A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Pupal and Adult Injections for RNAi and CRISPR Gene Editing in Nasonia vitripennis
In This Article
Summary
Here, we describe methods for efficient pupal and adult injections in Nasonia vitripennis as accessible alternatives to embryo microinjection, enabling functional analysis of genes of interest using either RNA-silencing via RNA interference (RNAi) or gene knockout via CRISPR/Cas9 genome editing.
Abstract
The jewel wasp, Nasonia vitripennis, has become an efficient model system to study epigenetics of haplo-diploid sex determination, B-chromosome biology, host-symbiont interactions, speciation, and venom synthesis. Despite the availability of several molecular tools, including CRISPR/Cas9, functional genetic studies are still limited in this organism. The major limitation of applying CRISPR/Cas9 technology in N. vitripennis stems from the challenges of embryonic microinjections. Injections of embryos are particularly difficult in this organism and in general in many parasitoid wasps, due to small embryo size and the requirement of a host pupa for embryonic development. To address these challenges, Cas9 ribonucleoprotein complex delivery into female ovaries by adult injection, rather than embryonic microinjection, was optimized, resulting in both somatic and heritable germline edits. The injection procedures were optimized in pupae and female wasps using either ReMOT Control (Receptor-Mediated Ovary Transduction of Cargo) or BAPC (Branched Amphiphilic Peptide Capsules). These methods are shown to be effective alternatives to embryo injection, enabling site-specific and heritable germline mutations.
Introduction
CRISPR/Cas9 gene editing is a powerful technology for functional genetic studies, especially in many rising model organisms such as the jewel wasp, Nasonia vitripennis. The ease of rearing and the availability of a complete genome makes the jewel wasp an important experimental system for elucidating the molecular mechanisms of different biological processes. For example, N. vitripennis has recently been used to unravel the epigenetic basis of the haplodiploid sex determination system1,2, the biology of B-chromosomes3,4,5,6, and the genetic basis for circadian and seasonal regulation7,8. Some of the features that make N. vitripennis amenable to work with include short generation time (~2 weeks at 25 °C), high reproduction rates, easy sex separation at the pupal stage, and the ability to diapause and store strains at 4 °C. The life cycle begins with female wasps parasitizing the pupae of the blowfly, Sarcophaga bullata. Through their ovipositor, females lay up to 50 eggs in the pupal case of the blowfly. Eggs develop into larvae that feed on the S. bullata pupa, continue to develop over the next several days, and then pupate, followed by adult eclosion and emergence from the host puparium9.
Molecular tools to perform functional genetic studies in N. vitripennis, such as RNA interference (RNAi)10 and CRISPR/Cas911,12, are available, but are limited, primarily due to difficulties in performing embryonic microinjections13. As N. vitripennis eggs require a pupal host for development, egg manipulation is very challenging. Pre-blastoderm stage embryos must be collected from the host blowfly pupae, quickly microinjected, and immediately transferred back to the host for development13. These steps require precision and specialized training to avoid damaging the microinjected embryos or the pupal hosts13. Moreover, the eggs are very small and fragile, especially after microinjections, with a very viscous cytoplasm causing a continuous clogging of the injecting needle13. These features make embryonic microinjections exceptionally challenging, requiring highly trained operators and specialized equipment that is absent in most N. vitripennis laboratories.
The optimization of alternative injection methods for the delivery of CRISPR reagents would contribute to the consolidation of N. vitripennis as a model organism. The manipulation of pupae and adults is less challenging than manipulating embryos and can be accomplished with a basic injection setup. Here, two protocols are described for injection of pupae and adults: one involving specialized equipment for injections, and the other involving the use of an aspirator tube assembly fitted with a glass capillary needle. The use of an aspirator tube is particularly suited for laboratories that do not have access to specialized equipment for embryo microinjections. Efficient injections of different developmental stages of N. vitripennis, including white or black pupae and adult wasps, are demonstrated. Wasps at the white pupal stage are particularly suited for RNAi-mediated knockdown experiments. Although RNAi in Nasonia was first described by Lynch and Desplan in 200610, there is no visual procedure available for how RNAi injections are performed. RNAi was recently used to discover the haploidizer gene of the B-chromosome PSR (Paternal Sex Ratio)3 and to study the involvement of the clock gene, period, in N. vitripennis biological rhythms7.
Black pupae and adult wasps can be used to induce CRISPR/Cas9 germline gene editing using ReMOT Control (Receptor-Mediated Ovary Transduction of Cargo) and BAPC (Branched Amphiphilic Peptide Capsules) protocols. These two ovary delivery methods have been recently described to be effective in Nasonia for generating germline mutations in the target gene, cinnabar7,12. Here, a simplified protocol is provided for injections including a visual procedure of a step-by-step methodology for both pupal and adult injections that can be utilized to generate functional genetics studies in Nasonia and likely in other parasitoid wasps, without requiring specialized equipment and bypassing embryonic microinjections.
Protocol
1. Nasonia rearing
- Set up ~20 mated females singularly in small glass test tubes plugged with cotton.
NOTE: To reduce rearing space, 10 x 85 cm, 4 mL tubes are optimal. Females are easily distinguished from males because of the larger wings and the presence of the ovipositor (Figure 1A). Detailed protocols for rearing N. vitripennis can also be found in other literature13,14,15.- Add two S. bullata hosts per tube, and maintain the wasps at 25 ± 1 °C and 30% relative humidity, with a 12:12 light-dark cycle for 2-3 days.
NOTE: Wasps can be maintained at lower temperature or room temperature; however, development will be slowed down.
- Add two S. bullata hosts per tube, and maintain the wasps at 25 ± 1 °C and 30% relative humidity, with a 12:12 light-dark cycle for 2-3 days.
- After 2-3 days, with the help of a fine-tip paint brush, gently remove the females to avoid continuous oviposition and asynchrony in offspring development.
NOTE: Removed females can optionally be re-hosted or stored at 5 °C for up to a month.- Maintain the parasitized host at 25 °C and 30% relative humidity, with a 12:12 light-dark cycle for 7 days if the desired injection stage requires white pupae; for 13 days if the required developmental stage is black pupae; or 14 days for young, newly emerged adults.
NOTE: Yellow and black pupae can also be stored at 5 °C for up to one week. Longer storage will increase the frequency of diapause in larvae for the next generation, making the post-injection screening and establishment of mutant lines more difficult. - Crack open the host puparium with a dissecting needle to recover N. vitripennis pupae and adults at the desired stage (Figure 1A).
NOTE: For injections at a particular adult age or for collecting virgin females, it is recommended to remove the dark pupae on day 13 from the host, separate by sex, and collect adults after emergence.
- Maintain the parasitized host at 25 °C and 30% relative humidity, with a 12:12 light-dark cycle for 7 days if the desired injection stage requires white pupae; for 13 days if the required developmental stage is black pupae; or 14 days for young, newly emerged adults.
2. Alignment of white and black pupae
- Prepare a glass slide by applying a line of school glue at the center. Spread the glue with a dissecting needle to obtain a thick layer (Figure 1B), which will be used to align the pupae. Let the glue dry for ~2 min before transferring the pupae.
NOTE: Do not overdry the glue, otherwise the pupae will not be properly attached to the slide and will slip over during the injections. - Under a dissecting microscope, using the dissecting needle, apply glue on the back of the head of the pupa.
- Attach the pupa to the glue layer on the slide with its abdomen facing up. Align 20-30 pupae side by side on the slide (Figure 1C).
NOTE: Avoid touching the abdomen with the glue, and avoid submerging the pupae into the glue; otherwise, the adults will not be able to emerge. - Place the slide with the pupae in a Petri dish to let the glue dry for ~10 min. Before starting the injections, test the adherence of the pupae to the glue by pushing them gently with the dissecting needle. If most of the pupae are loosened, prepare a new slide. Alternatively, remove all the pupae that are loose, and proceed to inject those that are properly attached to the slide.
3. Needle preparation
- Load one capillary glass tube into a needle puller, and pull needles following the manufacturer's instructions.
NOTE: A P-1000 platinum filament needle puller and aluminum silicate glass capillaries are used in this demonstration. The operation manual for this instrument (see Table of Materials) explains how to properly load capillary glass tubes and set up programs on this instrument. Use the following parameters (Heat: 536; Pull: 80; Vel: 100; Delay: 70) on aluminum silicate glass capillaries to inject yellow pupae, black pupae, and adults.
In addition, the P-2000 needle puller was used to prepare quartz needles following the parameters (Heat: 805; Pull: 145; Vel: 50; Delay: 145). The manual provides instructions for loading capillary quartz tubes and setup of programs in this instrument. In the absence of a puller, customizable capillary glass is available. - Load the needle with 5 µL of the injection mixture (prepared in advance following previous protocols for RNAi10 or CRISPR/Cas9 ribonucleoprotein12 (RNP) injections in N. vitripennis) using microloader pipette tips.
NOTE: During the demonstration, the needle is loaded with double-stranded RNA (dsRNA) combined with red dye (see Table of Materials) for injection of white stage pupae and with BAPC-Cas9-sgRNA for injection of black pupae wasps and adults. These reagents will target the cinnabar gene. Wasps that are successfully knocked down/out will show red eyes instead of the wild type brown eyes. - Open the needle, sliding the tip on a surface made from two overlapping slides (Figure 1D). Alternatively, open the tip of the needle with a pair of fine forceps to create a sharp edge or by slowly piercing the thorax or abdomen of the wasp.
4. Pupal injection with femtojet
- Place the slide with the aligned pupae under a dissecting microscope. Insert the needle into the femtojet's injection tube and tighten the grip head.
- Looking at the needle under the microscope, turn on the femtojet and set up Pc and Pi injection parameters by rotating the rotary knobs.
NOTE: A Pc value of 600 hPa is recommended for a continuous flow of the liquid. The Pc value depends on the opening of the needle. For needles prepared using the indicated parameters in the P-1000 needle puller, Pc values between 500 hPa and 700 hPa produce a constant flow. If lower values of Pc are required, this indicates that the opening may be too large and could damage the insects, whereas higher values indicate that the needle is still closed, or that the opening is too small. - Carefully insert the needle between the 2 and 3 visible abdominal segments with a vertical angle of about 30° (Figure 1E). Inject with a continuous flow until the whole abdomen turns pink in the case of white-stage pupae, or until the abdomen increases in size in the case of black pupae. Stop injecting when it is clear that the abdomen cannot take any more liquid, or when the liquid starts flowing out of the body. Move carefully to the next pupa and repeat these steps.
NOTE: Avoid touching and damaging the ovipositor with the needle during injection. - Transfer the slide with the injected pupae to a Petri dish containing a paper towel soaked with deionized water to maintain humidity. Cover the dish with its lid, and place it at 25 °C until wasp emergence (Figure 1F).
5. Pupal injection with aspirator tube
- Calculate the amount of injection mix using the factor: 4 pupae injected/1 µL solution.
NOTE: A typical injection of 20-30 pupae requires ~ 5-8 µL of ribonucleoprotein (RNP) complex-saponin mix or RNP with BAPC12. - Align the pupae, as suggested in section 2, and place one slide with the aligned pupae under a dissecting microscope (Figure 1E).
- Take one of the capillary needles and break the tip between two glass slides, as indicated in step 3.4 (Figure 1D). Ensure that the needle tip is open enough to allow the injection solution to go out by blowing air with the mouth, but not too open to avoid losing liquid and damaging the pupae (Figure 1E).
NOTE: This step is critical because the user will be using air from the mouth to push the injection mixture into the insect hemolymph. It is better to practice so as to ascertain what type of needle aperture is optimal for the viscosity of a particular solution mix. Five sets of injections of 20 pupae per set may be enough to get used to the mouth injection system. - Load one needle with the ribonucleoprotein solution12 using a microloading tip, and insert the needle into the connector pack of the aspirator tube assembly.
NOTE: During the demonstration, the needle is loaded with BAPC-Cas9-sgRNA targeting the Nasonia cinnabar gene12. - Carefully insert the needle between the 2 and 3 visible abdominal segments with a vertical angle of about 30° (Figure 1E). Inject with a continuous flow until the whole abdomen turns pink in the case of white-stage pupae or until the abdomen increases in size in the case of black pupae. Stop injecting when it is clear that the abdomen cannot take any more liquid or when the liquid starts flowing out of the body. Move carefully to the next pupa and repeat these steps
NOTE: Avoid touching and damaging the ovipositor with the needle during injection. - Transfer the slide with the injected pupae to a Petri dish containing a paper towel soaked with deionized water to maintain humidity. Cover the dish with its lid and place it at 25 °C until wasp emergence (Figure 1F).
6. Adult injection with aspirator tube
- For adult preparation, separate groups of 20 virgin females in a clean small test tube with a fine paintbrush. Place the tube on ice for 5 min until the females are anesthetized. Alternatively, females can be anesthetized and injected using CO2.
NOTE: Ice is preferable as an anesthetic as adult wasps are cold-tolerant and recover easily after the injections. Females can take up more injected liquid than the pupae: 1 µL of solution can be used for three females instead of for four pupae. Prepare volumes accordingly. - Prepare an ice bucket, placing one glass slide on top of the ice. Align the females side by side on the cold slide with abdomens facing up (Figure 1E) under a dissecting scope.
- Load one needle with the ribonucleoprotein solution12 using a microloading tip, and insert the needle into the connector pack of the aspirator tube assembly. Support the females from the opposite side with blunted dissecting needles while injecting slowly into the abdomen from the other side. Avoid touching the ovipositor, this will severely damage this critical reproductive structure (Figure 1E).
NOTE: Either orientation is acceptable, depending on operator preference. - Carefully insert the needle between the 2 and 3 visible abdominal segments with a vertical angle of about 30° (Figure 1E). Inject the solution into the female abdomen, stopping when no more liquid can enter, or when leaking of surplus solution is observed. Move carefully to the next wasp and repeat these steps
NOTE: Inject slowly, leaving the needle inside the abdomen for about 3 s before removing it very slowly. This will help to adjust the internal liquid pressure and avoid solution leaking from the injection site following needle removal. - When finished, gently transfer single injected females to a new tube with one host using a paintbrush. Leave to recover at room temperature for approximately 1 h, confirming survival, and then return the tubes to the rearing incubator.
NOTE: During the demonstration, the needle is loaded with BAPC-Cas9-sgRNA targeting the Nasonia cinnabar gene12.
7. Post-injection care and mutant screening
- After adult eclosion from the injected pupae, place single wasps into a glass tube plugged with cotton and insert one S. bullata host (Figure 1G). In contrast, place injected females in individual tubes with hosts immediately after injection.
- For CRISPR/Cas9 knockout experiments, allow females to parasitize the hosts for one day, and replace the host each day for three consecutive days.
NOTE: Owing to the haplodiploid sex determination system in N. vitripennis16, virgin females will produce haploid male broods, which facilitates the detection of mutations. For knockdown via RNAi of male-specific genes, the injected individuals can be singularly mated with wild type individuals and allowed to parasitize hosts. Use one host per day, and replace the hosts based on the experimental setup. - Place the parasitized hosts at 25 °C until emergence of the G0 male offspring (for ~13-14 days).
- Under a dissecting microscope, screen G0 males for the mutated phenotypes. The cinnabar gene is responsible for eye pigmentation12: wasps with brown eyes are wild type, and wasps with bright red eyes or variations between red and brown eyes are mutants (Figure 1H).
8. Post-injection crosses and rearing
- Place all the mutant G0 males with red eyes (the phenotypically disrupted cinnabar12 gene) with wild type virgin females for 1-2 days (Figure 2A).
NOTE: If the disrupted gene does not confer a visible phenotype, polymerase chain reaction (PCR) followed by sequencing of the target gene is required to identify the mutant animals. Before obtaining the DNA from G0 males for PCR, it would be ideal to mate them with wild type virgin females. Alternatively, extract DNA from a leg of a G0 male to identify mutants, and mate only those with verified mutations. - Add two S. bullata hosts per female, and allow to oviposit at 25 °C and 30% relative humidity with a 12:12 light-dark cycle. Replace the hosts every 2-3 days.
- Store parasitized hosts at 25 °C and 30% relative humidity, with a 12:12 light-dark cycle for ~10 days.
- After ~10 days, crack open the parasitized host with a dissecting needle, and remove each N. vitripennis pupa from the host.
- With the help of a fine-tip paintbrush, select female pupae (Figure 1A).
NOTE: Females are diploid and heterozygous, and thus the eye color will be wild type.
- Place 15-20 female pupae in glass tubes at 25 °C until emergence. After emergence, add ~20 S. bullata hosts and let the virgin G1 females parasitize the hosts for 2-3 days.
NOTE: These females will produce 50% G2 mutant males.- Store parasitized hosts at 25 °C and 30% relative humidity, with a 12:12 light-dark cycle until G2 male adult emergence.
- Place the remaining G1 pupae at 5 °C for 8-10 days . After this period, remove them and place them at 25 °C and 30% relative humidity, with a 12:12 light-dark cycle until emergence (Figure 2A).
- After emergence of the G2 males in step 8.3.1, screen for the presence of red-eyed mutant phenotype. With the help of a fine-tip paintbrush, separate red-eyed males from wild type males.
NOTE: The presence of red-eyed wasps in this generation (G2, Figure 2A) indicates that gene editing occurred in the germline, and that the mutation is hereditary. - Place red-eyed males with the G1 females newly emerged in step 8.3.2 (Figure 2A), and let them mate for 1-2 days. Add 2 S. bullata hosts per female, and place them at 25 °C and 30% relative humidity with a 12:12 light-dark cycle for 10-11 days.
- After 10-11 days, crack open the parasitized host with a dissecting needle, and remove each N. vitripennis pupa from the host (Figure 2A).
- With the help of a fine-tip paintbrush, separate red-eyed wasps (males and females) from wild type (Figure 1H). Continue rearing of red-eyed wasps together in glass tubes, and do not mix with the wild type wasps.
NOTE: At this stage (G3, Figure 2A), haploid males and diploid females carry and show phenotypes for the cinnabar mutations. If the affected gene encodes for an invisible phenotype, cross G0 male singularly with one wild type female for ~1 day. Then, remove the male for molecular characterization of the mutation by PCR and sequencing, and let the female parasitize a host. Continue the crossing scheme only with the offspring of a confirmed mutant male (Figure 2B). If the goal is the knockdown by RNAi of a candidate gene, perform the desired functional assay (such as lethality, sterility, or diapause induction assay3,7) directly using individuals injected with dsRNA. As RNAi is transient, it is not possible to generate a colony with the desired phenotype (Figure 2C)3,7.

Figure 1: Timeline for pupal and adult Nasonia vitripennis microinjection. (A) Male and female white (top) and black (bottom) pupae are collected, (B-C) aligned and glued to a glass slide for injection. (D) Capillary needle is prepared and opened, sliding it on two overlapping slides. (E) Needle is attached either to the Femtojet (top) or to an aspirator tube (bottom) for injections.(F) The injected pupae on the slide are transferred to a Petri dish with a wet tissue on the bottom to keep humidity. Upon emergence, (G) females are placed singularly in small glass tubes and allowed to oviposit on Sarcophaga pupa. (H) Screening of the offspring to detect mutants. Please click here to view a larger version of this figure.
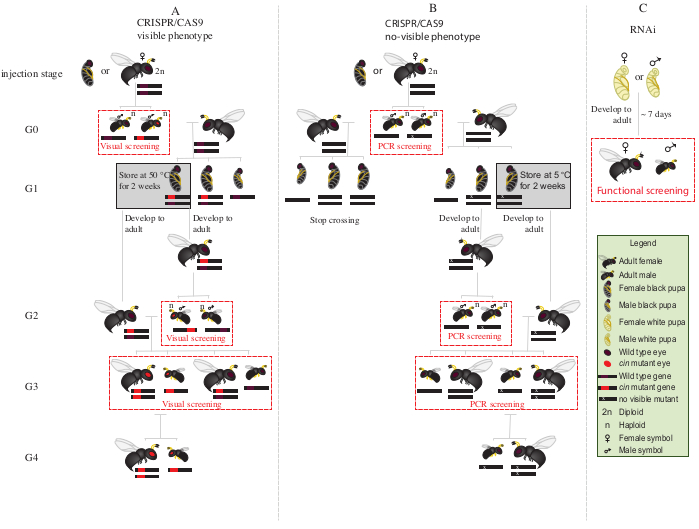
Figure 2: Crossing scheme after injection. Schematic representation of crossing scheme procedure in the case of CRISPR/Cas9-mediated gene editing of genes (A) that induce a visible phenotype and (B) that do not confer visible phenotype. (C) Schematic representation of RNAi screening procedure. Abbreviations: cin = cinnabar; PCR = polymerase chain reaction. Please click here to view a larger version of this figure.
Results
This paper presents two easy methods for pupal and adult microinjection, either using a femtojet or an aspirator tube. The first method allows a more precise injection of liquid, which is important for RNAi consistency, whereas the second one allows the injection of larger amounts of liquid into Nasonia pupae or adults.
Representative results presented in Table 1 show good survival rates (from 20% to 89...
Discussion
With the recent increased use of Nasonia vitripennis as a model organism for various biological questions2,3,7,17, there is a need to develop and optimize injection methods to enable a simplified and efficient protocol for the functional analysis of N. vitripennis genes. The current methods involving embryonic microinjection of gene editing reagents are challenging
Disclosures
JLR and DCR have filed for provisional patent protection on ReMOT Control technology. O.S.A is a founder of Agragene, Inc., has an equity interest, and serves on the company's Scientific Advisory Board. All other authors declare no competing interests.
Acknowledgements
This work was supported in part by UCSD startup funds directed to O.S.A. and NSF/BIO grant 1645331 to J.L.R.
Materials
| Name | Company | Catalog Number | Comments |
| 100 x 15 mm Stackable Petri Dishes, Polystyrene, Mono, Sterile | Sigma | 960-97693-083 | |
| Aluminosilicate glass capillary tubing 1 mm(outside diameter) x 0.58 mm (inner diameter) | Sutter Instruments | BF100-58-10 | Can also use Borosilicate or Quartz |
| Aspirator tube assemblies for calibrated microcapillary pipettes | Sigma | A5177-5EA | |
| BAPC | phoreus biotech | BAPtofect-25 0.5 mg Kit | |
| Cas9 Protein with NLS | PNABio | CP01 | |
| Dissecting needle | VWR | 10806-330 | |
| DNase/RNase-Free distilled Water | Invitrogen | 10977-015 | |
| Femtojet Express programmable microinjector | Eppendorf | ||
| Femtotips Microloader tips | Fisher Scientific | E5242956003 | |
| Fine-tip paintbrush | ZEM | 2595 | |
| Flesh fly pupae, Sarcophaga bullata | Ward's Science | 470180-392 | |
| Food colorant dye | |||
| Glass Test Tubes | Fisher Scientific | 982010 | |
| Glue | Elmer's | washable, no toxic school glue | |
| Micropipette Puller | Sutter Instruments | P-1000 or P-2000 | |
| Microscope Slides | Fisherbrand | 12-550-A3 | |
| Stereo Microscope | Olympus | SZ51 |
References
- Verhulst, E. C., Beukeboom, L. W., van de Zande, L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science. 328 (5978), 620-623 (2010).
- Verhulst, E. C., Lynch, J. A., Bopp, D., Beukeboom, L. W., van de Zande, L. A new component of the Nasonia sex determining cascade is maternally silenced and regulates transformer expression. PloS One. 8 (5), 63618 (2013).
- Dalla Benetta, E., et al. Genome elimination mediated by gene expression from a selfish chromosome. Science Advances. 6 (14), (2020).
- Aldrich, J. C., Leibholz, A., Cheema, M. S., Ausiό, J., Ferree, P. M. A "selfish" B chromosome induces genome elimination by disrupting the histone code in the jewel wasp Nasonia vitripennis. Scientific Reports. 7, 42551 (2017).
- Ferree, P. M., et al. Identification of genes uniquely expressed in the germ-line tissues of the jewel wasp Nasonnia vitripennis. G3. 3 (12), 2647-2653 (2015).
- Akbari, O. S., Antoshechkin, I., Hay, B. A., Ferree, P. M. Transcriptome profiling of Nasonia vitripennis testis reveals novel transcripts expressed from the selfish B chromosome, paternal sex ratio. G3. 3 (9), 1597-1605 (2013).
- Dalla Benetta, E., Beukeboom, L. W., van de Zande, L. Adaptive differences in circadian clock gene expression patterns and photoperiodic diapause induction in Nasonia vitripennis. The American Naturalist. 193 (6), 881-896 (2019).
- Paolucci, S., et al. Latitudinal variation in circadian rhythmicity in Nasonia vitripennis. Behavioral Sciences. 9 (11), (2019).
- Werren, J. H., Loehlin, D. W. The parasitoid wasp Nasonia: an emerging model system with haploid male genetics. Cold Spring Harbor Protocols. 2009 (10), (2009).
- Lynch, J. A., Desplan, C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nature Protocols. 1 (1), 486-494 (2006).
- Li, M., et al. Generation of heritable germline mutations in the jewel wasp Nasonia vitripennis using CRISPR/Cas9. Scientific Reports. 7 (1), 901 (2017).
- Chaverra-Rodriguez, D., et al. Germline mutagenesis of Nasonia vitripennis through ovarian delivery of CRISPR-Cas9 ribonucleoprotein. Insect Molecular Biology. , (2020).
- Li, M., Bui, M., Akbari, O. S. Embryo microinjection and transplantation technique for Nasonia vitripennis genome manipulation. Journal of Visualized Experiments. (130), (2017).
- Werren, J. H., Loehlin, D. W. Rearing Sarcophaga bullata fly hosts for Nasonia (Parasitoid Wasp). Cold Spring Harbor Protocols. 2009 (10), (2009).
- Werren, J. H., Loehlin, D. W. Strain maintenance of Nasonia vitripennis (Parasitoid Wasp). Cold Spring Harbor Protocols. 2009 (10), (2009).
- Beukeboom, L. W., van de Zande, L. Genetics of sex determination in the haplodiploid wasp Nasonia vitripennis (Hymenoptera: Chalcidoidea). Journal of Genetics. 89 (3), 333-339 (2010).
- Leung, K., van de Zande, L., Beukeboom, L. W. Life-history traits of the Whiting polyploid line of the parasitoid. Entomologia experimentalis et applicata. 167 (7), 655-669 (2019).
- Kennerdell, J. R., Carthew, R. W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 95 (7), 1017-1026 (1998).
- Yang, J., Zhao-jun, H. A. N. Efficiency of different methods for dsrna delivery in cotton bollworm (Helicoverpa armigera). Journal of Integrative Agriculture. 13 (1), 115-123 (2014).
- Brown, S., Holtzman, S., Kaufman, T., Denell, R. Characterization of the Tribolium Deformed ortholog and its ability to directly regulate Deformed target genes in the rescue of a Drosophila Deformed null mutant. Development Genes and Evolution. 209 (7), 389-398 (1999).
- Hughes, C. L., Kaufman, T. C. RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the hemipteran head. Development. 127 (17), 3683-3694 (2000).
- Dzitoyeva, S., Dimitrijevic, N., Manev, H. Intra-abdominal injection of double-stranded RNA into anesthetized adult Drosophila triggers RNA interference in the central nervous system. Molecular Psychiatry. 6 (6), 665-670 (2001).
- Chaverra-Rodriguez, D., et al. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nature Communications. 9 (1), 3008 (2018).
- Macias, V. M., et al. Cas9-mediated gene-editing in the malaria mosquito anopheles stephensi by ReMOT control. G3. 10 (4), 1353-1360 (2020).
- Heu, C. C., McCullough, F. M., Luan, J., Rasgon, J. L. CRISPR/Cas9-based genome editing in the silverleaf whitefly (Bemisia tabaci). CRISPR Journal. 3 (2), 89-96 (2020).
- Shirai, Y., Daimon, T. Mutations in cardinal are responsible for the red-1 and peach eye color mutants of the red flour beetle Tribolium castaneum. Biochemical and Biophysical Research Communications. 529 (2), 372-378 (2020).
- Hunter, W. B., Gonzalez, M. T., Tomich, J. BAPC-assisted CRISPR/Cas9 system: targeted delivery into adult ovaries for heritable germline gene editing (Arthropoda: Hemiptera). bioRxiv. , 478743 (2018).
- Chaverra Rodriguez, D., Bui, M., Li, M., Raban, R., Akbari, O. Developing genetic tools to control ACP. Citrus Research Board Citrograph Magazine. 11 (1), 64-68 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved