A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Histology Basics and Cell Death Detection in Honeybee Tissue
In This Article
Summary
Immunohistochemical methods are useful in honeybee research to detect and assess the level of apoptosis and necrosis in the midgut and hypopharyngeal glands of adult bees.
Abstract
Honeybees (Apis mellifera L.) inside the hive (nurse workers and other hive bees) and outside the hive (foragers) are exposed to climate and weather changes, various pesticides, pathogens, and malnutrition, mainly entering through the mouth and primarily affecting the digestive tracts of adult bees. To understand and prevent the effects of such external and internal stressors on honeybees, one useful research method is the immunohistochemical method. A basic protocol is described to prepare the midgut (ventriculus) and hypopharyngeal glands (HPGs) of adult bees for histological analysis. A detailed methodology is described to assess the level of cell damage and distinguish necrosis from programmed cell death (apoptosis) as a natural process of tissue regeneration. The results of adult honeybee treatment with oxalic acid and pesticides (insecticide and acaricide) and the determination of cell death in the ventriculus and HPGs are presented. The pros and cons of the methodology are also discussed.
Introduction
Honeybees (Apis mellifera L.) are, among other wild pollinators, the most important pollinators of agricultural plants. Over thousands of years, the changing environment has influenced bees to adapt their morphology, physiology, behavior, and tolerance to several pathogens and parasites. Therefore, honeybees have developed a highly diverse range of species and subspecies around the globe1. These results are consistent with previous findings, that there is genetic variation in the honeybee's digestive tract structure, but also suggest that alterations of the midgut are due to environmental factors2,3.
The digestive tract of the honeybee has three main parts: foregut, midgut (ventriculus), and hindgut4. The ventriculus is an essential organ for the digestion of pollen and nectar/honey; in the hindgut, osmotic control takes place through absorption of water and ions2. The hypopharyngeal glands (HPGs) of honeybee workers are located in the head and synthesize and secrete royal jelly components to feed the brood, the queen, and members of the colony. Their size changes with age and tasks and depends on proper nutrition (quality pollen). Nurse workers aged 6 to 18 days perform brood rearing, and the size of HPGs increases5,6. In forager bees, the HPGs degenerate and only secrete enzymes that are important to convert the complex sugars into simple ones (α-glucosidases, leucine arylamidase, invertase) in honey7.
Honeybees are exposed to several biotic and abiotic stressors8, and the digestive tract can be affected by several negative stimulants. The first barrier that protects the organism from pathogens is the peritrophic membrane in the midgut, which consists of intestinal mucosa to protect against pathogens4. The development and function of HPGs depend on diet, age, and colony condition9, and are affected by insecticides, acaricides10, and pathogens11,12,13. Acaricide residues in the hive due to varroa control treatment and pesticides from the environment affect forager bees and nurse bees14,15. The greatest threat to honeybee colonies is the mite Varroa destructor, both as a vector of viruses contributing to colony losses16 and as a consumer of the host's fat body (an important vital organ in honeybees), which consequently affects the individual's body and colony functions17.
However, intensive farmland habitats can provide a short-term food supply for honeybees. Therefore, agri-environmental schemes should enhance the availability of honey flowers in agricultural landscapes18. To assess the morphology of different subspecies6,19,20,21 or sublethal effects of these factors at the cell or tissue levels, especially midgut and HPGs, histological and immunohistochemical methods are practical and sufficiently accurate to be used in histology research in honeybees.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Basic histology for honeybee research
- Dissection of honeybee tissue
NOTE: For the dissection of worker bees, use a dissecting microscope with an LED light source. The most useful magnification is ~20x.- Manipulation and dissection
- Carefully take a worker bee with forceps and put it on ice (or into the freezer at -20 °C) for 2 min to immobilize it22. Pin the bee on the Petri dish diagonally through the uppermost back portion of the thorax twice, from left to right and from right to left.
- Pour insect saline to cover the body. Place the Petri dish under the microscope, focus, and adjust.
- Prepare the instruments (see the Table of Materials).
- Dissection of midgut
- Start with the abdomen by inserting one point of the scissors under the tergite A5 (Figure 1) in the center of the right side of the bee body. Cut to the tergite A2.
- Keep the inner blade of the scissors parallel with the side of the body to avoid damaging the internal organs. Turn the scissors left and make one cut; turn right and make another cut. Gently open the left part of the abdomen and pin it. Repeat on the other side.
- Using forceps with one hand, gently pull the honeybee stomach upward, and with scissors in the other hand, cut at the very end of the esophagus. Pull the stomach and midgut away from the abdomen and cut at the rectum. Use a pipette with insect saline solution and remove any feces or parts of the tissue.
- Dissection of HPGs
- Immobilize a worker bee on ice as described in step 1.1.1. Cut the head off and place it on the smaller plate with the antennae facing up. Secure the head with two pins: one through the left compound eye and the second through the right compound eye.
- Make a cut across the first compound eye on the inner side of the pins, continue to the labrum, and then make another cut on the other side across the second compound eye (Figure 2).
- Cut off the antennae. Lift off the mask and cut where still attached. Take the forceps and carefully remove the glands together with the brain and part of the compound eyes.
- Manipulation and dissection
- Fixation, dehydration, and paraffin embedding
NOTE: Wear protective gloves.- Place the tissue in penicillin bottles, filled 3/4 with 10% formalin. Keep in a refrigerator at 4 °C.
- After 24 h, dehydrate the tissue in a series of alcohols: 70%, 80%, 90%, 100%, for 1 h each, 100% 2-propanol for 1 h, 100% 2-propanol for 12 h, and finally 100% 2-propanol for 1 h.
- Place the tissue in histocassettes; mark and place them in the glass chambers with 2-propanol and paraffin (1:1) in an incubator at 60 °C for 24 h.
- Move the histocassettes to another chamber with paraffin (I.) for another 24 h. Repeat the procedure with fresh paraffin twice more (II. and III.), both for 24 h.
- Finally, prepare the mounting station and start embedding the tissue into wax.
- Open each histocassette and remove the cover. Fill the mold with wax and carefully put the tissue with warm forceps in the middle of the mold.
- Place the histocassette on the mold and slightly cover it with wax. Immediately place the mold on the cold surface of the mounting station for a few seconds, then place it on the cold plate for a few minutes until the wax hardens and separates from the mold together and the histocassette.
- Store the finished samples in a box, away from dust and heat.
- Cut 4 µm thin sections on a microtome: first, two sections attached to each other and then one separately. Transfer the sections with forceps and let them float on distilled water (42 °C), then collect them on clean slides by placing two sections together on the left side of the objective glass and the third one on the right side, remaining distinctly separate. Leave the marked slides overnight on the heating device and finally store them in a box dedicated for histology samples.
- Dewaxing and rehydration
NOTE: Wear protective gloves.- Prepare nine Coplin jars and put the sections into a series of clearing agents (I., II., III.) for 5 min each.
- Put into 2-propanol, ethanol 96% (I., II.), alcohol 90% and 80%, and distilled water for 3 min each.
- Dyeing with hematoxylin and eosin
NOTE: Wear protective gloves.- Prepare six Coplin jars.
- For hematoxylin and eosin (H&E) staining, put the dewaxed, rehydrated sections in hematoxylin for 5 min, then carefully place them under the running tap water for 2 min. Then put them into distilled water for 1 min and eosin for 4 min (for eosin, the Coplin jar is not necessary).
- Place the slides in ethanol 96% for 1 min, then 2-propanol for 2 min, and finally into the clearing agent for 2 min.
- Add mounting medium and a cover glass and let them dry. Observe under a light microscope.
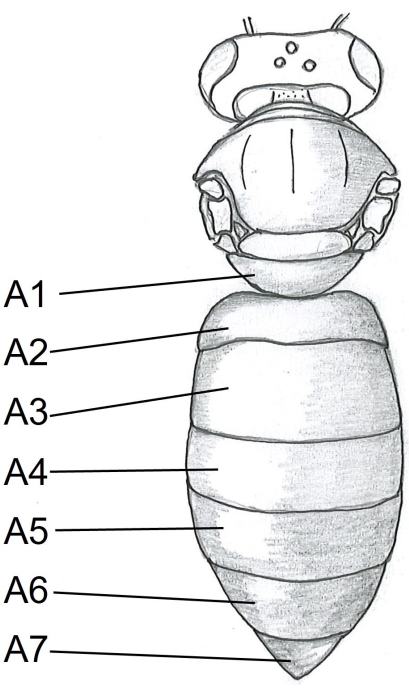
Figure 1: Dorsal view of honeybee body. A1-A7 tergites. The detailed instructions on honeybee dissection can be found in Carreck et al.24. Please click here to view a larger version of this figure.
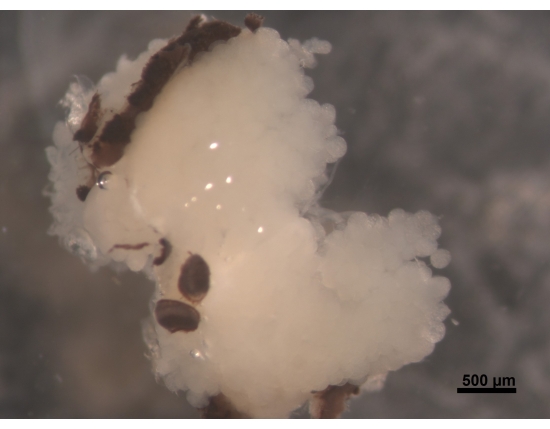
Figure 2: Dorsal view of HPGs, parts of compound eyes attached to the brain (not visible). A young worker bee aged 5 to 6 days has plump and creamy white HPGs. The acini are located on the brain and fill the head area with branches reaching the back of the brain. In foraging bees, these glands are greatly shrunken and leave only thin thread-like remains. For this reason, it is better to remove glands together with the brain to make it easier in further procedures to avoid losing the tissue. Scale bar = 500 µm. Please click here to view a larger version of this figure.
2. Cell death detection in tissue sections
- Apoptosis detection kit (Assay A)
NOTE: Follow the manufacturer's protocol (see the Table of Materials).- Prepare the Coplin jars.
- After dewaxing and rehydration (see step 1.3), immerse the slides in 0.85% NaCl solution, and then in phosphate-buffered saline (PBS) (5 min).
- Put the slides in 4% paraformaldehyde 2 x 15 min.
- Place the slides flat in the container and add 100 µL of a Proteinase K (20 µg/mL) solution, then leave them for 10-30 min.
- Place the slides in PBS (5 min).
- Place the slides in 4% paraformaldehyde in PBS (5 min).
- Immerse the slides in PBS (2 x 5 min).
- Place the slides flat in the container, add 100 µL of equilibration buffer, and leave them for 5-10 min.
- Add 100 µL of TdT reaction mix. Put paper towels inside the container, around the slides, moisten the towels with water, and then cover with plastic wrap. Incubate slides for 60 min at 37 °C.
- Place the slides back in the staining rack and immerse in 2x saline-sodium citrate (SSC) for 15 min.
- Immerse the slides 3 x 5 min in PBS, then in 0.3% hydrogen peroxide for 3-5 min, and then in PBS again, 3 x 5 min.
- Again, place the slides flat in the container, add 100 µL of Streptavidin HRP (horseradish peroxidase), and leave for 30 min (cover with plastic wrap).
- Immerse the slides 3 x 5 min in PBS.
- Place the slides flat in the container and add 100 µL of 3,3'-diaminobenzidine (DAB) solution. Look for a light brown background.
- Return the slides to the rack and wash them several times in water (double-distilled).
- Mount the slides under glass coverslips in mounting medium and leave flat to dry.
- Observe under a light microscope.
- Apoptosis detection kit (Assay B)
NOTE: Follow the manufacturer's protocol (see the Table of Materials).- Prepare Coplin jars.
- Prepare Proteinase K (20 µg/mL diluted in PBS).
- After dewaxing and rehydrating the sections (step 1.3), place the slides in PBS for 5 min.
- Place the slides flat in the container and add Proteinase K (20 µg/mL, 60 µL per 5 cm² specimen).
- Wash the slides 2 x 2 min in distilled water.
- Quench in endogenous peroxidase (in 3% hydrogen peroxidase) at room temperature.
- Rinse the slides 2 x 5 min with PBS or water.
- Place the slides flat in the container and apply equilibration buffer (75 µL/5 cm2) for 10 s at room temperature.
- Carefully wipe around the tissue.
- Add the TdT enzyme (terminal deoxynucleotidyl transferase) to each section and incubate in a humidified chamber for 1 h at 37 °C. Put paper towels inside the tray, around the slides, moisten the towels with water, and cover them with plastic wrap.
- After incubation, put the specimens in the rack and leave them in stop/wash buffer (10 min).
- Warm the anti-digoxigenin conjugate to room temperature.
- Wash the slides in PBS (3 x 1 min).
- Carefully wipe off around the tissue.
- Add two drops of Anti-Digoxigenin-Peroxidase conjugate (65 µL/5 cm²) to the sections and incubate for 30 min in a humidified container.
- After washing in PBS 4 x 2 min, prepare working-strength peroxidase substrate, and gently tap off excess liquid and aspirate around the section.
- Cover the sections with peroxidase substrate (75 µL/5 cm²) and stain for 5 min. Place a slide under the microscope and determine the optimal staining time.
- Wash the slides in a staining rack in distilled water (3 x 1 min).
- Incubate the slides in distilled water for 5 min.
- Counterstain using hematoxylin for 2 min.
- Place the slide under running tap water for 3 min.
- Wash the slide in distilled water.
- Mount the slides under glass coverslips in mounting medium and leave flat to dry.
- Observe under a light microscope.
- Apoptosis detection kit (Assay C)
NOTE: Follow the manufacturer's protocol (see the Table of Materials).- Prepare the Coplin jars.
- Dewax and rehydrate the tissue sections (see step 1.3).
- Incubate the tissue with Proteinase K (15-30 min at 37 °C).
- Place the slides back on the rack and rinse 2x in PBS.
- Cover with 50 µL of 'TUNEL reaction mixture'. Place wet paper towels inside the container, cover them with plastic wrap, and leave them for 60 min at 37 °C.
- Rinse 3x with PBS.
- Place the slides in the container and dry the area around the tissue sample.
- Add 50 µL of Converter-AP to the sample and incubate in a humidified container for 30 min at 37 °C.
- Rinse 3x in PBS.
- Add 50-100 µL of substrate solution and leave for 10 min in the dark.
NOTE: Observe the staining under a light microscope. - Rinse the slides 3x with PBS.
- Counterstain by transferring sections into hematoxylin for 2 min and then carefully rinse in running tap water for 5 min.
- Mount the slides under glass coverslips in an aqueous mounting medium and leave them flat to dry.
- Observe under a light microscope. Assess the affected (positive) cells by counting 70 to 100 cells in each sample of the midgut or HPGs under a light microscope.
Access restricted. Please log in or start a trial to view this content.
Results
Cell death detection in the midgut
Newly emerged worker bees (Apis mellifera carnica) from the experimental apiary at the Agricultural Institute of Slovenia in Ljubljana were individually treated with 3% oxalic acid (OA)23. OA is frequently used in beekeeping for Varroa destructor control. After the treatment, the worker bees (three from each group) were immobilized on ice. The midgut was dissected and fixed it in 10% formalin. The tissue was then dehydrate...
Access restricted. Please log in or start a trial to view this content.
Discussion
In living organisms, cell death is defined as apoptosis or necrosis25 and can be accompanied by autophagy26. The difference between apoptotic and necrotic cells is that apoptosis is a form of programmed cell death and appears in normal cells, whereas necrosis occurs due to lethal conditions (e.g., accident, disease)27,28. Apoptosis can be detected using assay kits based on the TUNEL technique (detection ofDNA f...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The author has no conflicts of interest.
Acknowledgements
I gratefully acknowledge the support of the Slovenian Research Agency, grant no. P4-133.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 2-Propanol | |||
| ApopTag Peroxidase kit (ApopTag Peroxidase In Situ Apoptosis Detection) | Sigma-Aldrich | S7100 | Assay B, https://www.sigmaaldrich.com/SI/en/product/mm/s7100?gclid=CjwKCA jw7vuUBhBUEiwAEdu2pPanI9SE j81ZTl-nLHEoxXAv7ViKwPA_QRx H7fciMRNcYwR7lbPQbhoCqcQQA vD_BwE; Positive controls included in S7101 |
| Covers | |||
| DeadEnd Colorimetric TUNEL system | Promega | G7360 | Assay A, https://worldwide.promega.com/products/cell-health-assays/apoptosis-assays/deadend-colorimetric-tunel-system/?catNum=G7360 |
| Dissecting microscope (for bee dissection) | Zeiss | ||
| Distilled water | |||
| Embedding cassette | |||
| EnVision System alkaline phosphatase kit | Dako | ||
| Eosin Y Solution | Sigma-Aldrich | alcoholic | |
| Ethanol | 95% (or less pure), 90%, 80% | ||
| Faramount mounting medium, aqueous | Dako | mounting medium | |
| Flattening table | Leica | HI1220 | |
| Forceps (for bee dissection) | Fine science tools | 11294-00 | Standard #4 |
| Formalin 10% | Formaldehyde | ||
| Hematoxylin | Sigma-Aldrich | ||
| HistoChoice Clearing Agent | Sigma-Aldrich | clearing agent | |
| Hydrogen peroxidase 3% | |||
| Incubator | BioRad | ||
| Insect pins (for bee dissection) | Entosphinx | 44594 | Insect pins stainless steel – white, size 2 |
| ISCDDK, AP (In Situ Cell Death Detecteion Kit, Alkaline Phosphatase) | Roche | 11684809910 | Assay C, https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/362/737/11684809910b ul.pdf |
| KH2PO4 | |||
| Lab clock | |||
| Light microscope | Leica | ||
| Microscope slides | Box with the slides must be preserved in a plastic wrap to prevent dust | ||
| Microtome | Leica | ||
| Modular tissue embedding station | Leica | ||
| Na2HPO4 | |||
| NaCl | |||
| Paraformaldehyde 4% | |||
| Paraplast | Leica | ||
| Pasteur pipettes | 1.5 mL; 3 mL | ||
| PBS | |||
| Petri dish (for bee dissection) | Filled with condensation silicon (Xantoprene L blue and Universal liquid plus activator) | ||
| Proteinase K | Merck | 21627 | |
| Ringers' solution (for bee dissection) | 7.5 g NaCL, 2.38 g Na2HPO4, 2.72 g KH2PO4, 1 L distilled water | ||
| Scissors (for bee dissection) | Fine science tools | 1406-09, 14061-09 | Straight and curved, 9 cm |
| Universal liquid plus activator (for bee dissection) | Kulzer | ||
| Watchmaker’s forceps (for bee dissection) | Fine science tools | 91100-12 | |
| Water bath | Leica | ||
| Watercolor brush | 2x | ||
| Xantoprene L blue (for bee dissection) | Kulzer |
References
- Ruttner, F. Naturgeschichte der Honigbienen. , Ehrenwirth. München. (1992).
- Jordan, R. Kleine Bienenkunde. Österreichischer Agrarverlag Wien München. , 41-45 (1964).
- Snodgrass, R. E. The Anatomy of the Honey Bee. The Hive and the Honey Bee. , Dadant and Sons. 111-113 (1975).
- Snodgrass, R. E. The Anatomy of the Honey Bee. , Cornell University Press. (2004).
- Hrassnigg, N., Crailsheim, K. Adaptation of hypopharyngeal gland development to the brood status of honeybee (Apis mellifera L.) colonies. Journal of Insect Physiology. 44 (10), 929-939 (1998).
- Smodiš Škerl, M. I., Gregorc, A. Characteristics of hypopharyngeal glands in honeybees (Apis mellifera carnica) from a nurse colony. Slovenian Veterinary Research. 52 (2), 67-74 (2015).
- Kubo, T. Change in the expression of hypopharyngeal-gland proteins of the worker honeybees (Apis mellifera L.) with age and/or role. Journal of Biochemistry. 119 (2), 291-295 (1996).
- Sammataro, D., Yoder, J. A. Honey bee colony health: Challenges and sustainable solutions. , Taylor & Francis Group. 302(2012).
- Crailsheim, K., Stolberg, E. Influence of diet, age and colony condition upon intestinal proteolytic activity and size of the hypopharyngeal glands in the honeybee (Apis mellifera L.). Journal of Insect Physiology. 35 (8), 595-602 (1998).
- Smodiš Škerl, M. I., Gregorc, A. Heat shock proteins and cell death in situ localisation in hypopharyngeal glands of honeybee (Apis mellifera carnica) workers after imidacloprid or coumaphos treatment. Apidologie. 41 (1), 73-86 (2010).
- Gregorc, A., Bowen, I. D. The histochemical characterisation of cell death in honeybee larvae midgut after treatment with Paenibacillus larvae, Amitraz and Oxytetracycline. Cell Biology International. 24 (5), 319-324 (2000).
- Higes, M., et al. Apoptosis in the pathogenesis of Nosema ceranae (Microsporidia: Nosematidae) in honey bees (Apis mellifera). Environmental Microbiology Reports. 5 (4), 530-536 (2013).
- Kurze, C., et al. Infection dynamics of Nosema ceranae in honey bee midgut and host cell apoptosis. Journal of Invertebrate Pathology. 154, 1-4 (2018).
- Johnson, R. M. Honey bee toxicology. Annual Review of Entomology. 60 (1), 415-434 (2005).
- Gashout, H. A., Guzman-Novoa, E., Goodwin, P. H. Synthetic and natural acaricides impair hygienic and foraging behaviors of honey bees. Apidologie. 51 (6), 1155-1165 (2020).
- McMenamin, A. J., Genersch, E. Honey bee colony losses and associated viruses. Current Opinion in Insect Science. 8, 121-129 (2015).
- Ramsey, S. D., et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proceedings of the National Academy of Sciences USA. 116 (5), 1792-1801 (2019).
- Requier, F., et al. Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecological Applications. 25 (4), 881-890 (2015).
- Santos, C., Serrão, J. Histology of the ileum in bees (Hymenoptera, Apoidea). Brazilian Journal of Morphological Sciences. 23 (3), 405-413 (2006).
- Suwannapong, G., Saichon, C., Benbow, M. Histochemical comparison of the hypopharyngeal gland in Apis cerana Fabricius, 1793 workers and Apis mellifera Linnaeus, 1758 workers. Psyche: A Journal of Entomology. , (2010).
- Ceylan, A., Sevin, S., Özgenç, Ö Histomorphological and histochemical structure of the midgut and hindgut of the Caucasian honey bee (Apis mellifera caucasia). Turkish Journal of Veterinary and Animal Sciences. 43 (6), 747-753 (2019).
- Human, H., et al. Miscellaneous standard methods for Apis mellifera research. Journal of Apicultural Research. 52 (4), 1-53 (2013).
- Gregorc, A., Smodiš Škerl, M. I. Toxicological and immunohistochemical testing of honeybees after oxalic and rotenone treatments. Apidologie. 38 (3), 296-305 (2007).
- Carreck, N. L., et al. Standard methods for Apis mellifera anatomy and dissection. Journal of Apicultural Research. 52 (4), 1-40 (2013).
- Bowen, I. D., Bowen, S. M., Jones, A. H. Mitosis and apoptosis: Matters of Life and Death. , Chapman & Hall. London. (1998).
- Eisenberg-Lerner, A., et al. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death and Differentiation. 16 (7), 966-975 (2009).
- Bowen, I. D., Mullarkey, K., Morgan, S. M. Programmed cell death in the salivary gland of the blow fly Calliphora vomitoria. Microscopy Research Techniques. 34, 202-207 (1996).
- D'Arcy, M. S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biology International. 43 (6), 582-592 (2019).
- Matylevitch, N. P., et al. Apoptosis and accidental cell death in cultured human keratinocytes after thermal injury. American Journal of Pathology. 153 (2), 567-577 (1998).
- Perry, S. W., Epstein, L. G., Gelbard, H. A. Simultaneous in situ detection of apoptosis and necrosis in monolayer cultures by TUNEL and trypan blue staining. BioTechniques. 22 (6), 1102-1106 (1997).
- Cuello-Carrion, F. D., Ciocca, D. Improved detection of apoptotic cells using a modified in situ TUNEL technique. Journal of Histochemistry and Cytochemistry. 47 (6), 837-839 (1999).
- Gregorc, A., Bowen, I. D. Programmed cell death in the honeybee (Apis mellifera L.) larvae midgut. Cell Biology International. 21 (3), 151-158 (1997).
- Gregorc, A., Pogačnik, A., Bowen, I. D. Cell death in honey bee (Apis mellifera) larvae treated with oxalic or formic acid. Apidologie. 35 (5), 453-460 (2004).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved