A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Monocular Visual Deprivation and Ocular Dominance Plasticity Measurement in the Mouse Primary Visual Cortex
* These authors contributed equally
In This Article
Summary
Here, we present detailed protocols for monocular visual deprivation and ocular dominance plasticity analysis, which are important methods for studying the neural mechanisms of visual plasticity during the critical period and the effects of specific genes on visual development.
Abstract
Monocular visual deprivation is an excellent experimental paradigm to induce primary visual cortical response plasticity. In general, the response of the cortex to the contralateral eye to a stimulus is much stronger than the response of the ipsilateral eye in the binocular segment of the mouse primary visual cortex (V1). During the mammalian critical period, suturing the contralateral eye will result in a rapid loss of responsiveness of V1 cells to contralateral eye stimulation. With the continuing development of transgenic technologies, more and more studies are using transgenic mice as experimental models to examine the effects of specific genes on ocular dominance (OD) plasticity. In this study, we introduce detailed protocols for monocular visual deprivation and calculate the change in OD plasticity in mouse V1. After monocular deprivation (MD) for 4 days during the critical period, the orientation tuning curves of each neuron are measured, and the tuning curves of layer four neurons in V1 are compared between stimulation of the ipsilateral and contralateral eyes. The contralateral bias index (CBI) can be calculated using each cell's ocular OD score to indicate the degree of OD plasticity. This experimental technique is important for studying the neural mechanisms of OD plasticity during the critical period and for surveying the roles of specific genes in neural development. The major limitation is that the acute study cannot investigate the change in neural plasticity of the same mouse at a different time.
Introduction
Monocular visual deprivation is an excellent experimental paradigm to examine V1 plasticity. To study the importance of visual experience in neural development, David Hubel and Torsten Wiesel1,2 deprived kittens of normal vision in one eye at various time points and for varying periods of time. They then observed the changes in response intensity in V1 for the deprived and nondeprived eyes. Their results showed an abnormally low number of neurons reacting to the eye that had been sutured shut in the first three months. However, the responses from the neurons in the kittens remained identical in all respects to those of a normal adult cat's eye that was sutured shut for a year, and the kittens did not recover. MD in adult cats cannot induce OD plasticity. Therefore, the impact of visual experience on V1 wiring is strong during a brief, well-defined phase of development, before and after which the same stimuli have less influence. Such a phase of increased susceptibility to visual input is known as the critical period in visual cortex.
Although the mouse is a nocturnal animal, individual neurons in mouse V1 have similar properties to neurons found in cats3,4,5. In recent years, with the rapid development of transgenic technology, an increasing number of studies in visual neuroscience have used mice as an experimental model6,7,8. In mouse visual studies, neuroscientists use mutants and knockout mouse lines, which allow control over the genetic makeup of the mice. Although mice V1 lack OD columns, single neurons in the V1 binocular zone show significant OD properties. For example, most cells respond more strongly to contralateral stimulation than to ipsilateral stimulation. Temporary closure of one eye during the critical period induces a significant shift in the OD index distribution9,10,11. Therefore, MD can be used to establish an OD plasticity model to investigate how genes involved in neural developmental disorders influence cortical plasticity in vivo.
Here, we introduce an experimental method for MD and suggest a commonly used method (electrophysiological recording) to analyze the change in OD plasticity during monocular visual deprivation. The method has been widely used in many laboratories for more than 20 years12,13,14,15,16. There are other methods used in measuring the OD plasticity as well, such as chronic visual evoked potential (VEP) recording17, and intrinsic optical imaging (IOI)18. The significant advantage of this acute method is that it is easy to follow, and the results are remarkably reliable.
Access restricted. Please log in or start a trial to view this content.
Protocol
In this protocol, male C57Bl/6 mice were obtained from the Institute of Laboratory Animals of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee, University of Electronic Science and Technology of China.
1. Monocular deprivation (MD) at postnatal day 28 in mice
- Put the surgical tools, the suture needle (0.25 mm diameter, string diameter 0.07 mm) and cotton swabs in an aluminum box and autoclave them at 120 °C for 0.5 h. Sterilize the hood with 75% ethanol. Dry the surgical tools in a drying oven.
- Prepare a 2% agarose solution, put it in a water bath at 75 °C to avoid solidifying.
- Use isoflurane mixed with oxygen to anesthetize the mouse (2% induction and 1.2–1.5% maintenance). Fix the mouse on the stereotaxic apparatus and use a heat regulating device to maintain the mouse body temperature at 37 °C and prevent hypothermia.
- Apply a thin layer of petroleum-based eye ointment to both eyes.
- Under the anatomical microscope with illumination, suture the eyelid on one eye. Make the needle pass though both sides of the eyelid 2x (Figure 1A) and make about four stitches.
- Knot the thread 2–3x and then trim the thread. Apply 3 μL of instant drying glue on the knot to increase its stability. Then cut the extra suturing thread.
- Provide an intraperitoneal injection of buprenorphine (1 mg/kg) to the mouse.
- Transfer the mouse onto a heating pad to maintain its body temperature at 37 °C and prevent hypothermia and monitor it until it regains consciousness.
- When the mouse is fully awake place it into a separate holding cage.
- Check the eyelids daily to ensure that they remain shut and uninfected. Exclude the mouse if an eyelid opening is found.
- Before electrophysiological recording, use isoflurane mixed with oxygen to anesthetize the mouse (2% induction and 1.2–1.5% maintenance).
- Remove the stitches with eye scissors to expose the eyeball. Carefully trim the eye lids.
- Flush the eye with lens solution and check the eye under a microscope for clarity. Exclude mice with corneal opacities or signs of infection.
2. Craniotomy in the mouse V1 binocular region after monocular deprivation on the 4th day
- After anesthetizing the mouse, check for the depth of anesthesia by the lack of response to a toe pinch.
- Place and fix the mouse on the stereotaxic apparatus. Adjust the height of the ear bar and tooth rod to keep the brain flat and stable.
- Use a heating pad to maintain the body temperature.
- Apply a petroleum-based eye ointment on the surface of the eyes to keep them moist.
- Remove the hair on the mouse's head to expose its skin. Rub the skin with alternating scrubs of iodine and 70% ethanol 3x.
- Incise an 8 x 8 mm area of the skin between the ears to expose the skull and remove the scalp tissue. Then remove the overlying connective tissue with 30% hydrogen peroxide.
- Drill a 1 x 1 mm hole in the skull above the cerebellum. Affix a small bone screw in the hole as a reference.
- Perform a small craniotomy of 1 mm in diameter in the V1 binocular region from the contralateral hemisphere to the deprived eye (Figure 1B, A-P: lambda -0.51–lambda +1.67 mm; M-L: -2.6– -3.0 mm; D-V: 0–1 mm). Carefully remove the skull fragment without hurting the brain.
- Cover the exposed cortical surface with 75 μL of 2% agarose at 40 °C to prevent drying.
- Fix a tungsten electrode on the stereotaxic frame. Place the tungsten electrode vertically on the surface of the exposed cortex, the binocular region of V1, to make sure that the cells that are recorded react to both eyes.
- Use cotton swabs to remove the eye gel and apply silicon oil to the eye every 2 h.
3. Visual stimulation and electrophysiological recording
- Mask the one eye with nontransparent plastic plate. Position an LCD monitor 23 cm from the mouse's eye.
- Reduce the anesthesia to 0.5–0.8% when the mouse is fully anesthetized.
- Advance the microelectrode electrode slowly with an oil hydraulic micromanipulator. Stop it when a high signal-to-noise ratio is observed and the electrode is advanced to layer 4 (Figure 1C, approximately 250–450 μm in depth). Ensure that the amplification factor is set at 1,000, the filter at 300–100 Hz, and the sample rate at 40 Hz.
- Present a full-field moving sinusoidal grating (Figure 1D, 12 directions, 100% contrast, 2 Hz of temporary frequency, 0.04 cycles per degree of spatial frequency) on the LED monitor.
- Measure the cell's response by stimulating the ipsilateral and contralateral eye separately. Present 3–5x total.
- Measure the responses of five to eight cells in each penetration. Perform four to six penetrations in each mouse.
- After the recording, adjust the isoflurane flow rate to 5% or greater, continue isoflurane exposure for 1 min, and then perform the cervical dislocation.
NOTE: Separate penetrations were spaced at least 200 μm apart in the V1 binocular zone.
4. Off-line spike sorting and data analysis
- Detect spikes when the raw signal crosses a threshold level. Align captured spikes on the first positive or negative peak. Use software to detect spikes from different cells.
- Set two cursors: one for positive and the other for negative deflection. Set the spike template (Figure 2A). Set the template area to that with the most significant variation between different classes of spikes.
- Use principal component analysis to separate them into clusters. Clustering methods can vary between different laboratories.
- Classify the spike of a boundary by using the K-means algorithm.
- Correlate the orientation with the spike firing rate and plot the orientation tuning curves for the ipsilateral and contralateral eye.
- Calculate the OD index for the single unit, which represents the contralateral/ipsilateral response strength ratio:
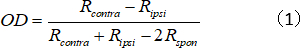
where Rcontra and Ripsi are the cell's optimal response for the contralateral and ipsilateral eye, respectively, and Rspon is the cell's spontaneous activity. - Assign OD scores to 1–7 as follows: − 1 to −0.75 = 1; −0.75 to −0.45 = 2; −0.45 to −0.15 = 3; −0.15 to 0.15 = 4; 0.15 to 0.45 = 5; 0.45 to 0.75 = 6; and 0.75 to 1 = 7.
- Calculate the contralateral bias index (CBI):
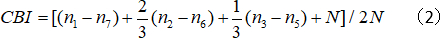
where N is the cell number, and nx equals the cell number with OD scores equal to x.
Access restricted. Please log in or start a trial to view this content.
Results
The experimental results described here enable successful MD and OD plasticity measurements from a deprived and nondeprived mouse during the critical period (P19–P32). Figure 1 shows how to perform single unit recordings in layer 4 from V1 the binocular zone for comparing responses in the ipsilateral and contralateral eye 4 days after MD. Figure 2 shows the spike sorting and orientation tuning measurements for stimulating the ipsilateral and contralateral ...
Access restricted. Please log in or start a trial to view this content.
Discussion
We present a detailed protocol for MD and measuring OD plasticity by single unit recording. This protocol is widely used in visual neuroscience. Although the MD protocol is not complicated, there are some critical surgical procedures that must be followed carefully. First, there are two important details ensuring the quality of the stitching. The suture is sufficiently stable if the stitches are concentrated in the medial portion of the eyelid. Moreover, 3 μL of glue is applied to the head of the knot to increase th...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81571770, 81771925, 81861128001).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 502 glue | M&G Chenguang Stationery Co., Ltd. | AWG97028 | |
| Acquizition card | National Instument | PCI-6250 | |
| Agarose | Biowest | G-10 | |
| Amplifier | A-M system | Model 1800 | |
| Atropine | Aladdin Bio-Chem Technology Co., Ltd | A135946-5 | |
| Brain Stereotaxic Apparatus | RWD Life Science Co.,Ltd | 68001 | |
| Cohan-Vannas spring scissors | Fine Science Tools | 15000-02 | |
| Contact Lenses Solutions | Beijing Dr. Lun Eye Care Products Co., Ltd. | GM17064 | |
| Cotton swabs | Henan Guangderun Medical Instruments Co.,Ltd | ||
| Fine needle holder | SuZhou Stronger Medical Instruments Co.,Ltd | CZQ1370 | |
| Forcep | 66 Vision Tech Co., Ltd. | 53320A | |
| Forcep | 66 Vision Tech Co., Ltd. | 53072 | |
| Forcep | 66 Vision Tech Co., Ltd. | #5 | |
| Heating pad | Stryker | TP 700 T | |
| Illuminator | Motic China Group Co., Ltd. | MLC-150C | |
| Isoflurane | RWD Life Science Co.,Ltd | R510-22 | |
| LCD monitor | Philips (China) Investment Co., Ltd. | 39PHF3251/T3 | |
| Microscope | SOPTOP | SZMT1 | |
| Noninvasive Vital Signs Monitor | Mouseox | ||
| Oil hydraulic micromanipulator | NARISHIGE International Ltd. | PC-5N06022 | |
| Petrolatum Eye Gel | Dezhou Yile Disinfection Technology Co., Ltd. | 17C801 | |
| Spike2 | Cambridge Electronic Design, Cambridge, UK | Spike2 Version 9 | |
| Surgical scissors | 66 Vision Tech Co., Ltd. | 54010 | |
| Surgical scissors | 66 Vision Tech Co., Ltd. | 54002 | |
| Suture Needle | Ningbo Medical Co.,Ltd | 3/8 arc 2.5*8 | |
| Tungsten Electrode | FHC, Inc | L504-01B | |
| Xylocaine | Huaqing |
References
- Hubel, D. H., Wiesel, T. N. Effects of monocular deprivation in kittens. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 248 (6), 492-497 (1964).
- Daw, N. W., Fox, K., Sato, H., Czepita, D. Critical period for monocular deprivation in the cat visual cortex. Journal of Neurophysiology. 67 (1), 197-202 (1992).
- Guire, E. S., Lickey, M. E., Gordon, B. Critical period for the monocular deprivation effect in rats: assessment with sweep visually evoked potentials. Journal of Neurophysiology. 81 (1), 121-128 (1999).
- Wang, L., Sarnaik, R., Rangarajan, K. V., Liu, X., Cang, J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. Journal of Neuroscience. 30 (49), 16573-16584 (2010).
- Niell, C. M. Cell Types, circuits, and receptive fields in the mouse visual cortex. Annual Review of Neuroscience. 38 (1), 413-431 (2015).
- Lee, S. H., et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 488 (8), 379-383 (2012).
- Cossell, L., et al. Functional organization of excitatory synaptic strength in primary visual cortex. Nature. 518 (2), 399-403 (2015).
- Lacaruso, M. F., Gasler, L. T., Hofer, S. B. Synaptic organization of visual space in primary visual cortex. Nature. 547 (7), 449-452 (2017).
- Metin, C., Godement, P., Imbert, M. The primary visual cortex in the mouse: Receptive field properties and functional organization. Experimental Brain Research. 69 (3), 594-612 (1988).
- Marshel, J. H., Garrett, M. E., Nauhaus, I., Callaway, E. M. Functional specialization of seven mouse visual cortical areas. Neuron. 72 (6), 1040-1054 (2011).
- Gordon, J. A., Stryker, M. P. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. The Journal of Neuroscience. 16 (10), 3274-3286 (1996).
- McGee, A. W., Yang, Y., Fischer, Q. S., Daw, N. W., Strittmatter, S. M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 309 (5744), 2222-2226 (2005).
- Sawtell, N. B., et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 38 (6), 977-985 (2003).
- Hofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T., Hubener, M. Prior experience enhances plasticity in adult visual cortex. Nature Neuroscience. 9 (12), 127-132 (2006).
- Crozier, R. A., Wang, Y., Liu, C., Bear, M. F. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proceedings of the National Academy of Sciences. 104 (4), 1383-1388 (2007).
- Tagawa, Y., Kanold, P. O., Majdan, M., Shatz, C. J. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience. 8 (3), 380-388 (2005).
- Lickey, M. E., Pham, T. A., Gordon, B. Swept contrast visual evoked potentials and their plasticity following monocular deprivation in mice. Vision Research. 44, 3381-3387 (2004).
- Cang, J., Kalatsky, V. A., Lowel, S., Stryker, M. P. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vision Neuroscience. 22 (5), 685-691 (2005).
- Khan, I. U., et al. Evaluation of different suturing techniques for cystotomy closure in canines. Journal of Animal & Plant Sciences. 23 (4), 981-985 (2013).
- Weisman, D. L., Smeak, D. D., Birchard, S. J., Zweigart, S. L. Comparison of a continuous suture pattern with a simple interrupted pattern for enteric closure in dogs and cats: 83 cases (1991-1997). Journal of the American Veterinary Medical Association. 214 (10), 1507-1510 (1999).
- Heneghan, C. P. H., Thornton, C., Navaratnarajah, M., Jones, J. G. Effect of isoflurane on the auditory evoked response in man. BJA: British Journal of Anaesthesia. 59 (3), 277-282 (1987).
- Mitzdorf, U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomenal. Physiological Reviews. 65 (1), 37-100 (1985).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved