A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Murine Surgical Model of Topical Elastase Induced Descending Thoracic Aortic Aneurysm
In This Article
Summary
We describe a surgical protocol to consistently induce robust descending thoracic aortic aneurysms in mice. The procedure involves left thoracotomy, thoracic aorta exposure, and placement of a sponge soaked in porcine pancreatic elastase on the aortic wall.
Abstract
According to the Center for Disease Control, aortic aneurysms (AAs) were considered a leading cause of death in all races and both sexes from 1999-2016. An aneurysm forms as a result of progressive weakening and eventual dilation of the aorta, which can rupture or tear once it reaches a critical diameter. Aneurysms of the descending aorta in the chest, called descending thoracic aortic aneurysms (dTAA), make up a large proportion of aneurysm cases in the United States. Uncontained dTAA rupture is almost universally lethal, and elective repair has a high rate of morbidity and mortality. The purpose of our model is to study dTAA specifically, to elucidate the pathophysiology of dTAA and to search for molecular targets to halt the growth or reduce the size of dTAA. By having a murine model to study thoracic pathology precisely, targeted therapies can be developed to specifically test dTAA. The method is based on the placement of porcine pancreatic elastase (PPE) directly on the outer murine aortic wall after surgical exposure. This creates a destructive and inflammatory reaction, which weakens the aortic wall and allows for aneurysm formation over weeks to months. Though murine models possess limitations, our dTAA model produces robust aneurysms of predictable size. Furthermore, this model can be used to test genetic and pharmaceutical targets which may arrest dTAA growth or prevent rupture. In human patients, interventions such as these could help avoid aneurysm rupture, and difficult surgical intervention.
Introduction
The purpose of this method is to study the development, pathophysiology, and structural changes in the murine descending thoracic aorta during aortic aneurysm formation. Our model offers a reproducible and consistent method to induce thoracic aortic aneurysms (dTAA) in mice thereby allowing for the testing of various genetic and pharmacologic inhibitors. This work can help identify drugs and gene-therapies which could be translated to a viable treatment strategy for humans with dTAA disease.
dTAAs form when the wall of the thoracic aorta becomes weakened and dilates over time until reaching a critical diameter when tearing or rupture can then occur. Clinically, dTAA can progress in silence, increasing in size until the structure of the aortic wall is so distorted that it eventually fails, with catastrophic consequences. Concerningly, symptoms usually develop only when the aneurysm has reached a perilous size (100-150% dilation) and is at high risk for dissection or rupture1,2. dTAA rupture is almost universally lethal3, and elective surgical repair carries significant morbidity4,5. Furthermore, most patients carry the diagnosis of an aortic aneurysm for approximately 5 years before surgical repair6,7. This window represents an opportune time to intervene non-surgically. Thus, medical therapies to treat or slow progression of dTAA are needed and would represent a significant advancement to the field of aneurysm research. There are currently no medical treatments for dTAA available, mostly because of an incomplete understanding of dTAA pathogenesis.
Over the last 20 years, several dTAA animal models have been developed, but each of these models were distinct from our own and did not produce robust aneurysms. A murine dTAA model most similar to ours was developed by Ikonomidis et al.8, which includes direct application of CaCl2 to the adventitia of the aorta. Though our model was adapted from many of the techniques set forth by Ikonomidis, our model is unique in three separate ways. First, in our model the aorta is exposed to topical elastase for 3-5 minutes, compared to 15 minutes of CaCl2 exposure. Second, aortic dilation occurs in 2 weeks, compared to 16 weeks in the CaCl2 model. Last, our model consistently produces aneurysms of approximately 100% dilatation, compared to the aortic dilatations of 20-30% produced by CaCl2 application (which cannot be truly considered aneurysms as they are defined as an increase in aortic diameter >50%). There are other non-surgical murine models of aneurysm formation, such as the Apo E knockout mouse, which form robust aneurysms with infusion of angiotensin II. However, these mice develop supra-renal or ascending thoracic aortic aneurysms rather than aneurysms specifically in the descending thoracic aorta9,10.
The rational for this protocol is to have a simple, inexpensive, and time suitable way to study dTAA in a murine model. The mouse model provides a unique opportunity to utilize many genetic and cell-specific knockouts that have been found to be impactful in other vascular diseases. The use of our specific TAA model has been well received and experiments utilizing it have been published in high impact journals11,12. To this point, the model has been used to investigate possible genetic and pharmacologic treatments that had a significant effect in the abdominal aortic aneurysm (AAA) murine models; however, as our lab has expanded use of the dTAA model, we are finding targets unique to dTAA formation which could be used as targeted therapies in humans.
This model is most appropriate for labs that have murine micro-surgical capabilities. Though it is technically challenging, it can be executed consistently even by researchers with no prior surgical experience. For a researcher with no murine surgical experience the model can be mastered in approximately 20 operative sessions (or approximately 50 mice). For the researcher with prior surgical experience, the model can be mastered in 5 operative sessions (approximately 20 mice). We believe with a high-quality video, the time to mastery can be further reduced. After proficiency is achieved, the procedure can be completed in 35 minutes for the surgery, and 20 minutes for the terminal harvest. The surgeons in our lab can complete 10-12 full surgeries per day, with an operative mortality rate of 5-10%. The most common cause of mortality is lung injury upon entry to the chest, anesthetic toxicity, or tear of the aorta during dissection. In addition to dTAA research, this model also serves as a guide for safe and easy access to the thoracic aorta and lung hilum for researchers studying other interventions in the chest.
Protocol
Animal protocols were approved by the University of Virginia Institutional Animal Care and Use Committee (No. 3634).
1. Induction of anesthesia and intubation
- Place an 8-10-week-old male C57BL/6 mouse in a closed chamber with continuous flow of 5% isoflurane and oxygen mixture for 5 min, until respirations are visibly slowed.
NOTE: Different strains, genders and ages of mice can be used depending on the experimental protocol. Female mice may be more difficult to intubate because of smaller size and thus smaller airway. - Intubate the mouse as described by Vandivort et al.13.
NOTE: The intubation step is the most difficult portion of this model to both learn and perform. The above referenced authors do an excellent job explaining the steps in their video.
2. Securing the mouse to the surgical board
- Connect the endotracheal (ET) tube, confirm chest rise, and lay the mouse in the right lateral decubitus position. Turn isoflurane to approximately 3% and apply lubrication to both eyes. The ventilator should be set to provide approximately 200 breaths per minute and 225 µL of tidal volume.
NOTE: Toxicity is rapid if isoflurane is left at 5%. However, response to isoflurane is variable so anesthetic flow rate should be titrated such that spontaneous diaphragmatic contractions occur approximately every 10-20 s and oxygenation appears adequate on inspection of mucous membranes and exposed tissue. - Secure the ET tube to the board with tape. Extend the right arm rostrally so the front paw is in line with the nose and secure it with tape.
- Pull the tail caudally in order to create a line of tension between the right arm and tail, producing extension of the spine.
NOTE: Securing both the tail and the right paw in line prevents over insertion or dislodgement of the ET tube. - Tape the left leg in its natural position. Draw the left arm ventrally over rolled gauze and tape down.
3. Preparation for surgery
- Shave the left flank of the mouse with electric clippers from left shoulder to left abdomen.
- Use a cotton tipped applicator to brush betadine solution over the surgical site. Use a new cotton tipped applicator to brush 70% ethanol solution over the surgical site. Allow to dry. Place a sterile drape.
- CAUTION: Ensure that all ethanol is completely dried before proceeding as electrocautery is used and can ignite the ethanol.
4. Entry into thorax
- Make a 1 cm lateral incision at the midpoint of the hemithorax using surgical scissors. Use handheld electrocautery to divide muscle layers until the ribs are visible.
- Directly cauterize a 2 mm portion of the rib.
NOTE: When using electrocautery on the rib, observe the spontaneous breathing frequency. If the mouse has a diaphragmatic contraction during cautery, the tip may enter the thorax and puncture the lung, which is usually fatal in this model. - Using a wetted, fine cotton-tipped applicator in the rib space superior to the cauterized portion, rotate the tip upon the tissue to break into the pleural space. Place wetted 3 mm x 2 mm sponge into the thorax to help collapse the lung.
NOTE: Only let soft, wet, sponges contact the lung as it is extremely delicate. - Use scissors to cut along the top aspect of the cauterized rib, until diaphragm is visible.
5. Aortic exposure
- Place rib retractors and use them to open the thoracotomy incision. Carefully remove sponge from the surface of the lung.
- Place wetted 6 mm x 4 mm sponge so that it lays covering the lung, with ends pointing rostrally and caudally. Place the (wide flat) lung retractor on the sponge and gently slide the retractor ventrally until the descending thoracic aorta is exposed.
- Use #7 forceps to dissect the connective tissue and fat off the aorta for an approximately 5 mm section.
NOTE: Small veins may be running transversely across the aorta; avoid tearing them during dissection (using at least 14x magnification can help to avoid this complication).
6. Elastase exposure
- Saturate 0.5 mm x 1 mm sponge with 12 μL of porcine pancreatic elastase and place it upon the exposed surface of the aorta.
NOTE: Do not let the sponge touch the contralateral lung. - After the predetermined time (usually 3-5 min), remove the elastase sponge with #7 forceps. Remove the lung retractor. Irrigate the chest cavity with 1 mL of sterile saline.
NOTE: Remove the lung retractor before irrigation with saline as it will allow the lung sponge to become saturated and soft, making it easier to remove from the surface of the lung. - Use rolled 2" x 2" gauze to absorb the remaining saline irrigation. Turn isoflurane down to 2%.
7. Closure of chest
- Remove the lung sponge. Remove the caudal lung retractor. Remove the rostral lung retractor.
- Place 3 interrupted 6-0 non-absorbable sutures to oppose the ribs, tie a loose knot in each but do not tie down. Take great care to not touch the lung with the suture needle.
- Re-inflate the lung by occluding the outflow tube on ventilator or by rapidly blowing 0.5-1.0 mL of air through the ET tube by utilizing the 3 way stop cock.
NOTE: To avoid barotrauma, avoid excessive use of the air-filled syringe, and use no more than 1.0 mL of air. - Tie non-absorbable sutures. Reapproximate the muscle layers with a running 5-0 absorbable multifilament suture. Turn isoflurane down to 1%.
- Close skin with 7-10 interrupted 5-0 non-absorbable sutures. Turn isoflurane to 0%.
8. Recovery
- Administer 6 µg (0.02 mL of a 0.3 mg/mL suspension) of buprenorphine subcutaneously. Remove the tape from the right foot, tail, and left arm followed by the right arm tape.
- When the mouse moves extremities, extubate by pulling it by its tail to slide off the ET tube. Place in the high oxygen content warmer chamber in the supine position.
NOTE: It is safe to move mouse from oxygen chamber into cage when it can turn itself over to normal standing position. Furthermore, mice should be monitored for signs of pain, distress, or failure to thrive frequently for the first 24-48 hours after surgery and provided additional analgesia or soft food as indicated.
9. Exposure of aortic aneurysm (terminal harvest procedure)
NOTE: In general, tissue harvest is carried out at 14 days, as this represents the period of maximal dilatation. However, depending on the experiment, the harvest procedure timing can be carried out at any time between 3 days and 4+ weeks, depending on the experiment.
- Intubate and secure mouse to operative field as described above (sections 1-3). Use scissors to incise skin medially from left flank to central abdomen taking care to not enter the peritoneum.
- Incise skin from dorsal left flank rostrally to the level of the left shoulder. Then incise at a 90° angle through the axilla to the sternum.
NOTE: This incision should completely encircle the original skin incision. - Using cautery, dissect skin flap toward the ventral aspect of the mouse, exposing the left hemithorax. Use scissors to enter the abdomen and incise along the left costal margin from ventral to dorsal to expose the underside of the left diaphragm. The lateral most edge of the diaphragm may open which is desired.
- If not created with the prior step, incise the diaphragm at its most lateral edge. Place tip of cautery in this hole and cauterize the diaphragm off the costal margin to the xyphoid process. Using a damp fine tipped cotton-tipped applicator, gently free the lung from adhesions to the chest wall and push lung medially.
NOTE: If adhesions do not easily come off the chest wall, use cautery to remove them; doing so helps avoid tearing the lung which can cause heavy bleeding. - Cauterize the inside of the chest wall from rib one to the costal margin, dorsal to the mid axillary line but at least 2 mm from the aorta. Cut chest wall along the cauterized line.
NOTE: This technique avoids bleeding from the intercostal arteries. - Cut along the superior margin of rib one and then caudally along the lateral edge of the sternum, removing the left rib cage. Place retractors on the lung and pull medially. Place retractor on diaphragm and draw caudally to expose as much aorta as possible.
- Use a dry cotton-tipped applicator to remove adhesions from aortic aneurysm and an unaffected distal segment. Measure the diameter of the unaffected control segment and the widest portion of the elastase treated aneurysm using video micrometry.
NOTE: The video micrometry measurements are used to calculate the percent dilation of the aneurysmal segment compared to a control segment with Equation 1. A control segment that is 0.5 mm distal to the aneurysmal segment 1 is selected.
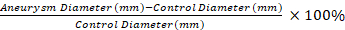 Equation 1
Equation 1
- Grasp aorta with Harms forceps, just distal to the treated segment. Use scissors to cut distal to forceps, then dissect the aorta off spinal column. Cut aorta proximal to treated segment and remove aneurysmal aorta.
- Using a tuberculin syringe and needle, wash the aortic lumen with saline and process tissue as desired.
Results
The application of our protocol results in robust dTAA in mice compared to saline controls. The TAAs developed are fusiform in shape and occur only in the treated portion of the aorta (Figure 1 and Figure 2)11. Figure 2 shows an example of a video micrometry measurement at tissue harvest. Using Equation 1, the aortic dilation is 130% in this example.
The original study by Johnston ...
Discussion
The thoracic and abdominal aorta are cellularly and embryologically distinct, which is relevant to aneurysmal disease14,15,16. Therefore, a specific animal model to study TAA is needed. Though other murine dTAA models have been published8, ours is the only model to create descending thoracic aortic dilatation which can be considered truly aneurysmal (over 50% dilation). Furthermore, our model is relativel...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by AHA Scientist Development Grant 14SDG18730000 (M.S.), NIH K08 HL098560 (G.A.) and RO1 HL081629 (G.R.U.) grants. This project was supported by the Thoracic Surgery Foundation for Research and Education (TSFRE) Research Grant (PI: G. Ailawadi). The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI or the TSFRE. We thank Anthony Herring and Cindy Dodson for their knowledge and technical expertise.
Materials
| Name | Company | Catalog Number | Comments |
| Angiocatheter (22G) | Used for ET Tube | ||
| Dumont Tweezers; Pattern #7 x2 | Roboz | RS-4982 | |
| Graefe Tissue Forceps | Roboz | RS-5158 | |
| Harms Forceps x2 | Roboz | RS-5097 | |
| Intracardiac Needle Holder; Extra Delicate; Carbide Jaws; 7" Length | Roboz | RS-7800 | |
| KL 1500 LED Light Source | Leica | 150-400 | |
| M205A Dissction Microscope | Leica | CH 94-35 | |
| Iris Scissors, 11cm, Tungsten Carbide | World Precision Instruments | 500216-G | |
| Metal Clip board | Use with the Mouse Retractor Set | ||
| Mouse Retractor Set | Kent | SURGI-5001 | Need 2 short and 1 tall fixators |
| Mouse Ventilator MiniVent Type 845, 115 V, Power Supply with US Connector | Harvard Apparatus | 73-0043 | MiniVent Ventilator for Mice (Model 845), Single Animal, Volume Controlled |
| Sigma Aldrich | Elastase from porcine pancreas | E0258-50MG | Can be purchased in various size bottles |
| Small Vessel Cauterizer Kit | Fine Science Tools | 18000-00 | Recommend using rechargable AA batteries |
| Spring Scissors, 10.5cm | World Precision Instruments | 14127 | |
| Steril Swabs (Sponges) | Sugi | 31603 | Can be cut to size |
| Surgi Suite Surgical Platform | Kent | Attach to clip board | |
| Tech IV Isoflurane Vap | Jorgensen Laboratories | J0561A | Anesthesia vaporizer |
References
- Coady, M. A., et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms. Journal of Thoracic and Cardiovascular Surgery. 113 (3), 489-491 (1997).
- Aggarwal, S., Qamar, A., Sharma, V., Sharma, A. Abdominal aortic aneurysm: A comprehensive review. Experimental and Clinical Cardiology. 16 (1), 11-15 (2011).
- Bickerstaff, L. K., et al. Thoracic aortic aneurysms: a population-based study. Surgery. 92 (6), 1103-1108 (1982).
- Cheng, D., et al. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. Journal of the American College of Cardiology. 55 (10), 986-1001 (2010).
- Walsh, S. R., et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. Journal of Vascular Surgery. 47 (5), 1094-1098 (2008).
- Absi, T. S., et al. Altered patterns of gene expression distinguishing ascending aortic aneurysms from abdominal aortic aneurysms: complementary DNA expression profiling in the molecular characterization of aortic disease. The Journal of Thoracic and Cardiovascular Surgery. 126 (2), 344-357 (2003).
- Ailawadi, G., Eliason, J. L., Upchurch, G. R. Current concepts in the pathogenesis of abdominal aortic aneurysm. Journal of Vascular Surgery. 38 (3), 584-588 (2003).
- Ikonomidis, J. S., et al. A murine model of thoracic aortic aneurysms. Journal of Surgical Research. 115 (1), 157-163 (2003).
- Daugherty, A., Manning, M. W., Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. Journal of Clinical Investigation. 105 (11), 1605-1612 (2000).
- Trachet, B., et al. Ascending Aortic Aneurysm in Angiotensin II-Infused Mice: Formation, Progression, and the Role of Focal Dissections. Arteriosclerosis, Thrombosis, and Vascular Biology. 36 (4), 673-681 (2016).
- Johnston, W. F., et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 130 (11), 51-59 (2014).
- Pope, N. H., et al. Interleukin-6 Receptor Inhibition Prevents Descending Thoracic Aortic Aneurysm Formation. Annals of Thoracic Surgery. 100 (5), 1620-1626 (2015).
- Vandivort, T. C., An, D., Parks, W. C. An Improved Method for Rapid Intubation of the Trachea in Mice. Journal of Visualized Experiments. (108), e53771 (2016).
- Ailawadi, G., et al. A nonintrinsic regional basis for increased infrarenal aortic MMP-9 expression and activity. Journal of Vascular Surgery. 37 (5), 1059-1066 (2003).
- Ruddy, J. M., Jones, J. A., Spinale, F. G., Ikonomidis, J. S. Regional heterogeneity within the aorta: relevance to aneurysm disease. Journal of Thoracic and Cardiovascular Surgery. 136 (5), 1123-1130 (2008).
- Trigueros-Motos, L., et al. Embryological-origin-dependent differences in homeobox expression in adult aorta: role in regional phenotypic variability and regulation of NF-kappaB activity. Arteriosclerosis, Thrombosis, and Vascular Biology. 33 (6), 1248-1256 (2013).
- Lu, G. S. G., Davis, J. P., Schaheen, B., Downs, E., Roy, R. J., Ailawadi, G., Upchurch, G. R. A novel chronic advanced staged abdominal aortic aneurysm murine model. Journal of Vascular Surgery. 66 (1), 232-242 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved