A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Calcium Carbonate Formation in the Presence of Biopolymeric Additives
In This Article
Summary
We describe a protocol for the precipitation and characterization of calcium carbonate crystals that form in the presence of biopolymers.
Abstract
Biomineralization is the formation of minerals in the presence of organic molecules, often related with functional and/or structural roles in living organisms. It is a complex process and therefore a simple, in vitro, system is required to understand the effect of isolated molecules on the biomineralization process. In many cases, biomineralization is directed by biopolymers in the extracellular matrix. In order to evaluate the effect of isolated biopolymers on the morphology and structure of calcite in vitro, we have used the vapor diffusion method for the precipitation of calcium carbonate, scanning electron microscopy and micro Raman for the characterization, and ultraviolet-visible (UV/Vis) absorbance for measuring the quantity of a biopolymer in the crystals. In this method, we expose the isolated biopolymers, dissolved in a calcium chloride solution, to gaseous ammonia and carbon dioxide that originate from the decomposition of solid ammonium carbonate. Under the conditions where the solubility product of calcium carbonate is reached, calcium carbonate precipitates and crystals are formed. Calcium carbonate has different polymorphs that differ in their thermodynamic stability: amorphous calcium carbonate, vaterite, aragonite, and calcite. In the absence of biopolymers, under clean conditions, calcium carbonate is mostly present in the calcite form, which is the most thermodynamically stable polymorph of calcium carbonate. This method examines the effect of the biopolymeric additives on the morphology and structure of calcium carbonate crystals. Here, we demonstrate the protocol through the study of an extracellular bacterial protein, TapA, on the formation of calcium carbonate crystals. Specifically, we focus on the experimental set up, and characterization methods, such as optical and electron microscopy as well as Raman spectroscopy.
Introduction
Biomineralization is the formation of minerals in the presence of organic molecules, often related with functional and/or structural roles in living organisms. Biomineralization may be intracellular, as in the formation of magnetite inside magnetotactic bacteria1, or extracellular, as in the formation of calcium carbonate in sea urchin spikes2, of hydroxyapatite that is related with collagen in bones3 and of enamel that is associated with amelogenin in teeth4. Biomineralization is a complex process that depends on many parameters in the living organism. Therefore, in order to simplify the system under study, it is necessary to evaluate the effect of separate components on the process. In many cases, biomineralization is induced by the presence of extracellular biopolymers. The purpose of the method presented here are as follows: (1) To form calcium carbonate crystals in the presence of isolated biopolymers in vitro, using a vapour diffusion method. (2) To study the effect of the biopolymers on the morphology and structure of calcium carbonate.
Three principal methods to precipitate calcium carbonate in vitro in the presence of organic additives are used5,6. The first method, which we will refer to as the solution method, is based on mixing a soluble salt of calcium (e.g., CaCl2) with a soluble salt of carbonate (e.g., sodium carbonate). The mixing process may be performed in several ways: inside a reactor with three cells that are separated by porous membranes7. Here, each of the outer cells contains a soluble salt and the central cell contains a solution with the additive to be tested. Calcium and carbonate diffuse from the outer to the middle cell, resulting in the precipitation of the less soluble calcium carbonate when the concentrations of calcium and carbonate exceed their solubility product, Ksp = [Ca2+][CO32-]. An additional mixing method is the double-jet procedure8. In this method, each soluble salt is injected from a separate syringe to a stirred solution containing the additive, where calcium carbonate precipitates. Here, the injection and therefore the mixing rate is well controlled, in contrast with the previous method where mixing is controlled by diffusion.
The second method used to crystallize CaCO3 is the Kitano method9. This method is based on the carbonate/hydrogen carbonate equilibrium (2HCO3- (aq) + Ca2+(aq) 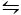 CaCO3 (s) + CO2 (g) + H2O (l)). Here, CO2 is bubbled into a solution containing CaCO3 in a solid form, shifting the equilibrium to the left and therefore dissolving the calcium carbonate. The undissolved calcium carbonate is filtered and the desired additives are added to the bicarbonate-rich solution. CO2 is then allowed to evaporate, thereby shifting the reaction to the right, forming calcium carbonate in the presence of the additives.
CaCO3 (s) + CO2 (g) + H2O (l)). Here, CO2 is bubbled into a solution containing CaCO3 in a solid form, shifting the equilibrium to the left and therefore dissolving the calcium carbonate. The undissolved calcium carbonate is filtered and the desired additives are added to the bicarbonate-rich solution. CO2 is then allowed to evaporate, thereby shifting the reaction to the right, forming calcium carbonate in the presence of the additives.
The third method of calcium carbonate crystallization, which we will describe here, is the vapor diffusion method10. In this set-up, the organic additive, dissolved in a solution of calcium chloride, is placed in a closed chamber near ammonium carbonate in a powder form. When ammonium carbonate powder decomposes into carbon dioxide and ammonia, they diffuse into the solution containing calcium ions (e.g., CaCl2), and calcium carbonate is precipitated (see Figure 1 for illustration). The calcium carbonate crystals can grow by slow precipitation or by fast precipitation. For the slow precipitation, a solution containing the additive in CaCl2 solution is placed in a desiccator next to the ammonium carbonate powder. In the fast precipitation, described in length in the protocol, both the additive solution and the ammonium carbonate are placed closer together in a multi-well plate. The slow precipitation method will produce fewer nucleation centers and larger crystals, and the fast precipitation will result in more nucleation centers and smaller crystals.
The methods described above differ in their technical complexity, in the level of control and in the rate of the precipitation process. The mixing method requires a special set-up6 for both the double jet and the three-cell system. In the mixing method, the presence of other soluble counter ions (e.g., Na+, Cl-)6 is inevitable, whereas in the Kitano method, calcium and (bi) carbonate are the only ions in solution and it does not involve the presence of additional counter ions (e.g., Na+, Cl-). Furthermore, the mixing method requires relatively large volumes and therefore it is not suitable for working with expensive biopolymers. The advantage of the double jet is that it is possible to control the rate of solution injection and that it is a rapid process in comparison to other methods.
The advantage of the Kitano method and the vapor diffusion method is that the formation of calcium carbonate is controlled by diffusion of CO2 into/out of a CaCl2 solution, thus allowing to probe slower nucleation and precipitation processes11,12. Furthermore, calcium carbonate formation by diffusion of CO2 may resemble calcification processes in vivo13,14,15. In this method, well-defined and separated crystals are formed16. Last, the effect of single or multiple biopolymers on calcium carbonate formation can be tested. This enables a systematic study of the effect of a series of additive concentrations on calcium carbonate formation as well as a study of mixtures of biopolymers - all performed in a controlled manner. This method is suitable for use with a large range of concentrations and volumes of additives. The minimal volume used is approximately 50 µL and therefore this method is advantageous when there is a limited amount of the available biopolymers. The maximal volume depends on the accessibility of a larger well-plate, or the desiccator into which the plate or beaker containing CaCl2 are to be inserted. The method described below has been optimized for working in a 96-well plate with a biopolymer chosen to be the protein TapA17.
Protocol
1. Calcium carbonate crystallization
- Control preparation and optimization
- Prepare clean glass pieces. Use the same cleaning procedure to clean the glassware.
- Use a diamond pen to cut pieces of a glass microscope slide so that they fit in a well of a 96-well plate.
NOTE: 5 mm x 5 mm pieces should largely fit. - Place the glass pieces in a beaker with triple distilled water (TDW) so that water covers the glass slides and sonicate in a bath sonicator for 10 min.
- Decant the water, add ethanol to cover the glass slides, and sonicate in a bath sonicator for 10 min.
- Dry the slides and the glassware with a stream of nitrogen gas and place them in an air plasma cleaner for 10 min at 130 W.
- Use a diamond pen to cut pieces of a glass microscope slide so that they fit in a well of a 96-well plate.
- Optimize the concentration of the CaCl2 used in the calcification experiments performed under the desired experimental conditions to achieve a sample rich with smooth-faceted calcite crystals (without or at least with a scarce number of vaterite crystals).
- Fill the wells at the corners of a 96-well plate with ammonium carbonate powder and seal the plate using aluminum foil; cover the foil with paraffin film. Clean any residual ammonium carbonate using nitrogen gas.
CAUTION: Ammonium carbonate irritates nose and lungs; use only inside the fume hood. - Prepare a stock solution of 0.5 M CaCl2. This stock solution will be used to prepare a gradient of concentrations of CaCl2 solutions in the multi-well plate.
NOTE: A 10 mL stock solution is sufficient for the whole experiment. - Place the previously cut and cleaned glass pieces into five different wells. Use the closest wells to the center.
- Fill each well bearing a glass piece with 100 µL of a CaCl2 solution16. Mix TDW and 0.5 M CaCl2 (stock) to achieve an increasing concentration gradient of CaCl2 across the different wells. If a different sized well-plate is used, adjust the concentration of CaCl2 to achieve separate calcite crystals (step 1.1.2.10, and see Discussion section).
NOTE: An increasing CaCl2 gradient of 10, 20, 30, 40 ,50 mM concentrations in separate wells is used in this protocol. To increase the concentration range or the number of concentrations tested, use additional wells. - Puncture the cover of each of the wells containing ammonium carbonate 3x with a needle.
- Put back the lid, seal the borders with paraffin film and keep it at 18 °C in an incubator for 20 h.
- After the incubation, open the lid carefully inside a fume hood and remove the crystals formed at the water/air interface with a loop.
- Use a tweezer to transfer the glass pieces into a beaker containing double distillated water (DDW). Remove the samples from the beaker and use a double-sided tape to fix the glass pieces onto the bottom of the Petri dish.
- Dry excessive water touching the borders of the slide with tissue wipes. Cover the petri dish and place it in a desiccator for 24 h.
- Observe the crystals formed on the glass pieces with a stereoscope (3.5x magnification) and/or an upright optical microscope (10x-40x magnification). If the control solutions are clean, rhombohedral crystals (most likely calcite) will be observed with an optical microscope (Figure 2A).
- If in addition to the rhombohedral crystals, the control contains spherical crystals (most likely vaterite, Figure 2B), or if scanning electron microscope (SEM) images show rhombohedral crystals with rough rather than smooth faces (Figure 3A,B), repeat the crystallization protocol making sure that the cleaning step (1.1.1) is performed correctly. Furthermore, make better care that there is no ammonium carbonate in areas on the plate other than the dedicated wells. Otherwise, continue to the next step.
- Fill the wells at the corners of a 96-well plate with ammonium carbonate powder and seal the plate using aluminum foil; cover the foil with paraffin film. Clean any residual ammonium carbonate using nitrogen gas.
- Prepare clean glass pieces. Use the same cleaning procedure to clean the glassware.
- Crystallization in the presence of the additives
- To study the effect of the additives on the crystallization of CaCO3, set up a multi-well plate that contains (in different wells), a control CaCl2 solution without the additives, and CaCl2 solutions with the additives. Use the optimal concentration of CaCl2 found in section 1.1.2 for the experiment.
NOTE: The protocol below uses optimal conditions as those reported in a previous study16. - Repeat step 1.1.2.2.
- Place ammonium carbonate powder in the corners of the plate as described in step 1.1.2.1.
- In each well where precipitation will occur, place a glass piece that was cut and cleaned as described in section 1.1.1.
- To prepare control wells, pipette 90 µL of TDW into the control wells. Prepare at least one replicate of each well including the control. If the additive used is in a buffer solution, then pipette 90 µL of buffer instead of TDW water.
- Prepare the additive-containing wells. Repeat step 1.2.5 by adding 90 µL of the additive solution in water. If the additive is in buffer (instead of TDW), pre-adjust the concentration of the additive with buffer to meet the desired final concentration. Keep a total volume of 90 µL; pipette first the additive, then the buffer.
NOTE: A final concentration of 10 µM of the protein TapA in 100 mM NaCl, 25 mM Tris pH 8.0 buffer16 is used in this protocol. - Add 10 µL of the 0.5 M CaCl2 stock solution (prepared in step 1.2.2) to both the controls and the additives-containing wells to reach a final concentration of 50 mM CaCl2.
- Repeat steps 1.1.2.5-1.1.2.9.
- To study the effect of the additives on the crystallization of CaCO3, set up a multi-well plate that contains (in different wells), a control CaCl2 solution without the additives, and CaCl2 solutions with the additives. Use the optimal concentration of CaCl2 found in section 1.1.2 for the experiment.
2. Characterization of calcium carbonate crystals
- With a scanning electron microscope, observe the calcium carbonate crystals formed in the presence of the additives at a higher resolution than that obtained by optical microcopy (see step 1.1.2.10).
- Mount the glass pieces containing the crystals on an aluminum stub with double-sided carbon tape.
- Coat with a layer of Au/Pd for 40-50 s.
- Acquire the images at 5 kV acceleration voltage.
NOTE: Figure 3A shows a representative SEM image of calcium carbonate crystals formed in a proper control experiment, while Figure 4 shows representative images of calcium carbonate crystals formed in the presence of the protein TapA.
- Perform micro Raman spectroscopy to determine the calcium carbonate polymorphs formed. Micro Raman allows the collection of a Raman spectrum from single crystals rather than from a whole powder.
- Use a 20x objective of the microscope to choose the crystal of interest.
- Collect the Raman spectrum in a range of 100−3200 cm-1 using a 514 nm argon laser.
NOTE: Figure 5 shows representative spectra of calcite (A) and vaterite (B). For the spectrum of aragonite, refer to reference18.
- Quantification of the mass percentage of the additives in the CaCO3 precipitates
- Verify/measure the extinction coefficient (ε) of the additive used. The extinction coefficient of a protein can be given by online servers19. If the extinction coefficient is unknown, measure the absorbance of the additive at different concentrations, plot the absorbance vs. concentration and calculate the extinction coefficient from the slope of the curve.
- Weigh the glass pieces where the crystals formed, preferably use a microbalance.
- Scrap the crystals off the glass into 1.2 mL of 0.1 M acetic acid solution, vortex and sonicate the sample. Store the sample at room temperature for 24 h.
CAUTION: Acetic acid is very hazardous in case of skin or eye contact; handle with caution and dispose following the regulations. - Weigh the glass slide after scraping off the crystals.
- Measure the UV/vis absorbance (A) spectrum of the solution. If the additive is a protein, measure the absorbance at 280 nm and calculate its concentration (C), using the Beer-Lambert equation:
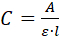
where l is the optical path inside the cuvette. - Use the concentration (C) found in 2.3.5 and the volume used (V = 1.2 mL) to calculate the mass (m) of the additives in/on the crystals. If the concentration is in mg/mL, use the equation C ● V = m.
- If the concentration is in mol/L, then calculate the moles (n) applying C ● V = n. Then use the molecular weight (Mw) to calculate the mass (m) of the additives (m = n ● Mw).
- Calculate the weight percentage of the additives in/on the crystals using the equation:
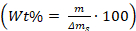 , where m is the mass of the additives, and Δms is the mass of the calcium carbonate crystals that were scrapped off the glass piece.
, where m is the mass of the additives, and Δms is the mass of the calcium carbonate crystals that were scrapped off the glass piece.
Results
A schematic of the experimental set up is shown in Figure 1. Briefly, the diffusion method is used in order to form calcium carbonate crystals in 96-well plates and test the effect of biopolymers on the morphology and structure of the calcium carbonate crystals. In these experiments, ammonium carbonate is decomposed into ammonia and CO2, which diffuse into calcium carbonate solutions, resulting in the formation of calcium carbonate crystals (Figure 1 ...
Discussion
The method described here is aimed at forming calcium carbonate crystals in the presence of organic additives and evaluating the effect of organic biopolymers on the morphology and structure of calcium carbonate crystals in vitro. The method is based on the comparison of the crystals formed in the presence of the organic additives to the calcite crystals formed in the control experiment. We have shown how to use the diffusion method to form the calcium carbonate crystals, how to characterize their morphology using optica...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Prof. Lia Addadi, Prof. Jonathan Erez, and Dr. Yael Politi for fruitful discussions. This research has been supported by the Israeli Science Foundation (ISF), grant 1150/14.
Materials
| Name | Company | Catalog Number | Comments |
| Acetic acid | Gadot | 64-19-7 | |
| Ammonium carbonate | Sigma-Aldrich | 506-87-6 | |
| Calcium chloride dihydrate | Merck KGaA | 10035-04-8 | |
| Ethanol Absolute | Gadot | 64-17-5 | |
| Micro-Raman | Renishaw | inVia Reflex spectrometer coupled with an upright Leica optical microscope | |
| Microscope | Nikon | Eclipse 90i model | |
| Nis elements Br software | Nikon | For microscope imaging | |
| Scanning Electron Microscope | ThermoFisher Scientific | FEI Sirion microscope | |
| Spectrophotometer | JASCO | V-670 model | |
| Sputter coater | Polaron | SC7640 model |
References
- Blakemore, R. Magnetotactic bacteria. Science. 190 (4212), 377-379 (1975).
- Politi, Y., Arad, T., Klein, E., Weiner, S., Addadi, L. Sea Urchin Spine Calcite Forms via a Transient Amorphous Calcium Carbonate Phase. Science. 306 (5699), 1161-1164 (2004).
- Nudelman, F., Lausch, A. J., Sommerdijk, N. A. J. M., Sone, E. D. In vitro models of collagen biomineralization. Journal of Structural Biology. 183 (2), 258-269 (2013).
- Sigel, A., Sigel, H., Sigel, R. K. . Biomineralization: from nature to application. 12, (2008).
- Nielsen, M. H., Lee, J. R. I., De Yoreo, J. J. . Methods in Enzymology. 532, 209-224 (2013).
- Page, M. G., Cölfen, H. Improved Control of CaCO3 Precipitation by Direct Carbon Dioxide Diffusion: Application in Mesocrystal Assembly. Crystal Growth & Design. 6 (8), 1915-1920 (2006).
- Wang, H., Huang, W., Han, Y. Diffusion-reaction compromise the polymorphs of precipitated calcium carbonate. Particuology. 11 (3), 301-308 (2013).
- Sedlák, M., Antonietti, M., Cölfen, H. Synthesis of a new class of double-hydrophilic block copolymers with calcium binding capacity as builders and for biomimetic structure control of minerals. Macromolecular Chemistry and Physics. 199 (2), 247-254 (1998).
- Kitano, Y., Park, K., Hood, D. W. Pure aragonite synthesis. Journal of Geophysical Research. 67 (12), 4873-4874 (1962).
- Politi, Y., Mahamid, J., Goldberg, H., Weiner, S., Addadi, L. Asprich mollusk shell protein: in vitro experiments aimed at elucidating function in CaCO3 crystallization. CrystEngComm. 9 (12), 1171-1177 (2007).
- Gehrke, N., Cölfen, H., Pinna, N., Antonietti, M., Nassif, N. Superstructures of Calcium Carbonate Crystals by Oriented Attachment. Crystal Growth & Design. 5 (4), 1317-1319 (2005).
- Rudloff, J., et al. Double-Hydrophilic Block Copolymers with Monophosphate Ester Moieties as Crystal Growth Modifiers of CaCO3. Macromolecular Chemistry and Physics. 203 (4), 627-635 (2002).
- Boquet, E., Boronat, A., Ramos-Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria is a General Phenomenon. Nature. 246, 527 (1973).
- Cohen, A. L., McConnaughey, T. A. Geochemical Perspectives on Coral Mineralization. Reviews in Mineralogy and Geochemistry. 54 (1), 151-187 (2003).
- Erez, J. Vital effect on stable-isotope composition seen in foraminifera and coral skeletons. Nature. 273, 199 (1978).
- Azulay, D. N., et al. Biopolymers from a Bacterial Extracellular Matrix Affect the Morphology and Structure of Calcium Carbonate Crystals. Crystal Growth & Design. 18 (9), 5582-5591 (2018).
- Abbasi, R., et al. The Bacterial Extracellular Matrix Protein TapA Is a Two-Domain Partially Disordered Protein. ChemBioChem. , (2018).
- Gauldie, R. W., Sharma, S. K., Volk, E. Micro-raman spectral study of vaterite and aragonite otoliths of the coho salmon, Oncorhynchus kisutch. Comparative Biochemistry and Physiology Part A: Physiology. 118 (3), 753-757 (1997).
- Gasteiger, E., et al. . The Proteomics Protocols Handbook. , 571-607 (2005).
- Gunasekaran, S., Anbalagan, G., Pandi, S. Raman and infrared spectra of carbonates of calcite structure. Journal of Raman Spectroscopy. 37 (9), 892-899 (2006).
- Trushina, D. B., Bukreeva, T. V., Kovalchuk, M. V., Antipina, M. N. CaCO3 vaterite microparticles for biomedical and personal care applications. Materials Science and Engineering: C. 45, 644-658 (2014).
- Weiss, I. M., Tuross, N., Addadi, L., Weiner, S. Mollusc larval shell formation: amorphous calcium carbonate is a precursor phase for aragonite. Journal of Experimental Zoology. 293 (5), 478-491 (2002).
- Yamamoto, Y., Nishimura, T., Saito, T., Kato, T. CaCO3/chitin-whisker hybrids: formation of CaCO3 crystals in chitin-based liquid-crystalline suspension. Polymer Journal. 42, 583 (2010).
- Magnabosco, G., et al. Insights on the interaction of calcein with calcium carbonate and its implications in biomineralization studies. CrystEngComm. 20 (30), 4221-4224 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved