A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fabrication and Use of Dry Macroporous Alginate Scaffolds for Viral Transduction of T Cells
In This Article
Summary
Herein is a protocol for creating dry macroporous alginate scaffolds that mediate efficient viral gene transfer for use in genetic engineering of T cells, including T cells for CAR-T cell therapy. The scaffolds were shown to transduce activated primary T cells with >85% transduction.
Abstract
Genetic engineering of T cells for CAR-T cell therapy has come to the forefront of cancer treatment over the last few years. CAR-T cells are produced by viral gene transfer into T cells. The current gold standard of viral gene transfer involves spinoculation of retronectin-coated plates, which is expensive and time-consuming. There is a significant need for efficient and cost-effective methods to generate CAR-T cells. Described here is a method for fabricating inexpensive, dry macroporous alginate scaffolds, known as Drydux scaffolds, that efficiently promote viral transduction of activated T cells. The scaffolds are designed to be used in place of gold standard spinoculation of retronectin-coated plates seeded with virus and simplify the process for transducing cells. Alginate is cross-linked with calcium-D-gluconate and frozen overnight to create the scaffolds. The frozen scaffolds are freeze-dried in a lyophilizer for 72 h to complete the formation of the dry macroporous scaffolds. The scaffolds mediate viral gene transfer when virus and activated T cells are seeded together on top of the scaffold to produce genetically modified cells. The scaffolds produce >85% primary T cell transduction, which is comparable to the transduction efficiency of spinoculation on retronectin-coated plates. These results demonstrate that dry macroporous alginate scaffolds serve as a cheaper and more convenient alternative to the conventional transduction method.
Introduction
Immunotherapy has emerged as a revolutionary cancer treatment paradigm due to its ability to specifically target tumors, limit off-target cytotoxicity, and prevent relapse. Particularly, chimeric antigen receptor T (CAR-T) cell therapy has gained popularity due to its success in treating lymphomas and leukemias. The FDA approved the first CAR-T cell therapy in 2017, and, since then, has approved four more CAR-T cell therapies1,2,3,4,5. CARs have an antigen recognition domain usually consisting of a single chain variable fragment of a monoclonal antibody that is specific for a tumor associated antigen3,4. When a CAR interacts with its tumor-associated antigen, the CAR-T cells become activated, leading to an antitumor response involving cytokine release, cytolytic degranulation, transcription factor expression, and T cell proliferation. To produce CAR-T cells, blood is collected from the patient to obtain their T cells. CARs are genetically added to the patient's T cells using a virus. The CAR-T cells are grown in vitro and infused back into the patient2,3,4,6. Successful generation of CAR-T cells is determined by the transduction efficiency, which describes the number of T cells that are genetically modified into CAR-T cells.
Currently, the gold standard for CAR-T cell generation is spinoculation of activated T cells and virus on retronectin-coated plates7,8. Transduction begins when viral particles engage with the surface of the T cells. Retronectin promotes colocalization of virus and cells by increasing the binding efficiency between the viral particles and the cells, enhancing transduction7,8. Retronectin does not work well on its own and needs to be accompanied by spinoculation, which enhances gene transfer by concentrating the viral particles and increasing the surface permeability of the T cell, allowing for easier viral infection8. Despite the success of spinoculation on retronectin-coated plates, it is a complex process that requires multiple spin cycles and expensive reagents. Therefore, alternate methods for viral gene transfer that are quicker and cheaper are highly desirable.
Alginate is a natural anionic polysaccharide extensively used in the biomedical industry due to its low cost, good safety profile, and ability to form hydrogels upon mixing with divalent cations9,10,11,12. Alginate is a GMP-compliant polymer and is generally recognized as safe (GRAS) by the FDA13. Cross-linking alginate with cations creates stable hydrogels often used in wound healing, delivery of small chemical drugs and proteins, and cell transportation9,10,11,12,14,15,16. Due to its excellent gelling properties, alginate is the preferred material to create porous scaffolds by freeze-drying10,17. These characteristics of alginate make it an attractive candidate for producing a scaffold that can mediate viral gene transfer of activated cells.
Described here is a protocol for making dry macroporous alginate scaffolds, known as Drydux scaffolds, that statically transduce T cells by viral gene transfer17,18. The process for making these scaffolds is shown in Figure 1. These scaffolds eliminate the need for spinoculation of retronectin-coated plates. The macroporous alginate scaffolds encourage the interaction of viral particles and T cells to enable efficient gene transfer in a single step without affecting functionality and viability of the engineered T cells17. When followed correctly, these macroporous alginate scaffolds have a transduction efficiency of at least 80%, simplifying and shortening the viral transduction process.
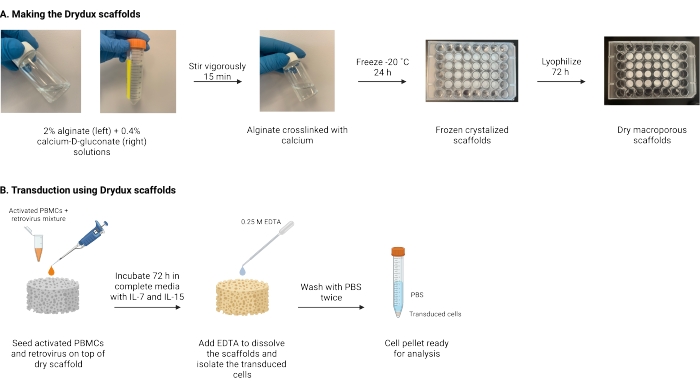
Figure 1: Schematic and timeline of the protocol. (A) Timeline for making the dry macroporous alginate scaffolds. Alginate is cross-linked with calcium-D-gluconate and frozen overnight. The frozen scaffolds are lyophilized for 72 h to create the Drydux scaffolds. (B) Timeline for viral transduction of activated cells. Activated cells and virus (MOI 2) are seeded on top of the scaffold and incubated in complete media supplemented with IL-7 and IL-15. The scaffolds absorb the mixture and promote viral gene transfer. EDTA is used to dissolve the scaffolds and isolate the transduced cells. After washing twice with PBS, the cell pellet can be used for analysis. Abbreviations: PBS = phosphate-buffered saline; PBMCs = peripheral blood mononuclear cells. Please click here to view a larger version of this figure.
Protocol
All the procedures involving human primacy cells and retroviral vectors were performed in compliance with North Carolina State University's Biological Safety guidelines and approved by the Environmental Health and Safety Office. Human peripheral blood mononuclear cells were purchased as buffy coats from commercial sources. Primary human cells must be isolated from human buffy coat fractions and require Biosafety Level 2 clearance and detailed standard operating procedures and approval from the institution where the work is to take place. Viral vectors, including the retroviral vector supernatants used for the transduction prepared as previously described19, can be classified either as Biosafety Level 1 or Biosafety Level 2 depending on the encoded protein and require approval from the relevant institutional biosafety committee.
1. Making the macroporous alginate scaffolds
- Prepare a stock solution of 0.4% (weight per volume) calcium D gluconate by adding sterile-filtered deionized water to calcium D gluconate in a beaker. Stir on medium speed until the calcium gluconate has dissolved (~1.5 h), sterile-filter, and store at room temperature.
NOTE: It is not necessary to check the pH of the solution. - Prepare a solution of 2% (weight per volume) alginate in a screw cap vial or beaker. Carefully add the ultrapure alginate to the vessel and add sterile-filtered deionized water to the alginate. Choose the largest stir bar possible that will not impede mixing and add it to the vessel.
NOTE: Information on the type of ultrapure alginate used for this protocol can be found in the Table of Materials and on the Novamatrix website20. Different alginates were not explored for these scaffolds, as they can lead to undesired changes in porosity and biocompatibility of the scaffolds9,10,11. - Stir the solution on high speed until the alginate dissolves (~1 h). If there are large clumps on the sides of the vessel, tilt the vessel to dislodge them. Small amounts of alginate on the sides will dissolve while stirring and are not cause for concern.
NOTE: It is not necessary to check the pH of the solution. - When the alginate dissolves, reduce the stirring speed, slowly add an equal volume of 0.4% calcium D gluconate to the alginate solution to prevent crosslinking clumps from forming, and stir vigorously for 15 min.
- Tilt the beaker or vial sideways to remove any clumps that may have formed.
NOTE: It is recommended to use a homogenizer. The final solution should be optically clear. - Pipette the desired volume of the solution into each well of a 48-well plate or 24-well plate. Use 300 µL/well for a 48-well plate and 1 mL/well for a 24-well plate. Cast slowly and change tips frequently, as the solution will be viscous and will stick to the tip.
- Cover the plates with lids and freeze overnight at -20 ˚C.
- Prepare the plate for the lyophilizer by removing the lid and securing the top with wipes and rubber bands. Place the plates in 750 mL to 2,000 mL lyophilizer flasks, and place on the lyophilizer for 72 h.
- After 72 h, remove the plate from the lyophilizer and replace the lid. Seal the plate and store at 4 ˚C. For long term storage, vacuum-seal the plate before storing at 4 ˚C. Keep dry until needed.
NOTE: Make extra calcium-alginate solution due to volume error (some sticks to the walls of the vial, pipette tips). For example, to make four scaffolds in a 24-well plate, 4 mL of combined solution (2 mL alginate + 2 mL calcium D gluconate) is required, but it is recommended to prepare 2.5 mL of 2% alginate solution and 2.5 mL of 0.4% calcium D gluconate solution.
2. Transduction
- Concentration of viral supernatant using 100 kDa filters
- Hydrate the centrifugal filter for 2 min with 1 mL of 1x phosphate-buffered saline (PBS). Discard the PBS.
- Concentrate the viral suspension by adding 2 mL of viral suspension (1 × 106 TU/mL) and centrifuging it through the centrifugal filter at 1,500 × g for 10 min in a swinging bucket rotor.
NOTE: One to two hundred microliters of concentrated virus is needed per scaffold. - Spin for a few additional minutes if the concentrated virus suspension volume is greater than 200 µL.
- Repeat steps 2.1.1-2.1.3 for each scaffold.
NOTE: It is also acceptable to concentrate a large stock of virus and take 100-200 µL aliquots from the concentrated stock.
- Seed activated cells and virus together.
- For each scaffold, prepare an aliquot by suspending 1 × 106 activated peripheral blood mononuclear cells (PBMCs) in 50 µL of complete cell culture media (250 mL Click's media + 250 mL RPMI 1640 media + 50 mL fetal bovine serum + 5 mL glutamine substitute + 5 mL penicillin/streptomycin).
NOTE: See Supplemental File 1 for a protocol on how to activate PBMCs. - Add the concentrated viral supernatant (~2 × 106 TU, MOI 2) to the cell suspension. The total volume of the cell-virus suspension should not exceed 200 µL for a 48-well scaffold or 350 µL for a 24-well scaffold.
- Add the cell-virus mixture dropwise to the top of the dry scaffold. For negative control experiments, use complete cell culture media in place of the concentrated virus. Add the cell suspension to the top of the dry scaffold.
- Repeat steps 2.2.1-2.2.3 for each scaffold.
- Incubate the scaffolds in a cell culture incubator at 37 ˚C for 45-60 min. Remove the scaffolds from the incubator; the scaffolds will fully absorb the solution during this time.
- To induce proliferation, add complete cell culture media supplemented with IL-15 (5 ng/mL) and IL-7 (10 ng/mL) to each well; IL-2 can also be used. Add 500 µL to each 48-well scaffold or 1 mL to each 24-well scaffold.
- Incubate the scaffolds at 37 ˚C for 72 h. As the cells proliferate, the media will turn orange.
- For each scaffold, prepare an aliquot by suspending 1 × 106 activated peripheral blood mononuclear cells (PBMCs) in 50 µL of complete cell culture media (250 mL Click's media + 250 mL RPMI 1640 media + 50 mL fetal bovine serum + 5 mL glutamine substitute + 5 mL penicillin/streptomycin).
- Isolate cells from the scaffolds for analysis.
- Dilute 0.5 M EDTA to 0.25 M EDTA in 1x PBS.
- Remove excess medium from each well and collect into separate 15 mL centrifuge tubes.
- To isolate cells from the scaffold, add 0.25 M EDTA to each scaffold. Add 300 µL to 48-well scaffolds or 1 mL to 24-well scaffolds. Let the plate sit or gently agitate for 3-4 min.
- Once the scaffold is mostly dissolved, pipette in and out gently inside the well. A few in-and-out pipetting steps might be necessary to fully dissolve the scaffold. If it does not dissolve in 10 min, add another 200 µL of 0.25 M EDTA.
- Once the scaffold dissolves completely, transfer the solution to a 15 mL centrifuge tube.
- Repeat steps 2.3.4 and 2.3.5 for each scaffold.
- Wash the cells twice by adding 12 mL PBS to each centrifuge tube and centrifuging at 400 × g for 5 min. A cell pellet will form at the bottom of each tube. Aspirate the supernatant and repeat the wash. Be careful to fully aspirate the supernatant with every wash as it is crucial to remove all the EDTA.
- After the second wash with PBS, the cell pellet is now ready for analysis. Follow the appropriate protocol required for the analysis.
Results
These macroporous alginate scaffolds are easy to make and should come out of the lyophilizer as porous, fluffy, and white discs. Although not studied in this experiment, calcium-alginate solution can be cast into different molds to create scaffolds of varying shapes, depending on the needs of the user9,10. The scaffolds are electrostatic and may stick to the lid of the well-plate or to a gloved finger. Figure 2 demonstrates what the ...
Discussion
CAR-T cell therapy continues to gain interest for both research and commercial applications. Despite the success CAR-T cell therapy has had in treating blood cancers, the high cost of the procedure limits its use. The protocol presented here introduces a new method for viral gene transfer of T cells without the need for spinoculation of retronectin-coated plates. Producing dry macroporous alginate scaffolds to mediate transduction is relatively simple and is a suitable low-cost replacement for the conventional method.
Disclosures
P.A. and Y.B. are inventors on patents related to the use of biomaterials for generation of CAR-T cell therapeutics. Y.B. receives an industry-sponsored research grant related to CAR-T cell therapeutic technology (unrelated to this work). All other authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Institutes of Health through Grant Award Numbers R37-CA260223, R21CA246414. We thank the NCSU flow cytometry core for training and guidance on flow cytometry analysis. Schematics were created with Biorender.com
Materials
| Name | Company | Catalog Number | Comments |
| 0.5 M EDTA | Invitrogen | 15575-038 | UltraPure, pH 8.0 |
| 1x DPBS | Gibco | 14190-144 | No calcium chloride or magnesium chloride |
| 3% Acetic Acid with Methylene Blue | Stemcell Technologies Inc | 07060 | |
| Activated Periphreal Blood Mononuclear Cells | - | - | Fresh or frozen |
| Calcium-D-Gluconate | Alfa Aesar | A11649 | |
| CD28.2 Antibody | BD | 555725 | 1 mg/mL |
| CD3 Antibody | Miltenyi | 130-093-387 | 100 μg/mL |
| Click's Media | FUJIFILM IRVINE SCIENTIFIC MS | 9195 | |
| DI Water | - | - | |
| Glutamax | Gibco | 35-050-061 | |
| HyClone FBS | Cytvia | SH3039603 | |
| HyClone RPMI 1640 Media | Cytvia | SH3009601 | |
| Penicillin-streptomycin (P/S) | Gibco | 15-140-122 | |
| Peripheral Blood Mononuclear Cells | - | - | Fresh or frozen |
| PRONOVA UP MVG | NovaMatrix | 4200101 | Sodium alginate |
| Recombinant Human IL-15 | Peprotech | 200-15 | 5 ng/mL |
| Recombinant Human IL-7 | Peprotech | 200-07 | 10 ng/mL |
| Retrovirus | - | - | 1 x 106 TU/mL |
References
- Prinzing, B. L., Gottschalk, S. M., Krenciute, G. C. A. R. T-cell therapy for glioblastoma: ready for the next round of clinical testing. Expert Review of Anticancer Therapy. 18 (5), 451-461 (2018).
- Bagley, S. J., Desai, A. S., Linette, G. P., June, C. H., O'Rourke, D. M. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro-oncology. 20 (11), 1429-1438 (2018).
- Nair, R., Westin, J. CAR T cells. Advances in Experimental Medicine and Biology. 1342, 297-317 (2021).
- Jackson, H. J., Rafiq, S., Brentjens, R. J. Driving CAR T-cells forward. Nature Reviews. Clinical Oncology. 13 (6), 370-383 (2016).
- Sterner, R. C., Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer Journal. 11 (4), 69 (2021).
- Miliotou, A. N., Papadopoulou, L. C. CAR T-cell therapy: A new era in cancer immunotherapy. Current Pharmaceutical Biotechnology. 19 (1), 5-18 (2018).
- Lee, H. -. J., et al. Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals: Journal of the International Association of Biological Standardization. 37 (4), 203-209 (2009).
- Rajabzadeh, A., Hamidieh, A. A., Rahbarizadeh, F. Spinoculation and retronectin highly enhance the gene transduction efficiency of Mucin-1-specific chimeric antigen receptor (CAR) in human primary T cells. BMC Molecular and Cell Biology. 22 (1), 57 (2021).
- Sun, J., Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials. 6 (4), 1285-1309 (2013).
- Nayak, A. K., Mohanta, B. C., Hasnain, M. S., Hoda, M. N., Tripathi, G. Chapter 14 - Alginate-based scaffolds for drug delivery in tissue engineering. Alginates in Drug Delivery. , 359-386 (2020).
- Lee, K. Y., Mooney, D. J. Alginate: properties and biomedical applications. Progress in Polymer Science. 37 (1), 106-126 (2012).
- Kuo, C. K., Ma, P. X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 22 (6), 511-521 (2001).
- Soccol, C., et al. Probiotic nondairy beverages. Handbook of Plant-Based Fermented Food and Beverage Technology, Second Edition. , 707-728 (2012).
- Moody, C. T., et al. Restoring carboxylates on highly modified alginates improves gelation, tissue retention and systemic capture. Acta Biomaterialia. 138, 208-217 (2022).
- Brudno, Y., et al. Replenishable drug depot to combat post-resection cancer recurrence. Biomaterials. 178, 373-382 (2018).
- Moody, C. T., Palvai, S., Brudno, Y. Click cross-linking improves retention and targeting of refillable alginate depots. Acta Biomaterialia. 112, 112-121 (2020).
- Agarwalla, P., et al. Scaffold-mediated static transduction of T Cells for CAR-T Cell therapy. Advanced Healthcare Materials. 9 (14), 2000275 (2020).
- Agarwalla, P., et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nature Biotechnology. 40 (8), 1250-1258 (2022).
- Vera, J., et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 108 (12), 3890-3897 (2006).
- . PRONOVA UP MVG. IFF Nutrition Norge AS Available from: https://novamatrix.biz/store/pronova-up-mvg/ (2022)
- Zappasodi, R., Budhu, S., Abu-Akeel, M., Merghoub, T. In vitro assays for effector T cell functions and activity of immunomodulatory antibodies. Methods in Enzymology. 631, 43-59 (2020).
- Kong, B. S., Lee, C., Cho, Y. M. Protocol for the assessment of human T cell activation by real-time metabolic flux analysis. STAR Protocols. 3 (1), 101084 (2022).
- Bio-Rad cell activation protocols. Bio-Rad Available from: https://www.bio-rad-antibodies.com/cell-activation.html?JSESSIONID_STERLING=D6E538F76818E53C29884D6CC7334F24 (2022)
- Lin, H. -. R., Yeh, Y. -. J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, andin vitro studies. Journal of Biomedical Materials Research. 71 (1), 52-65 (2004).
- Wu, J., Zhao, Q., Sun, J., Zhou, Q. Preparation of poly(ethylene glycol) aligned porous cryogels using a unidirectional freezing technique. Soft Matter. 8 (13), 3620 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved