Method Article
Electroantennography-based Bio-hybrid Odor-detecting Drone using Silkmoth Antennae for Odor Source Localization
In This Article
Summary
This study introduces experimental protocols for a bio-hybrid odor-detecting drone based on silkmoth antennae. The operation of an experimental electroantennogram device with silkmoth antennae is presented, in addition to the structure of a bio-hybrid drone designed for odor source localization using the spiral-surge algorithm.
Abstract
Small drones with chemical or biosensor devices that can detect airborne odorant molecules have attracted considerable attention owing to their applicability in environmental and security monitoring and search-and-rescue operations. Small drones with commercial metal-oxide-semiconductor (MOX) gas sensors have been developed for odor source localization; however, their real-time-odor-detection performance has proven inadequate. However, biosensing technologies based on insect olfactory systems exhibit relatively high sensitivity, selectivity, and real-time response with respect to odorant molecules compared to commercial MOX gas sensors. In such devices, excised insect antennae function as portable odorant biosensor elements and have been found to deliver excellent sensing performance. This study presents experimental protocols for odorant-molecule detection in the air using a small autonomous bio-hybrid drone based on a mountable electroantennography (EAG) device incorporating silkmoth antennae.
We developed a mountable EAG device including sensing/processing parts with a Wi-Fi module. The device was equipped with a simple sensor enclosure to enhance the sensor directivity. Thus, odor source localization was conducted using the spiral-surge algorithm, which does not assume an upwind direction. The experimental bio-hybrid odor-detecting drone identified real-time odorant-concentration differences in a pseudo-open environment (outside a wind tunnel) and localized the source. The developed drone and associated system can serve as an efficient odorant molecule-detection tool and a suitable flight platform for developing odor source localization algorithms owing to its high programmability.
Introduction
With recent advances, small drones with chemical sensing devices have become highly applicable in environmental and security monitoring and gas-leak detection1. Small drones (with a diameter approximately < 20 cm) with commercial metal-oxide-semiconductor (MOX) gas sensors have been recently applied for performing odor mapping or odor source localization2,3,4. When searching for odor sources, a drone must trace odor plumes; however, odor source localization using small drones presents significant challenges. In an open environment, odor-plume structures are subjected to continual changes due to environmental factors such as the wind or landscape. Hence, drones should be capable of identifying odorant-concentration differences and directions varying over time; however, the odor detection performance of commercial MOX sensors is still inadequate for real-time sensing because of their slow recovery time5.
Bio-hybrid systems formed by the merging of biological and artificial systems are a recent trend in robotics and sensor technologies6, showing great potential for surpassing the capabilities of existing approaches. For instance, a bio-robotic sensor network has been developed based on cockroaches for application in disaster situations7. Experiments have been conducted in which cyborg rats with computationally enhanced intelligence were tasked with solving mazes8. The possibility of social integration of biomimetic robots into groups of real zebra fishes have been investigated9.
Naturally, this trend has been applied to develop odorant sensors10. For example, biosensors based on insect olfactory systems have relatively high sensitivity and selectivity with respect to various odorant molecules compared to existing MOX sensors11. Along these lines, we had previously developed bio-hybrid odorant biosensor systems based on a combination of insect cells expressing insect odorant receptors and a microscope or electronic devices12,13,14,15,16. Moreover, insect antennae can be independently used as portable odorant sensing parts with high sensitivity, selectivity, reproducibility, and quick response/recovery time, using the electroantennography (EAG) technique17,18,19. Several ground-mobile odor-sensing robots with EAG techniques based on insect antennae20,21,22,23 or small drones with EAG devices24,25 have been developed for odor detection and odor source localization. These robots displayed sensor sensitivity and real-time sensing ability. However, the mobility of ground mobile robots is significantly influenced by land features or obstacles. In addition, the flight performance and odor source localization algorithms of existing EAG-based bio-hybrid drones remain limited because experimental conditions have been limited to tethered flight24 or to being conducted in a small wind tunnel25.
This study presents experimental protocols for odor detection in the air and odor source localization using a recently developed bio-hybrid drone based on silkmoth (Bombyx mori) antennae26. We developed a mountable-sized and lightweight EAG device with a wireless communication function to detect the odor responses of silkmoth antennae. The EAG device was mounted on a small drone, installed in a simple sensor enclosure to enhance the sensor directivity for odorant molecules and reduce noise. The bio-hybrid drone reproducibly detected airborne odorant molecules and identified the maximum odorant concentration during spiral movements. Moreover, the drone localized the odor source using the spiral-surge algorithm without wind-direction information.
Protocol
1. Insects
NOTE: Eggs of silkmoths (Bombyx mori) were purchased from a domestic company. The silkmoths were used within 10 days after they emerged from cocoons. Prepare three adult silkmoths for the experiments (six antennae); however, this number can be changed depending on the experimental requirements.
- Incubate silkmoth eggs at 15 °C for 24 h and move them to an incubator at 25 °C.
NOTE: The silkworms hatch approximately 10-13 days later. - Place the silkworms on sliced artificial diets in a plastic dish.
- After 20-25 days of silkworm raising, observe the formation and pupation of the silkworms within cocoons.
NOTE: The cultivation procedure includes feeding, removal, and sanitization in an environment at 25 °C. The silkmoths emerge from the cocoons after 10-15 days.
2. Odorants and odor source preparation
NOTE: The principal component of the female silkmoth sex pheromone, bombykol ((E,Z)-10,12-hexadecadien-1-ol), was used as an odor source to perform stimulation. A male silkmoth (Figure 1A) can identify and discriminate bombykol27, and isolated silkmoth antennae have been used to act as a biosensor on mobile robots20,21,22. Store purified bombykol dissolved in hexane (10 mg/mL) in a high-sealed storage bottle in a refrigerator at −30 °C.
- Insert a syringe into the high-sealed storage bottle and withdraw and inject 2 mL of 2000 ng/µL bombykol into a 10 mL vial. Then, add 8 mL of hexane to the same vial.
- Dilute 400 ng/µL of bombykol to 2 ng/µL of bombykol with hexane in a 1 mL vial.
- Cut filter paper into 10 mm × 10 mm pieces, roll them into a cylindrical shape, and place them in a glass tube (internal diameter [ID]: 5 mm; outer diameter [OD]: 7 mm; length [L]: 100 mm).
- Drop a diluted sample (100 ng bombykol dissolved in 50 µL of hexane) onto a portion of the filter paper in the glass tube.
- Close both ends of the glass tube with the filter paper using poly-droppers cut in the middle.
3. EAG experiments on a fixed desk surface
NOTE: The mountable EAG device, which functions as a portable biosensor on a small drone, is shown in Figure 1B. The device included high-pass (0.1 Hz) and low-pass (300 Hz) filters. The detailed information of the electrical circuit is described in Terutsuki et al.26
- Perform data acquisition and analysis on a personal computer (PC) after the EAG device has sent the measurement data.
- To generate purified air, pass the airflow generated by a compact air pump with a cooling fan through cotton, activated carbon granules, and distilled water. Then, pass the purified air through a glass tube for stimulation.
NOTE: A photograph of the odor stimulation system is shown in Figure 1C. The airflow path is indicated by black arrows. The airflow path of the exhaust port of the solenoid valve is indicated by the dashed black arrow. - Set the flow rate as 5 L min-1 using a flowmeter for odor stimulation in the fixed experimental setup. Set a higher flow rate for generation, assuming odor stimulations of several meters for the drone experiments.
NOTE: That the flow rate (5 L min-1) did not affect the signal detection of the EAG device had been previously confirmed26. The maximum airflow velocity at the EAG device position during stimulation was measured as 3.9 m s-1 using an anemometer. - Use a solenoid valve with a microcontroller to stimulate the EAG device and conduct the stimulations automatically.
- Set the stimulation time to 0.5 s using the solenoid valve.
- Use electrically conductive gel to attach a silkmoth antenna to the electrode.
NOTE: This procedure does not require the insertion of micrometer-scale wires to both ends of a silkmoth antenna to attach it to the EAG device.- Isolate silkmoth antennae using postmortem scissors (Figure 2A,B) without anesthesia. See Figure 2C for an enlarged view of the antenna.
- Cut both sides of the isolated silkmoth antenna and attach it to the Ag/AgCl-coated electrodes of the sensing part of the EAG device (Figure 3A) using electrically conductive gel.
- Connect the glass tube containing bombykol to the odor stimulation system (ensure that the pump is already switched on).
- Fix the glass tube such that its tip is 10 mm from the silkmoth antenna on the EAG device (Figure 3B).
- Set the exhaust port (diameter of 60 mm) at 30 mm behind the EAG device to stabilize the airflow and prevent pheromone stagnation (Figure 3B).
- Switch on the EAG device. Connect the PC to the Wi-Fi access point.
- Run the data acquisition program on the PC. See Figure 3C for the graphical user interface (GUI) on the PC for the experiments.
- After pressing the Ground button in the Log menu to decide the experimental state, press the Log start button for data acquisition. Five seconds after pressing the Log start button, initiate odor stimulations.
- Press the Log stop button on the GUI to stop recording.
4. Drone
NOTE: A commercial drone flight platform (98 mm x 93 mm x 41 mm; weight 87 g; maximum flight time 13 min) was used in this study. The payload of the drone was approximately 30 g based on the experiments. The drone was equipped with a vision positioning system (VPS) consisting of a camera and an infrared sensor under its body, which allowed for stable hovering without an external positioning system.
- Remove the top cover of the drone and add a custom carbon fiber-reinforced plastic (CFRP) board using a three-dimensional (3D)-printed mount to attach the EAG device. See Figure 4A for an image of the bio-hybrid drone.
NOTE: The drone developer offers a software development kit (SDK) and sample Python programs (see the Table of Materials); therefore, the drone control program for flight experiments was based on these. - Send flight commands through the PC to control the drone.
NOTE: For safety, cut-resistant gloves are required for stopping (catching) the drone in an emergency abort. The GUI is equipped with an emergency stop button to immediately stop the rotation of the propellers of the drone (Figure 3C).
5. Flight experimental area preparation
- Prepare an experimental flight area (5.0 m x 3.2 m x 3.0 m) and equip it with a commercial surveillance camera on the ceiling.
- Set the flow rate of the odor stimulation system as 5 L min-1 and the stimulation time to 0.5 s using the solenoid valve.
6. EAG experiments on the drone
- Isolate silkmoth antennae using postmortem scissors and cut both sides of the antenna.
- Attach the isolated antennae to the Ag/AgCl-coated electrodes of the sensing part of the EAG device using electrically conductive gel.
- Connect the glass tube containing bombykol (50,000 ng in 250 µL of hexane/filter paper) to the odor stimulation system (with the pump already switched on).
- Set the glass tube so that the tube and its tip are parallel to and directly above the edge of the desk, respectively.
- Set the circulator so that the most protruding part (the center of the fan) is 15 cm from the edge of the desk.
- Set the wind speed of the circulator to 1 (minimum power) by pushing the button on the console.
- Mount the EAG device on the drone. Connect the PC to the Wi-Fi access point. Switch on the EAG device and the drone.
NOTE: The switch of the EAG device is in the processing part. - Run the drone control program on the PC.
- After the light on the drone blinks yellow, press the appropriate button in the Command menu on the GUI (Figure 3C) of the PC to execute the command.
NOTE: After the drone is connected to the PC, the light on the drone will turn green. - Press the Take off button on the GUI to hover the drone above the ground.
- After pressing the Flight button in the Log menu to decide the experimental state, press the Log start button for data acquisition.
NOTE: Odor stimulation will be initiated 5 s after pressing the Log start button. - Press the Log stop button on the GUI to stop recording.
- Send the Stop command in intervals of 5 s after the lift-off of the drone to maintain the hovering state, as the drone automatically lands if not operated for approximately 15 s.
- After the light on the drone blinks yellow, press the appropriate button in the Command menu on the GUI (Figure 3C) of the PC to execute the command.
7. Sensor enclosure
- Develop a sensor enclosure (L: 40 mm; ID: 20 mm; OD: 22 mm) based on a carbon fiber tube to enhance the sensor directivity. See Figure 4B,C for an image of the bio-hybrid drone with its sensor enclosure and configuration.
- Cover the sensing part with a heat-shrink insulation tube and fix it to the inner wall of the enclosure using double-sided tape.
- Insert the sensing part of the EAG device into the sensor enclosure.
- Set the distance between the tip of the electrodes and the tip of the enclosure as 10 mm.
8. Odor tracing demonstration using the bio-hybrid drone
- Isolate silkmoth antennae using postmortem scissors and cut both sides of the antenna.
- Attach the isolated antenna to the Ag/AgCl-coated electrodes of the sensing part of the EAG device using electrically conductive gel.
- Mount the EAG device with the sensor enclosure on the drone.
- Hover the drone so that it begins an approximately 90° pivoting motion to the left and right.
- Stimulate the EAG device on the drone using poly-droppers containing bombykol during these movements.
- Conduct four cycles of step 8.5.
NOTE: After step 8.6, the drone will rotate clockwise. When conducting the stimulation during this movement, the drone will perform one counterclockwise rotation and land.
9. Odor source localization using the bio-hybrid drone
- Connect the glass tube containing bombykol (50,000 ng in 250 µL of hexane/filter paper) to the pump that is already switched on.
- Fix the glass tube such that its tip is 150 mm from the circulator.
- Define the direction toward the odor source as 0°, and set the drone at an angle of clockwise 270° from the odor source at the starting point.
- Connect the PC to the Wi-Fi access point, and switch on the EAG device and the drone.
- Run the drone control program on the PC.
- After the light on the drone blinks yellow, press the appropriate button in the Command menu on the GUI of the PC (Figure 3C) to execute the command.
NOTE: After the drone is connected to the PC, the light on the drone will turn green. - Press the Take off button on the GUI to hover the drone above the ground.
- After pressing the Search button in the Log menu to decide the experimental state, press the Log start button for data acquisition. Then, press the Search start button in the Command menu to initiate odor source localization using the spiral-surge algorithm and cyclic odor stimulations (odor: 0.5 s; interval: 2.0 s) of the odor source.
- After landing the drone, press the Log stop button on the GUI to stop recording.
- After the light on the drone blinks yellow, press the appropriate button in the Command menu on the GUI of the PC (Figure 3C) to execute the command.
Results
This paper describes the protocols for signal measurements using the proposed EAG device mounted on a desk and drone. First, we evaluated the performance of the EAG device on a desk. A silkmoth antenna on the EAG device was stimulated by bombykol. Twenty-five continuous stimulations were conducted using 100 ng of bombykol dissolved in 50 µL of hexane with intervals of 5 s, as controlled by a microcontroller. The results indicated that the proposed EAG device reproducibly responded to the stimulations (Figure 5).
The odor detection performance of the EAG device was subsequently evaluated on the drone. The drone equipped with the EAG device hovered at the height of 95 cm from the floor and at a distance of 90 cm from the odor source (Figure 6A). By following the procedure described in section 6, the signals of the EAG device on the drone were measured relative to bombykol (50,000 ng in 250 µL of hexane/filter paper). The sensor performance of a commercial gas sensor on a drone was evaluated for comparison. A digital multi-pixel gas sensor28 was used to detect ethanol vapors. This sensor can be used for the detection of total volatile organic compounds (TVOCs).
According to the datasheet, the TVOC signal range of the sensor was 0-60,000 ppb. The drone with the gas sensor breakout board hovered under the same conditions as the EAG device. Moreover, 500 µL of ethanol (99.5% purity) was used as the odor source instead of bombykol. The typical signals of the EAG device and gas sensor on the drone are shown in Figure 6B. As the odorant molecules and sensor devices differed in this comparison, quantitative comparisons could not be performed. However, the experimental results suggest that it may be difficult for a drone with a commercial gas sensor to detect odorant molecules with a rapid response/recovery speed. In particular, the recovery time of the gas sensor in this study was significantly higher than that of the EAG device with silkmoth antennae.
We also evaluated the sensor directivity of the EAG device on the drone. In this study, the direction toward the odor source was defined as 0°, and the drone was rotated clockwise by 60° intervals to evaluate signal intensities at each angle. For the drone without a sensor enclosure, the signal intensity at 180°, while the drone faced in the opposite direction from the odor source, was occasionally higher than that at 0° (Figure 6C). However, for the drone equipped with the enclosure, the signal intensity of the EAG at 0° became higher than that at 180° (Figure 6D). Consequently, the sensor enclosure enhanced the sensor directivity of the EAG device on the drone.
An odor-tracing demonstration was conducted using the bio-hybrid drone with the sensor enclosure. The results indicated that the drone detected bombykol in the air outside a wind tunnel and identified the direction of the odor plume by pivoting movements (Figure 7, Supplemental Video S1). Finally, odor source localization was conducted based on the spiral-surge algorithm using the bio-hybrid drone (Figure 8A). The drone was set at 270° from the odor source at the starting point. After hovering, the drone started searching for the maximum value of the signal intensity during clockwise or counterclockwise spiral movements. Then, the drone moved forward in the direction of the maximum value of the signal intensity. After repeating the odor-searching spiral and surge movements six times, the drone landed on the ground. The flowchart of the spiral-surge algorithm is described in Terutsuki et al.26
The trajectory, yaw angles, and EAG signals during the odor source localization are presented in Figure 8B-D. Figure 8D shows that the detection time, including response and recover times of the EAG device on the drone, was approximately 1 s. The drone autonomously modified its movement by searching for the maximum odor concentration during the spiral movements. Readers can view videos of the odor source localization by the bio-hybrid drone described by Terutsuki et al.26.
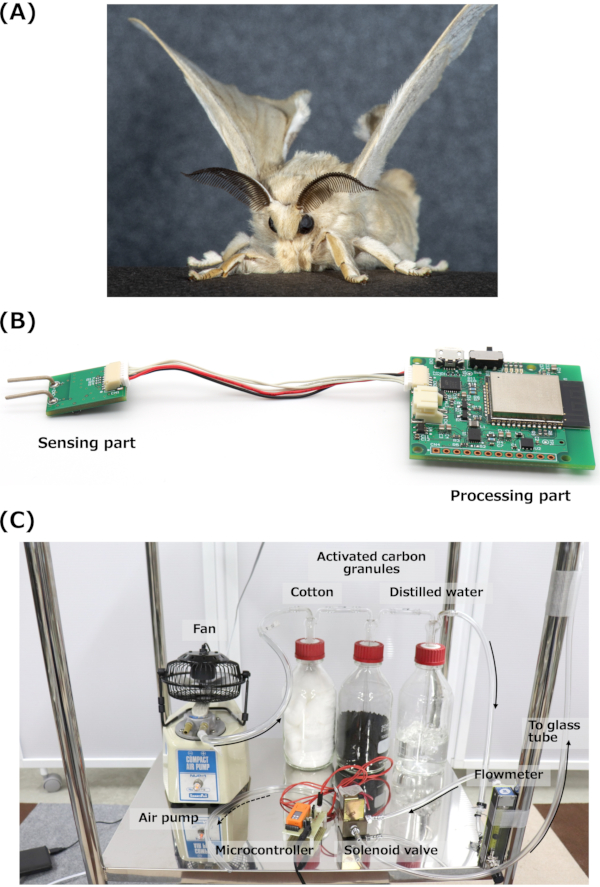
Figure 1: The silkmoth, EAG device, and odor stimulation system. (A) Image of a male silkmoth. (B) Image of the mountable EAG device for a small drone. (C) Image of the odor stimulation system with airflow directions. Abbreviation: EAG = electroantennography. Please click here to view a larger version of this figure.
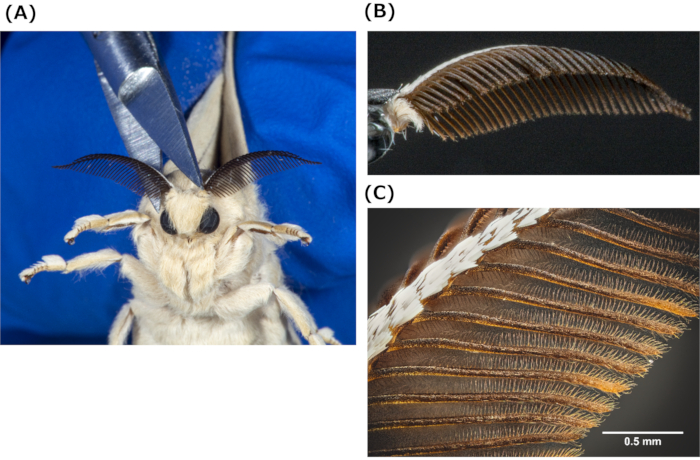
Figure 2: Isolation of silkmoth antenna. (A) Isolation of a silkmoth antenna using postmortem scissors. (B) Typical isolated silkmoth antenna. (C) Enlarged view of an isolated silkmoth antenna; scale bar = 0.5 mm. Please click here to view a larger version of this figure.
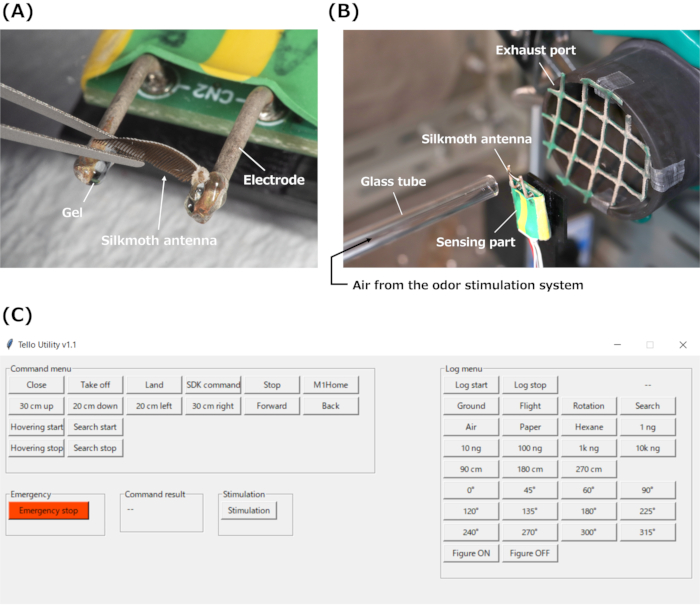
Figure 3: EAG device set up and GUI. (A) Installation of an isolated silkmoth antenna on the electrodes of the EAG device using gel. (B) Setup for odor stimulation using the EAG device on the desk. (C) The GUI for the experiments. Abbreviations: EAG = electroantennography; GUI = graphical user interface. Please click here to view a larger version of this figure.
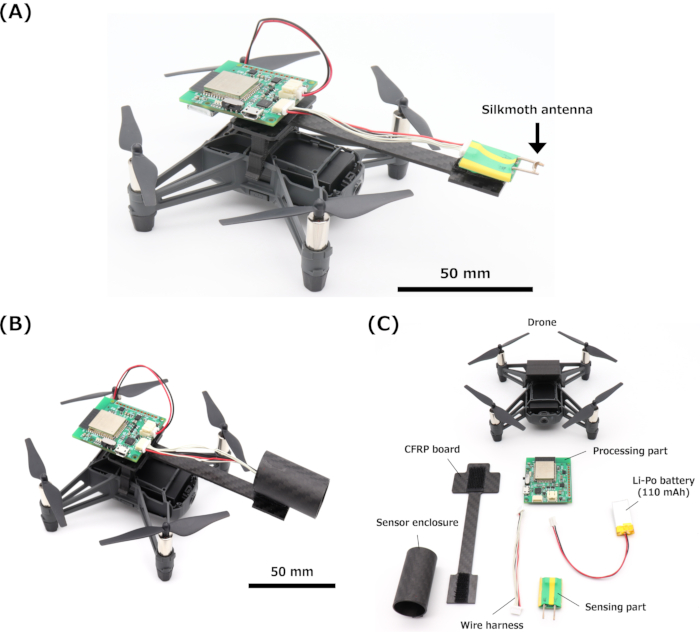
Figure 4: Bio-hybrid drone. (A) Bio-hybrid drone based on a silkmoth antenna. (B) Bio-hybrid drone with the sensor enclosure. (C) Configuration of the bio-hybrid drone. Scale bars (A, B) = 50 mm. Abbreviation: CFRP = carbon fiber-reinforced plastic. Please click here to view a larger version of this figure.
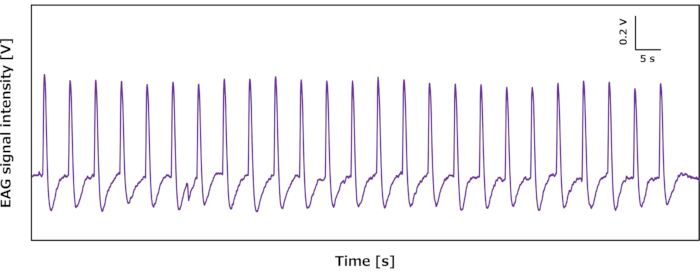
Figure 5: Typical continuous response profile of the EAG device on the desk stimulated by bombykol. Abbreviation: EAG = electroantennography. Please click here to view a larger version of this figure.
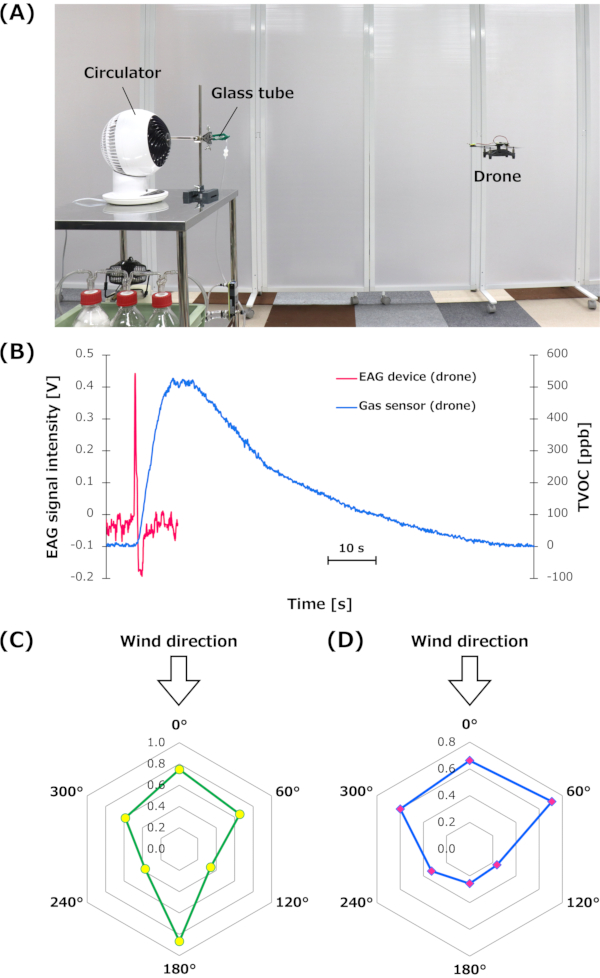
Figure 6: Experimental environment of the bio-hybrid drone and signal intensity of the EAG device. (A) Image of the experimental environment with the bio-hybrid drone, which autonomously hovered 95 cm above the ground at a distance of 90 cm from the odor source. (B) Comparison between the typical signals of the EAG device and commercial gas sensor on the drone. (C) Typical signal intensity of the EAG device without equipping the sensor enclosure on the drone at each angle (N = 1). (D) Average signal intensity of the EAG device with the enclosure on the drone at each angle (N = 3; individual tests). The unit of the signal intensities is V. C and D have been modified from Terutsuki et al.26. Abbreviations: EAG = electroantennography; TVOC = total volatile organic compounds. Please click here to view a larger version of this figure.
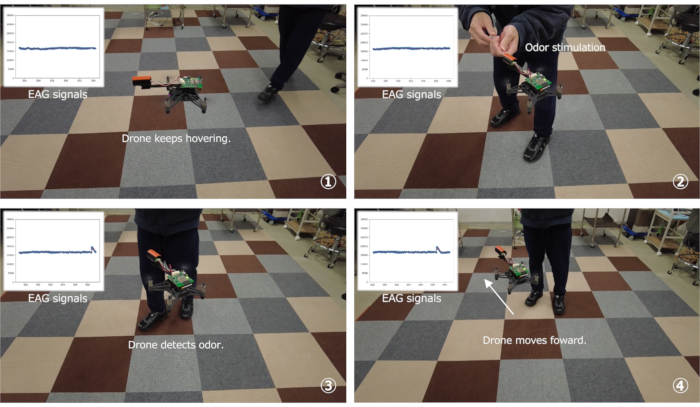
Figure 7: Manual odor stimulation to demonstrate detection and tracing of odor in a room by the bio-hybrid drone. Please click here to view a larger version of this figure.
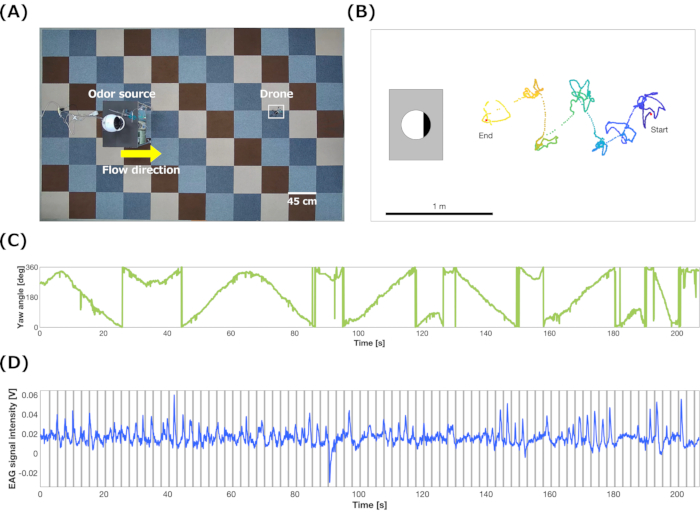
Figure 8: Odor source localization by the bio-hybrid drone. (A) Viewpoint from the ceiling camera of the flight area of the bio-hybrid drone. (B) Typical flight trajectory, (C) yaw angles, and (D) EAG signal intensities during odor source localization using the spiral-surge algorithm. These figures are representative results (N=1). A-D have been modified from Terutsuki et al.26. Please click here to view a larger version of this figure.
Supplemental Video S1: Demonstration of manual odor stimulation using the bio-hybrid drone. Please click here to download this Video.
Discussion
Mobile robots with EAG devices were first developed 25 years ago20. Since then, there have been significant advancements in robotic technologies, including drones. Considering these technological advancements, we developed an autonomous bio-hybrid drone with an EAG device based on a silkmoth antenna for odor detection and localization in air26. This study demonstrates the operation of the developed bio-hybrid drone and the tracing of manual stimulation of odors in a room using the drone.
In this study, as silkmoth antennae were attached to electrodes using electrically conductive gel, we verified that both ends of each antenna made contact with the electrodes securely before beginning EAG experiments on the desk or the drone. If signals from the EAG device were suddenly lost during the experiment, a researcher would first check the connection of the antenna with the electrodes. It is possible that this problem occurred with a higher probability in the EAG experiments on the drone. While the lifespan of isolated silkmoth antennae is more than an hour, because the gel dried out in a dozen to dozens of minutes in this study, the addition of gel to the connecting points of the antennae and the electrodes may help recover signal intensities.
The drone in this study was equipped with the VPS comprising a camera and an infrared sensor for flight stabilization. We found that the drone drifted during hovering on a smooth floor, which may have caused the instability of an infrared sensor under the body of the drone. The same problem sometimes arose when experiments were conducted using this drone in a room with a smooth floor such as tile. Therefore, we covered the floor with raised carpets (we used four-color carpets of 45 cm × 45 cm area) and reduced the drift of the drone. This process was found to be useful for flight stabilization of the EAG experiments on the drone.
The significance of the bio-hybrid drone in this study lies in its ability to recognize odor concentration and its sensor directivity toward odor sources. The drone identified real-time odorant-concentration differences outside a wind tunnel and localized the source using the spiral-surge algorithm (Figure 8). The spiral-surge algorithm29,30 does not require plume-location information during plume reacquisition and exhibits its relatively high reliability, compared to that of the casting algorithm, in a low-speed laminar flow30. This algorithm was previously installed on a ground mobile robot30; however, a wind direction sensor was required to recognize the upwind direction. Odor information was binarized, and concentration was ignored.
For the insect antenna-based drone, mounting additional sensors, such as wind sensors, is a trade-off between payload and battery consumption. In addition, odor information detected by the EAG on the drone was still assessed to determine whether it exceeded a threshold25. The bio-hybrid drone design used in this study enhanced the directivity of the EAG device itself and did not require a wind direction sensor. The sensor directivity enabled the drone to utilize odor concentration information during spiral movements in a room environment that was more complex than a wind tunnel. A cylindrical enclosure was used in this study; however, a more elaborate and lightweight enclosure should be developed in the future.
However, the bio-hybrid drone examined in this study has some limitations. For example, the distance of odor source localization was still limited. Owing to their high mobility, drones should be capable of searching for odors over long distances in the order of several tens of meters. However, the distance achieved by the insect antenna-based bio-hybrid drone was limited to 2 m26, and odor source localization tests were conducted in a wind tunnel with limited space25. Extending the searching distance is essential for the development of a practical odor-detecting flight platform.
For long-distance searches (over 10 m), a high sensor directivity and an efficient odor source localization algorithm are required, given that dilution of the odor concentration and complex distribution of the odor plume are expected. Stereo sensing using two antennae of the same insect can increase directionality23. Most odor source localization experiments using small drones with commercial gas sensors were conducted using a single sensor, and an EAG device array on drones was not conducted. Therefore, an EAG device array must be developed for small drones to increase their odor-sensing-application potential. The EAG device array would also facilitate the development of an efficient odor source localization algorithm as it allows for more precise localization of an odor plume.
Insect antenna-based bio-hybrid odor-detecting drones contribute to both fundamental and applied research. From the perspective of fundamental research, such drones can be used as test platforms to develop odor source localization algorithms. Various algorithms have been previously proposed31; however, test platforms using a mobile robot that conducted two-dimensional odor searches or commercial gas sensors have exhibited limited performance. In these setups, it is difficult for proposed algorithms to demonstrate their performance. The bio-hybrid drone in this study demonstrated odor concentration recognition ability as well as sensor directivity, sensitivity, and selectivity. Therefore, it shows great promise for installation in more advanced or three-dimensional odor source localization algorithms.
In terms of applications, bio-hybrid drones can be deployed on missions that living animals may have difficulty approaching, such as detecting toxic chemical/biological leaks, explosive materials, and search-and-rescue operations. To apply such drones to these missions, the insect antennae need to detect odorant molecules included in target odor sources. Silkmoth antennae can be genetically modified32 to have the potential to detect odorant molecules other than the female silkmoth sex pheromone; thus, these applications are now becoming reality.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported in part by a research grant from The Murata Science Foundation. The authors would like to acknowledge Smart Robotics Co., Ltd., Tokyo, Japan, for assisting in the development of the drone platforms and programming and Assist Technology Co., Ltd., Osaka, Japan, for assisting with the design of the electronic circuits. The authors would also like to thank Dr. Shigeru Matsuyama (Graduate School of Life and Environmental Sciences, University of Tsukuba) for providing purified bombykol; Mr. Takuya Nakajo (RCAST, The University of Tokyo) for support for silkmoth breeding; and Mr. Yusuke Notomi (Graduate School of Science and Technology, Tokyo University of Science) for supporting the acquisition of silkmoth images.
Materials
| Name | Company | Catalog Number | Comments |
| Anemometer | MK Scientific, Kanagawa, Japan | DT-8880 | |
| Circulator | IRIS OHYAMA Inc., Miyagi, Japan | PCF-SC15T | |
| Compact air pump | AS ONE Corporation, Osaka, Japan | NUP-1 | |
| Drone | Shenzhen Ryze Tech Co., Ltd. | Tello EDU | Ryze Tech opens Tello EDU SDK. Our source code is based on SDK 2.0 Use Guide. https://dl-cdn.ryzerobotics.com/downloads/Tello/Tello%20SDK%202.0%20User%20Guide.pdf You can download python code (Tello3.py.) and develop flight programs. |
| EAG device | Custom made | The EAG device has custom software to measure signals and communicate with the PC. | |
| Electrically conductive gel | Parker Laboratories, NJ, USA | Spectra 360 | |
| Ethanol | FUJIFILM Wako Pure Chemical Corporation, Ltd., Osaka, Japan | 057-00456 | |
| Flowmeter | KOFLOC, Kyoto, Japan | RK1600R-12-B-Air-20 | |
| Gas sensor | Sensirion AG, Stäfa, Switzerland | SGP30 | SGP30 breakout board can be used. You can refer the Adafruit_SGP30 github library. https://github.com/adafruit/Adafruit_SGP30 |
| High-sealed storage bottle | FUJIFILM Wako Pure Chemical Corporation, Ltd., Osaka, Japan | 290-35731 | |
| Microcontroller | M5Stack, Shenzhen, China | M5StickC | |
| Purebred silkworm diet | Nosan Corporation Life Tech Department, Kanagawa, Japan | Sausage type | |
| Silkmoth | Ueda-sansyu, Nagano, Japan | a hybrid strain of Kinshu × Showa | |
| Solenoid valve | Takasago Electric, Inc., Nagoya, Japan | YDV-3-1/8 | |
| Wi-Fi access point | Yamaha Corporation, Shizuoka, Japan | WLX313 |
References
- Burgués, J., Marco, S. Environmental chemical sensing using small drones: A review. Science of the Total Environment. 748, 141172(2020).
- Ercolani, C., Martinoli, A. 3D odor source localization using a micro aerial vehicle: system design and performance evaluation. 2020 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). , 6194-6200 (2020).
- Neumann, P. P., et al. Indoor air quality monitoring using flying nanobots: design and experimental study. IEEE International Symposium on Olfaction and Electronic Nose (ISOEN). , 1-3 (2019).
- Burgués, J., Hernández, V., Lilienthal, A. J., Marco, S. Smelling nano aerial vehicle for gas source localization and mapping. Sensors. 19 (3), 478(2019).
- Shigaki, S., Okajima, K., Sanada, K., Kurabayashi, D. Experimental analysis of the influence of olfactory property on chemical plume tracing performance. IEEE Robotics and Automation Letters. 4 (3), 2847-2853 (2019).
- Romano, D., Donati, E., Benelli, G., Stefanini, C. A review on animal-robot interaction: from bio-hybrid organisms to mixed societies. Biological Cybernetics. 113, 201-225 (2019).
- Bozkurt, A., Lobaton, E., Sichitiu, M. A biobotic distributed sensor network for under-rubble search and rescue. Computer. 49 (5), 38-46 (2016).
- Yu, Y., et al. Intelligence-augmented rat cyborgs in maze solving. PLoS One. 11 (2), 0147754(2016).
- Cazenille, L., et al. How mimetic should a robotic fish be to socially integrate into zebrafish groups. Bioinspiration & Biomimetics. 13, 025001(2018).
- Sankaran, S., Khot, L. R., Panigrahi, S. Biology and applications of olfactory sensing system: A review. Sensors and Actuators B: Chemical. 171-172, 1-17 (2012).
- Bohbot, J. D., Vernick, S. The emergence of insect odorant receptor-based biosensors. Biosensors. 10 (3), 26(2020).
- Terutsuki, D., et al. Increasing cell-device adherence using cultured insect cells for receptor-based biosensors. Royal Society Open Science. 5 (3), 172366(2018).
- Terutsuki, D., Mitsuno, H., Kanzaki, R. 3D-printed bubble-free perfusion cartridge system for live-cell imaging. Sensors. 20 (20), 5779(2020).
- Terutsuki, D., et al. Highly effective volatile organic compound dissolving strategy based on mist atomization for odorant biosensors. Analytica Chimica Acta. 1139, 178-188 (2020).
- Terutsuki, D., et al. Odor-sensitive field effect transistor (OSFET) based on insect cells expressing insect odorant receptors. 2017 IEEE 30thInternational Conference on Micro Electro Mechanical Systems (MEMS). , 394-397 (2017).
- Nagata, S., et al. A high-density integrated odorant sensor array system based on insect cells expressing insect odorant receptors. 2018 IEEE International Conference on Micro Electro Mechanical Systems (MEMS). , 282-285 (2018).
- Park, K. C., Ochieng, S. A., Zhu, J., Baker, T. C. Odor discrimination using insect electroantennogram responses from an insect antennal array. Chemical Senses. 27 (4), 343-352 (2002).
- Myrick, A. J., Park, K. C., Hetling, J. R., Baker, T. C. Real-time odor discrimination using a bioelectronic sensor array based on the insect electroantennogram. Bioinspiration & Biomimetics. 3, 046006(2008).
- Pawson, S. M., et al. Light-weight portable electroantennography device as a future field-based tool for applied chemical ecology. Journal of Chemical Ecology. 46 (7), 557-566 (2020).
- Kuwana, Y., Shimoyama, I., Miura, H. Steering control of a mobile robot using insect antennae. Proceedings 1995 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). 2, 530-535 (1995).
- Kuwana, Y., Shimoyama, I. A pheromone-guided mobile robot that behaves like a silkworm moth with living antennae as pheromone sensors. The International Journal of Robotics Research. 17 (9), 924-933 (1998).
- Kuwana, Y., Nagasawa, S., Shimoyama, I., Kanzaki, R. Synthesis of the pheromone-oriented behaviour of silkworm moths by a mobile robot with moth antennae as pheromone sensors. Biosensors and Bioelectronics. 14 (2), 195-202 (1999).
- Martinez, D., Arhidi, L., Demondion, E., Lucas, J. B. P. Using insect electroantennogram sensors on autonomous robots for olfactory searches. Journal of Visualized Experiments: JoVE. (90), (2014).
- Lan, B., Kanzaki, R., Ando, N. Dropping counter: A detection algorithm for identifying odour-evoked responses from noisy electroantennograms measured by a flying robot. Sensors. 19 (20), 1-16 (2019).
- Anderson, M. J., Sullivan, J. G., Horiuchi, T., Fuller, S. B., Daniel, T. L. A bio-hybrid odor-guided autonomous palm-sized air vehicle. Bioinspiration & Biomimetics. 16, 026002(2020).
- Terutsuki, D., et al. Real-time odor concentration and direction recognition for efficient odor source localization using a small bio-hybrid drone. Sensors and Actuators B: Chemical. 339, 129770(2021).
- Große-Wilde, E., Svatoš, A., Krieger, J. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chemical Senses. 31 (6), 547-555 (2006).
- Rüffer, D., Hoehne, F., Bühler, J. New digital metal-oxide (MOx) sensor platform. Sensors. 18 (4), 1052(2018).
- Hayes, A. T., Martinoli, A., Goodman, R. M. Distributed odor source localization. IEEE Sensors Journal. 2 (3), 260-271 (2020).
- Lochmatter, T., Raemy, X., Matthey, L., Indra, S., Martinoli, A. A comparison of casting and spiraling algorithms for odor source localization in laminar flow. 2008 IEEE International Conference on Robotics and Automation. , 1138-1143 (2008).
- Chen, X., Huang, J. Odor source localization algorithms on mobile robots: A review and future outlook. Robotics and Autonomous Systems. 112, 123-136 (2019).
- Sakurai, T., et al. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genetics. 7 (6), 1002115(2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved