A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Rapid Testing of Resistance of Timber to Biodegradation by Marine Wood-Boring Crustaceans
In This Article
Summary
This protocol presents a method for assessing the feeding rate of the wood-boring crustacean, Limnoria, by measuring faecal pellet production. This method is designed for use in non-specialist labs and has potential for incorporation into standard testing protocols, to evaluate enhanced wood durability under marine conditions.
Abstract
Wood-boring invertebrates rapidly destroy marine timbers and wooden coastal infrastructure, causing billions of dollars of damage around the globe every year. As treatments of wood with broad spectrum biocides, such as creosote and chromated copper arsenate (CCA), are now restricted in marine use by legislation, naturally durable timber species and novel preservation methods of wood are required. These methods undergo testing in order to meet regulatory standards, such as the European standard for testing wood preservatives against marine borers, EN 275. Initial investigation of durable timbers species or wood preservative treatments can be achieved quickly and inexpensively through laboratory testing, which offers many advantages over marine field trials that are typically costly, long-term endeavours. Many species of Limnoria (gribble) are marine wood-boring crustaceans. Limnoria are ideal for use in laboratory testing of biodegradation of wood by marine wood-borers, due to the practicality of rearing them in aquaria and the ease of measuring their feeding rates on wood. Herein, we outline a standardizable laboratory test for assessing wood biodegradation using gribble.
Introduction
Wood-borers can cause extensive damage to marine wooden structures, such as sea defences, piers, and aquaculture structures; the replacement or restoration of which costs billions of dollars per annum worldwide1,2,3. In order to protect these structures, timber is often treated to reduce biodegradation. However, due to the restriction of use of broad-spectrum biocides in Australia, EU, UK, and USA, in the marine environment, new modification techniques and species of wood that are naturally durable to borers are sought after4,5,6,7. Novel techniques for the preservation of wood in the marine environment require thorough testing in order to meet regulatory standards and limit environmental impacts from hazards such as leaching of any chemical preservative. For example, the European standard, EN 275, which is the current European standard from 1992, is used to evaluate wood preservation treatments against marine wood-borer damage8,9. This standard, along with other legislations against the use of biocidal compounds, such as CCA4,5,6,7 and creosote10, necessitates sustainable, non-toxic methods of wood protection and the use of naturally durable timber species to replace biocidal treatments11,12. Marine trials, such as those specified in EN 275, require long exposure periods and are thus expensive and slow to yield meaningful results. Laboratory tests, however, provide a much quicker alternative to test methods of preserving timber products against marine wood-borer attack, allowing rapid evaluation of adjustments to treatment schedules13. Results from this rapid laboratory experiment are designed to inform novel modification processes of wood and to identify timber species with natural durability to borer damage. A low feeding rate and vitality can indicate increased resistance in potential products and this information can then be fed back to industry partners to allow them to improve designs. Our method allows a nimble and rapid response, that is desirable in industry, and once promising products have been identified, results can be supplemented with those from marine trials.
Gribbles (Limnoria) are a genus of isopod crustacean in the family Limnoriidae. There are over 60 species of Limnoria worldwide13,14,15, with three common species found in the UK, Limnoria lignorum, Limnoria tripunctata and Limnoria quadripunctata16. They bore tunnels on the surface of wood that is submerged in seawater, often causing economically significant damage. Gribbles are highly abundant in coastal UK waters and are easy to maintain under laboratory conditions, making them ideal organisms for the study of wood biodegradation by marine wood-boring invertebrates. Evaluating the feeding rates and vitality of gribbles on different timber species and wood preservation methods can determine the efficacy of their resistance to biodegradation. The following protocol sets out a standard method for measuring gribble feeding rates, developed from that described by Borges and colleagues12,17, in addition to streamlining the introduction of image analysis to make the process operable in non-specialist labs. Image analysis is also used to reduce the practical limitations of manually counting large number of samples. Durability in long-term marine testing, according to the British Standard EN350-1:1994, are graded in reference to Pinus sylvestris sapwood18. In the short-term laboratory testing presented here, we use Scots pine (Pinus sylvestris L) sapwood as a control to testing heartwood of the species ekki (Lophira alata Banks ex C.F Gaertn), beech (Fagus sylvatica L), sweet chestnut (Castanea sativa Mill) and turpentine (Syncarpia glomulifera (Sm.) Nied). Average faecal pellet production and vitality among eight replicates per wood species was used as an indicator of durability. We provide illustrative data collected from a typical evaluation, using the gribble species Limnoria quadripunctata and a range of naturally durable timber species. Limnoria quadripunctata, identified by the keys provided by Menzies (1951), was selected as the optimal species for biodegradation trials due to the fact that it is the most well-studied member of the family and is well-established as a model species for use in biodegradation trials. This protocol is also applicable for testing woods of different treatments although the control used should be untreated replications of the same species.
Protocol
1. Preparing Test Sticks
- After any treatment processes are complete, cut dry wood into test sticks to size 2 mm x 4 mm x 20 mm (Figure 1). Air dry sticks to a constant weight, under laboratory conditions. Use at least 5 replicates of each wood being tested.

Figure 1: Test sticks used in short-term laboratory testing to assess gribble feeding rates. Test wood sticks sized 2 mm x 4 mm x 20 mm. From left to right: ekki, turpentine, sweet chestnut and beech heartwood and Scots pine sapwood. Scale bar 4 mm. Please click here to view a larger version of this figure.
- Vacuum Impregnation
- Post wood preparation (i.e., cutting and treatment, if applicable), place sticks under a mesh in a food-safe plastic container, inside the vacuum desiccator and replace lid ensuring there is a tight seal, facilitated by a coating of vacuum grease (Figure 2).
- Attach a three-way valve between the tubing connecting the desiccator and pump, with a third tube leading to open air (Figure 2). Ensure that the three-way valve is closed off to the air and run the pump to achieve a vacuum of between -0.75 to -1.0 bar within the vacuum desiccator and hold this vacuum for 45 minutes - 1 hour.
- Submerge the open end of the third tube into a container of seawater. Switch the pump off and close the valve leading to the pump, then slowly open the valve until seawater is drawn by the vacuum into the desiccator. Allow the water to flow until it fills the plastic container, above the level of the mesh.
- Then withdraw the tube from the seawater in the container, allowing air to enter, until the desiccator returns to atmospheric pressure. Keep the sticks submerged under the mesh until they sink to the bottom of the plastic container.
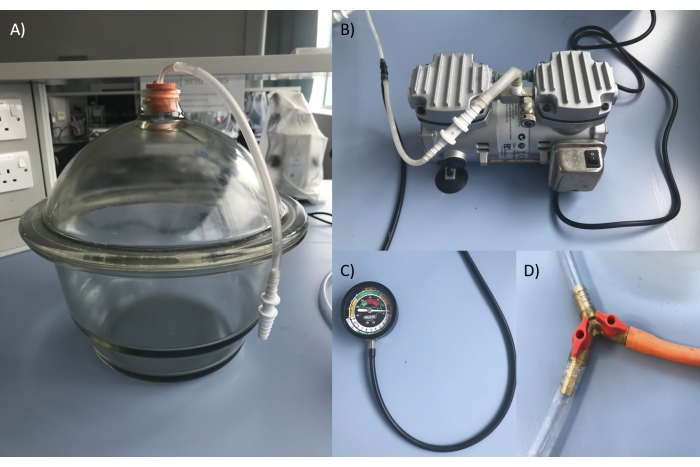
Figure 2: Equipment used to vacuum impregnate wood sticks with seawater, in preparation for feeding to gribbles during a laboratory feeding assay. A) Vacuum desiccator; B) Pump; C) Pressure gauge for the vacuum desiccator; D) The three-way valve leading to the vacuum desiccator, pump and to open air or seawater (orange tube). Please click here to view a larger version of this figure.
- Leaching Wood
- Submerge seawater-saturated test sticks in seawater contained in 50 mL tubes (Figure 3). Replace water regularly for a period of 20 days.
NOTE: The leaching process applies to any experimental wood under test, including treated or natural woods.
- Submerge seawater-saturated test sticks in seawater contained in 50 mL tubes (Figure 3). Replace water regularly for a period of 20 days.
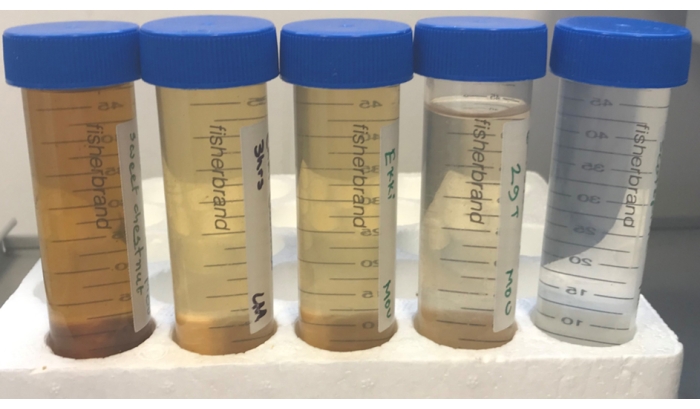
Figure 3: Leachate from wood sticks for preparation for feeding to gribbles during a laboratory feeding assay. Wood that was fully submerged in seawater contained in a 50 ml Falcon tube, with regular water change (1-3 days), produced distinctly coloured leachate. From left to right leachate from heartwood of; sweet chestnut, turpentine, ekki, and beech and Scots pine sapwood. Please click here to view a larger version of this figure.
2. Extracting Gribble
- Extract individual specimens of gribble from an infested wood block. Use a pair of fine forceps and a thin (size 000/0.4 mm or smaller) paintbrush. Carefully peel back any wood that is covering the gribble burrow with the forceps
NOTE: Burrows are found on the surface of wood and can be identified by small holes (Figure 4). - Once gribble have been exposed, use a paintbrush to gently pick up individuals from underneath and deposit in a petri dish filled with seawater. Check gribble under a microscope to identify species and to ensure no damage was caused while extracting.
NOTE: Beating pleopods are a sign of vitality.- Discard any females brooding eggs as gravid females have a reduced feeding capacity.
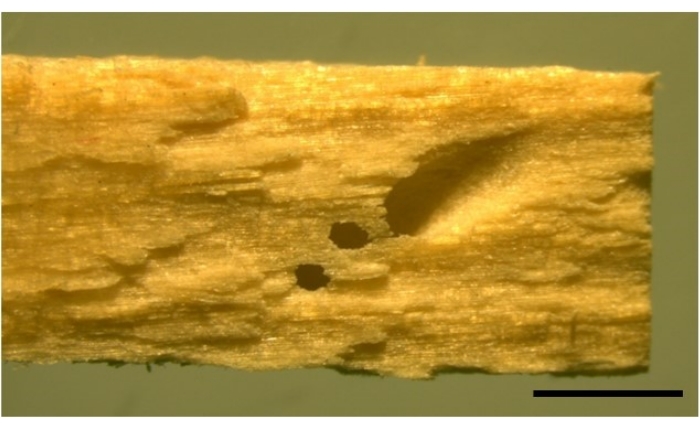
Figure 4: Image of a gribble burrow with two typical ventilation holes. L. quadripunctata burrow on a stick of Radiata pine wood, sized 2 mm x 4 mm x 20 mm. Two smaller ventilation holes can be seen next to the burrow entrance. Scale bar 2 mm. Please click here to view a larger version of this figure.
- Identifying Limnoria quadripunctata
- Identify Limnoria quadripunctata under a stereomicroscope by the four distinct tubercles, arranged in a square pattern, on the animal's pleotelson in addition to an X-shaped carina on the fifth pleonite19 (Figure 5).

Figure 5: Limnoria quadripunctata identifying features. Image of dorsal surface Limnoria quadripunctata, taken on a stereomicroscope at x20 magnification. Identifying features shown by red arrow - indicates the X- shaped carina and blue arrow - indicates four tubercles on pleotelson. Scale bar 1 mm. Please click here to view a larger version of this figure.
3. Preparing Well Plates
- In multi-well plates with wells of diameter 20 mm, place one test stick and 5 mL of unfiltered seawater, between 32-35 PSU, per well (Figure 6).
- Place treatments/species of wood systematically throughout the well plate so that each type of wood is represented at least once per plate. Add one gribble per well.
NOTE: Temperature should be kept stable in an incubator at 20 °C ± 2 °C for the species L. quadripunctata, other species of Limnoria can be used with adjustments to the temperature made to suit the specific species. - Keep plates in constant dark conditions as the photoperiod does not have an effect on gribble feeding rate15.

Figure 6: Experimental set up for gribble feeding assay. An example of a 12 multi-well plate used in the laboratory testing of gribble feeding rate. Each well contains 5 ml seawater and one test stick (20 mm x 4 mm x 2 mm) of different wood species; Scots Pine sapwood and ekki, beech, sweet chestnut, and turpentine heartwood. Scale bar 20 mm. Please click here to view a larger version of this figure.
4. Collecting and Counting Faecal Pellets and Assessing Vitality.
- Twice per week, remove the test stick and each gribble (one per well) from the well plate and place into a freshly pre-prepared well plate (containing 5 mL of seawater per well [32-35 PSU, 18-22 °C]).
- Use a paintbrush to gently brush off any faecal pellets from the stick before transferring and retain the faecal pellets within the original well.
NOTE: Prior to transferring the gribble to a fresh well plate, vitality can be assessed on a scale of 1-5; 1= dead, 2 = passive, not on the wood, 3 = actively swimming or beating pleopods, not on the wood, 4 = crawling on the surface of the wood, 5 = burrowed into the wood. - Image Processing
- Use a fine paintbrush to separate any clumps so that individual pellets are visible and brush pellets away from the very edges of the well. Take a detailed photograph under a stereo microscope, at magnification x4 and upload to a computer (Figure 7).
NOTE: Ensure the pellets are in focus and the background is uniform, with no shadows or light reflections on the surface of the water.
- Use a fine paintbrush to separate any clumps so that individual pellets are visible and brush pellets away from the very edges of the well. Take a detailed photograph under a stereo microscope, at magnification x4 and upload to a computer (Figure 7).
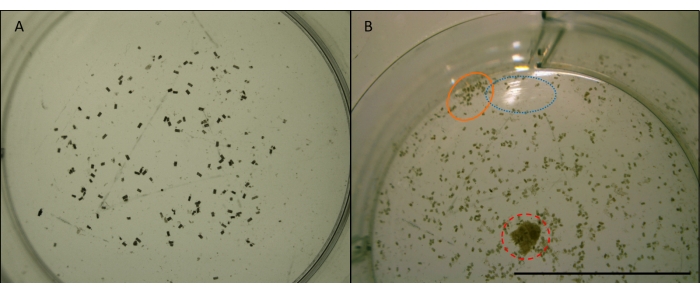
Figure 7: Image of gribble faecal pellets. L. quadripunctata faecal pellets (small, cylindrical, brown pellets) from feeding on Radiata pine wood in one well of a multi-well plate. Taken at x4 magnification. Images prior to manipulation for image analysis (see Figure 7). A) Example of a suitable image to be used for automated counting in ImageJ. Pellets are sufficiently spread out and away from the edges of the well. The well is centred and there are no obstructions or reflections. B) An example of an image that is unsuitable for image analysis. The well is off-centre, cutting off the bottom half. Blue (dotted) circle shows light reflection off the surface of the water. Orange (solid) circle shows pellets that are clumped too closely together and too near the edge of the well. Red (dashed) circle shows a wood chip that was not removed. Scale bar 10 mm. Please click here to view a larger version of this figure.
- Process to Generate Faecal Pellet Count Using ImageJ.
- Download ImageJ (latest version as of 03/08/21, 1.8.0_172) from https://imagej.nih.gov/ij/download.html or run from the computer’s browser.
- Upload a stack of images by dragging and dropping or by selecting File | Import | Image sequence | Browse. Do not change any parameters then select Okay.
- Next, use the circle tool to select the bottom section of the well containing the faecal pellets. Remove the well edges, select Edit | Clear outside. Make the image binary, select Process | Make binary.
- Calibrate by selecting Analyse | Set scale and choose the number of pixels per millimeter for the image (for example 10 pixels = 1 mm). Count the pellets, select Analyse | Analyse particles.
- In the box next to Size (unit2), select a lower threshold that is the same as the smallest size pellet, using the unit scale set earlier (for example, if 10 pixels = 1 mm and the smallest pellet is 0.5 mm, choose 5-infinity).
- In the Show drop down box, select Outlines and then tick Summarise and press Okay (Figure 8).
NOTE: Further information can be found at https://imagej.nih.gov/ij/docs/guide/index.html

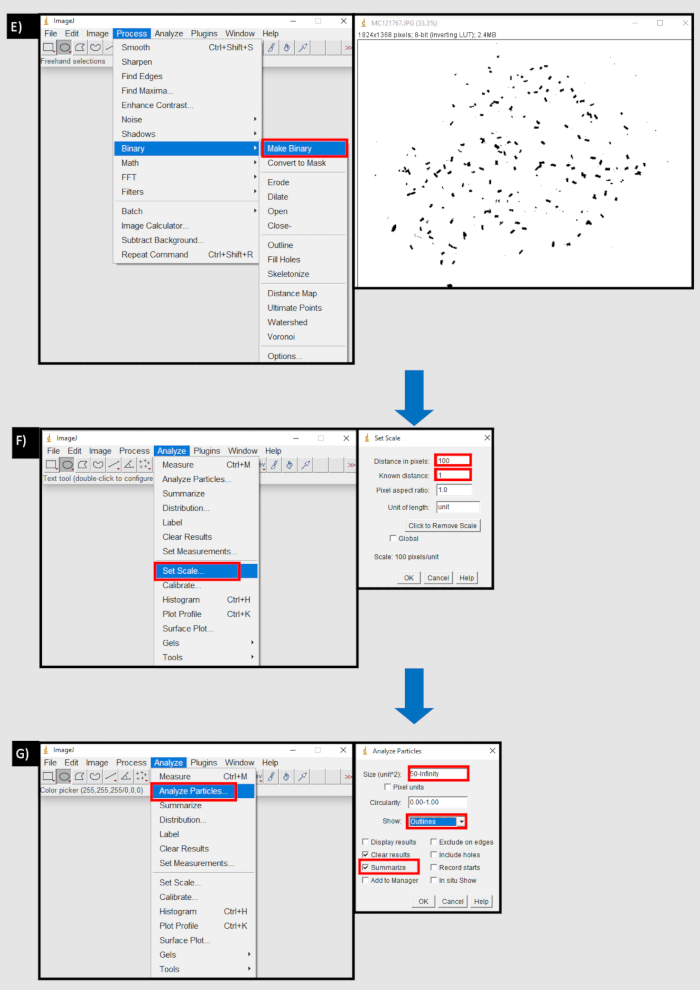
Figure 8: A flow diagram of the process used in ImageJ to count faecal pellets. A) Importing an image sequence in the File tab of ImageJ. B) The browse button in the 'Import Image Sequence' dialog box to import a sequence of images from a local device. C) Using the circle tool to select area containing faecal pellets D) Clear outside button in the edit tab area to remove outside of selected area. E) Make binary button in the process tab. F) Set scale button in the Analyse tab. Distance in pixels is equivalent to the number of pixels to one unit of measurement (mm). G) Analyse particles button in the Analyse tab. Size (unit^2) set to the lower threshold of faecal pellet size, in pixels, to infinity. Show 'outlines' and 'summarise' are selected. Please click here to view a larger version of this figure.
- Data Analysis
- Convert pellet counts to pellets per day, which gives and indirect measure of feeding rate. Discard data from any moulting individuals on days that moulting occurred (Figure 9).
NOTE: Moulting occurs over 1-3 days and can be identified when a full moult of the exoskeleton can be seen.
- Convert pellet counts to pellets per day, which gives and indirect measure of feeding rate. Discard data from any moulting individuals on days that moulting occurred (Figure 9).
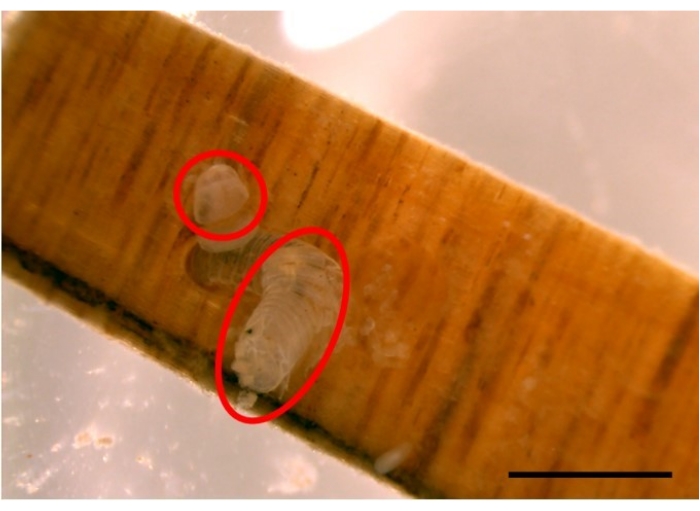
Figure 9: Example of a gribble moult. Gribble (L. quadripunctata) moulting, on a Radiata pine wood test stick sized 20 mm x 4 mm x 2 mm. Moults are indicated by red circles. Scale bar 2 mm. Please click here to view a larger version of this figure.
Results
A feeding experiment of L. quadripunctata was conducted over 20 days, using five different wood types (Scots pine (Pinus sylvestris L) sapwood, and heartwood of beech (Fagus sylvatica L), ekki (Lophira alata Banks ex C. F Gaertn), sweet chestnut (Castanea sativa Mil), and turpentine (Syncarpia glomulifera (Sm.) Neid)) (See Table of Materials), in November 2020. Eight replicate sticks were used per wood species and one specimen of Limnoria...
Discussion
Prior to selecting gribble specimens to be used in the feeding experiment, individuals should be screened to assess their suitability. There can be some variation in feeding rate between individuals due to differences in size, so only fully grown adult specimens should be selected. No significant difference between feeding rate of individuals between 1.5 mm and 3 mm length was detected by Borges et al., 200917. Female Limnoria brood their eggs, during which time have a reduced feedin...
Disclosures
The authors have no conflicts of interest related to this study.
Acknowledgements
Thank you to the Research Council of Norway (Oslo Regional Fund, Alcofur rffofjor 269707) and the University of Portsmouth (Faculty of Science PhD research bursary) for providing funding for the studies of Lucy Martin. Also, to Gervais S. Sawyer who provided the wood used to generate the representative results. Turpentine was provided by Prof. Philip Evans of the University of British Columbia.
Materials
| Name | Company | Catalog Number | Comments |
| 12-well cell culture plates | ThermoFisher Scientific | 150200 | |
| 50ml Falcon tubes | Fisher Scientific | 14-432-22 | |
| Adjustable volume pipette | Fisher Scientific | FBE10000 | 1-10 ml |
| Beech | G. Sawyer (consultant in timber technology) | Fagus sylvatica | Taxonomic authority: L |
| Ekki | G. Sawyer (consultant in timber technology) | Lophira alata | Taxonomic authority: Banks ex C. F. Gaertn. |
| Forceps | Fisher Scientific | 10098140 | |
| Incubator | LMS LTD | INC5009 | |
| Microporous specimen capsules | Electron Microscopy Sciences | 70187-20 | |
| Petri dish | Fisher Scientific | FB0875713 | |
| Scots Pine | G. Sawyer (consultant in timber technology) | Pinus sylvestris | Taxonomic authority: L. |
| Size 00000 paintbrush | Hobby Craft | 5674331001 | Size 000 or 0000 also acceptable |
| Sweet Chestnut | G. Sawyer (consultant in timber technology) | Castanea sativa | Taxonomic authority: Mill |
| Turpentine | P. Evans (Professor, Dept. Wood Science, University of British Columbia) | Syncarpia glomulifera | Taxonomic authority: (Sm.) Nied. |
| Vacuum desiccator | Fisher Scientific | 15544635 |
References
- Morrell, J. J., Kutz, M. Protection of wood-based materials. Handbook of environmental degradation of materials, 3rd ed. , 343-368 (2018).
- Distel, D. L., Goodell, B., Nicholas, D., Schultz, T. The biology of marine wood boring bivalves and their bacterial endosymbionts. Wood deterioration and preservation. , 253-271 (2003).
- Buslov, V., Scola, P. Inspection and structural evaluation of timber pier: case study. Journal of Structural Engineering. 117 (9), 2725-2741 (1991).
- Registration Eligibility Decision for Chromated Arsenicals. List A, Case No. 0132. US EPA - Office of prevention, pesticides and toxic substances Available from: https://swap.stanford.edu/20110202084/http://www.epa.gov/oppsrrd1/reregistration/REDs/cca_red.pdf (2008)
- Arsenic timber treatments (CCA and arsenic trioxide) review scope document, Review series 03.1. ISSN number 1443. Australian pesticides and veterinary medicines authority Available from: https://apvma.gov.au/sites/default/files/publication/14296-arsenic-timber-review-scope.pdf (2003)
- Commission directive 2003/2/EC of 6 January 2003 relating to restrictions on the marketing and use of arsenic (tenth adaptation to technical progress to Council Deretive 76/769/EEC). Official Journal of the European Communities Available from: https://www.legislation.gov.uk/eudr/2003/2/adopted (2003)
- The Hazardous Waste (England and Wales) Regulations 2005 No.894. Environmental Protection England and Wales Available from: https://www.legislation.gov.uk/uksi/2005/894/contents/made (2005)
- Palanti, S., Cragg, S. M., Plarre, R. Resistance against marine borers: About the revision of EN 275 and the attempt for a new laboratory standard for Limnoria. International Research Group on Wood Preservation, Document No. IRG/WP 20-20669. , (2020).
- The European Commission for Standardization. EN 275:1992. Wood preservatives- Determination of the protective effectiveness against marine wood borers. The European Commission for Standardization (CEN). , (1992).
- European Commission. Directive 98/8/EC concerning the placing of biocidal products on the market. Communication and Information Resource Centre for Administrations, Businesses and Citizens. , (2010).
- Mantanis, G. I. Chemical modification of wood by acetylation or furfurylation: A review of the present scaled-up technologies. BioResources. 12 (2), 4478-4489 (2017).
- Borges, L. M. S., Cragg, S. M., Bergot, J., Williams, J. R., Shayler, B., Sawyer, G. S. Laboratory screening of tropical hardwoods for natural resistance to the marine borer Limnoria quadripunctata: The role of leachable and non-leachable factors. Holzforschung. 62 (1), 99-111 (2008).
- Cragg, S. M., Pitman, A., Henderson, S. Developments in the understanding of the biology of marine wood boring crustaceans and in methods of controlling them. International Biodeterioration & Biodegradation. 43 (4), 197-205 (1999).
- Cookson, L. J., Vic, M. D. C. Additions to the taxonomy of the Limnoriidae. Memoirs of the Museum of Victoria. 56 (1), 129-143 (1997).
- Cookson, L. Australasian species of Limnoriidae (Crustacea: Isopoda). Memoirs of the Museum of Victoria. 52 (2), 137 (1991).
- Jones, L. T. The geographical and vertical distribution of British Limnoria [Crustacea: Isopoda]. Journal of the Marine Biological Association of the United Kingdom. 43 (3), 589-603 (1963).
- Borges, L. M. S., Cragg, S. M., Busch, S. A laboratory assay for measuring feeding and mortality of the marine wood borer Limnoria under forced feeding conditions: A basis for a standard test method. International Biodeterioration & Biodegradation. 63 (3), 289-296 (2009).
- BSI Standards Publication. BS EN 350:2016. Durability of wood and wood-based products - Testing and classification of the durability to biological agents of wood and wood-based materials. BSI Standards Publication. , (2016).
- Menzies, R. . The phylogeny, systematics, distribution, and natural history of limnoria. , 196-208 (1951).
- Palanti, S., Feci, E., Anichini, M. Comparison between four tropical wood species for their resistance to marine borers (Teredo spp and Limnoria spp) in the Strait of Messina. International Biodeterioration & Biodegradation. 104, 472-476 (2015).
- Delgery, C. C., Cragg, S. M., Busch, S., Morgan, E. Effects of the epibiotic heterotrich ciliate Mirofolliculina limnoriae and moulting on the faecal pellet production by the wood-boring isopods Limnoria tripunctata and Limnoria quadripunctata. Journal of Experimental Marine Biology and Ecology. 334 (2), 165-173 (2006).
- Morrell, J. J., Helsing, G. G., Graham, R. D. Marine wood maintenance manual: a guide for proper use of Douglas-fir in marine exposures. Forest Research Laboratory. , (1984).
- Slevin, C. R., Westin, M., Lande, S., Cragg, S. Laboratory and marine trials of resistance of furfurylated wood to marine borers. Eighth European Conference on Wood Modification. , 464-471 (2015).
- Westin, M., et al. Marine borer resistance of acetylated and furfurylated wood - results from up to 16 years of field exposure. International Research Group on Wood Preservation. , (2016).
- Westin, M., Rapp, A., Field Nilsson, T. Field test of resistance of modified wood to marine borers. Wood Material Science and Engineering. 1 (1), 34-38 (2006).
- Borges, L. M. S. Biodegradation of wood exposed in the marine environment: Evaluation of the hazard posed by marine wood-borers in fifteen European sites. International Biodeterioration & Biodegradation. 96 (1), 97-104 (2014).
- Treu, A., et al. Durability and protection of timber structures in marine environments in Europe: An overview. BioResources. 14 (4), 10161-10184 (2019).
- Williams, J. R., Sawyer, G. S., Cragg, S. M., Simm, J. A questionnaire survey to establish the perceptions of UK specifiers concerning the key material attributes of timber for use in marine and freshwater engineering. Journal of the Institute of Wood Science. 17 (1), 41-50 (2005).
- Purnell, P. The carbon footprint of reinforced concrete. Advances in Cement Research. 25 (6), 362-368 (2013).
- Hill, C. A. S. The environmental consequences concerning the use of timber in the built environment. Frontiers in Built Environment. 5, 129 (2019).
- Mercer, T. G., Frostick, L. E. Leaching characteristics of CCA-treated wood waste: a UK study. Science of the Total Environment. 427, 165-174 (2012).
- Brown, C. J., Eaton, R. A., Thorp, C. H. Effects of chromated copper arsenate (CCA) wood preservative on early fouling community formation. Marine Pollution Bulletin. 42 (11), 1103-1113 (2001).
- Brown, C. J., Eaton, R. A. Toxicity of chromated copper arsenate (CCA)-treated wood to non-target marine fouling communities in Langstone Harbour, Portsmouth, UK. Marine Pollution Bulletin. 42 (4), 310-318 (2001).
- Brown, C. J., Albuquerque, R. M., Cragg, S. M., Eaton, R. A. Effects of CCA (copper-chrome-arsenic) preservative treatment of wood on the settlement and recruitment of wood of barnacles and tube building polychaete worms. Biofouling. 15 (1-3), 151-164 (2000).
- Lebow, S. T., Foster, D. O., Lebow, P. K. Release of copper, chromium and arsenic from treated southern pine exposed in seawater and freshwater. Forest Products Journal. 49 (7), 80-89 (1999).
- Smith, P. T. Risk to human health and estuarine posed by pulling out creosote-treated timber on oyster farms. Aquatic Toxicology. 86 (2), 287-298 (2008).
- Brown, C. J., et al. Assessment of Effects of Chromated Copper Arsenate (CCA)-Treated Timber on Nontarget Epibiota by Investigation of Fouling Community Development at Seven European Sites. Archives of Environmental Contamination and Toxicology. 45 (1), 0037-0047 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved