Assay Buffer has reached room temperature, add 12 mL of Assay Buffer to one Substrate Mix bottle. Invert and shake gently until completely dissolved. 1 bottle is enough for two 96-well plates.
NOTE: Assay Buffer should be protected from light while being thawed. Mix immediately prior to use.
- After Assay Buffer has reached room temperature, add 12 mL of Assay Buffer to one Substrate Mix bottle. Invert and shake gently until completely dissolved. 1 bottle is enough for two 96-well plates.
- Add 50 µL of Assay Reagent to all wells in the assay plate from step 4.6. Cover the plate with foil to protect it from light and incubate for 30 min at room temperature.
NOTE: Newly made Assay Reagent should be stored in a freezer. Reagent is good for approximately 8 weeks. - Add 50 µL of Stop Solution to each well. Read plate within 1 hour after adding Stop Solution and record the absorbance at 490 nm. Representative data is shown in Table 2.
5. Calculation of Results
- Calculate the average absorbance value from the Culture Medium Background wells and subtract from all absorbance values for Experimental, Target Cell Spontaneous LDH Release and Effector Cell Spontaneous LDH Release wells.
- Calculate the average absorbance values for the Volume Correction Control wells and subtract from the absorbance values acquired for the Target Cell Maximum LDH Release Control wells.
- Use the corrected values from Steps 5.1 and 5.2 in the following formula to calculate percent cytotoxicity for each effector:target well.
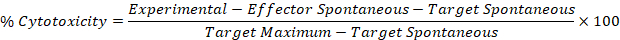
Results
Placental NK cells obtained from NP and RUPP rats were incubated for 5 h with target cells in their respective medias at a ratio of 50:1 (NK:target). Absorbance was recorded at 490 nm and the raw data is shown in Table 2. The average absorbance of the Culture Medium Background and the Volume Correction control wells were calculated. These averages were subtracted from the appropriate wells indicated in the manufacturer’s protocol and are represented in Table 3. The corrected values...
Discussion
There are a number of important key notes to consider for optimal results. The sterility of the cells utilized is very important. After collection of the placenta, it is important that preparation and isolation of the NK cells are performed under sterile conditions in a biosafety cabinet. Furthermore, because all cells release LDH upon cellular damage, care should be taken to obtain a high viability of NK cells after isolation and during the co-culture process. Too much spontaneous LDH release from the NK cells can often...
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgements
This work was supported by the National Heart Lung and Blood Institute of the National Institutes of Health under grant R00HL130456, the National Institute of General Medical Sciences of the National Institutes of Health under award P20GM104357, and by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institutes of General Medical Sciences of the National Institutes of Health under grant number P20GM103476. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 mL Eppendorf tube | Fisher | 5408129 | |
| 100 µL Filter | Fisher | 22363549 | Nylon Mesh |
| 15 mL conical tube | Fisher | 0553859A | |
| 3 mL syringe | Fisher | 14823436 | |
| 50 mL conical tube | Fisher | 7203510 | |
| 6-well cell culture plate | Corning | 720083 | |
| 96-well Tissue Culture Plate | CELLTREAT | 229190 | Sterile, Round Bottom |
| AOPI | Nexcelom | CS201065ML | |
| Cell scraper | Fisher | 8100241 | |
| Cellometer Disposable Counting Chambers | Nexcelom | CHT4-SD100 | |
| Cellometer Vision Image Cytometer | Nexcelom | N/A | |
| Cytotox 96 Non-Radioactive Cytotoxicity Assay Kit | Promega | G1780 | |
| Dynabeads Flowcomp Flexi Kit | Invitrogen | 11061D | |
| DynaMag-2 Magnet | Invitrogen | 12321D | |
| EDTA | Sigma Aldrich | EDS-100G | |
| FBS | Atlanta Biologicals | S11150H | |
| Flow Cytometry Tube | Corning | 352008 | |
| Lymphoprep | Fisher | NC0460539 | Density gradient medium; 4 x 250 mL |
| PBS | Fisher | SH3025801 | 10 x 500 mL |
| Penicillin/Streptomycin | Gibco | 15140122 | |
| Petri dishes | Fisher | 9720500 | Without Pad |
| Purified Mouse anti-Rat CD161a | BD Biosciences | 555006 | |
| Purified Mouse anti-Rat CD3 | BD Biosciences | 554829 | |
| Recombinant Rat IL-2 | R&D Systems | 502-RL | |
| RPMI | Gibco | 11875135 | 1640 Medium |
| T25 flask | Corning | 430639 | |
| Trypsin | ThermoFisher | 15090046 | |
| YAC-1 cell | ATCC | TIB-160 |
References
- Cornelius, D. C., Cottrell, J., Amaral, L. M., LaMarca, B. Inflammatory Mediators: A causal link to hypertension during pregnancy- Studies in Preeclampsia. British Journal of Pharmacology. , (2018).
- Elfarra, J., et al. Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure. Clinical Science. 131 (23), 2753-2762 (2017).
- Brunner, K. T., Mauel, J., Cerottini, J. C., Chapuis, B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 14 (2), 181-196 (1968).
- Kim, G. G., Donnenberg, V. S., Donnenberg, A. D., Gooding, W., Whiteside, T. L. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. Journal of Immunological Methods. 325 (1-2), 51-66 (2007).
- Somanchi, S. S., McCulley, K. J., Somanchi, A., Chan, L. L., Lee, D. A. A Novel Method for Assessment of Natural Killer Cell Cytotoxicity Using Image Cytometry. PLoS One. 10 (10), 0141074 (2015).
- Neri, S., Mariani, E., Meneghetti, A., Cattini, L., Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clinical and Diagnostic Laboratory Immunology. 8 (6), 1131-1135 (2001).
- Karimi, M. A., et al. Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay. PLoS One. 9 (2), 89357 (2014).
- Caporossi, C., Nogueira, P. L., Marques, J. C., Assis, R. M., Aguilar-Nascimento, J. E. Validation of the gastroschisis experimental model and the influence of the mother's diet enriched with glutamine in the fetal morphology. Acta Cirúrgica Brasileira. 29 (3), 158-165 (2014).
- Lv, L. H., et al. Functional distinction of rat liver natural killer cells from spleen natural killer cells under normal and acidic conditions in vitro. Hepatobiliary and Pancreatic Diseases International. 11 (3), 285-293 (2012).
- Flieger, D., et al. A novel non-radioactive cellular cytotoxicity test based on the differential assessment of living and killed target and effector cells. Journal of Immunological Methods. 180 (1), 1-13 (1995).
- Jang, Y. Y., et al. An improved flow cytometry-based natural killer cytotoxicity assay involving calcein AM staining of effector cells. Annals of Clinical Laboratory Science. 42 (1), 42-49 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved