A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Collection and Long-Term Maintenance of Leaf-Cutting Ants (Atta) in Laboratory Conditions
In This Article
Summary
Here, a protocol is described to successfully collect and maintain healthy Atta (Hymenoptera: Formicidae) ant colonies in laboratory conditions. Additionally, different nest types and configurations are detailed together with possible experimental procedures.
Abstract
Ants are one of the most biodiverse groups of animals on the planet and inhabit different environments. The maintenance of ant colonies in controlled environments enables an enriched comprehension of their biology that can contribute to applied research. This practice is usually employed in population control studies of species that cause economic loss, such as Atta ants. To cultivate their mutualistic fungus, these leaf-cutting ants collect leaves and for this are considered agricultural pests widely distributed throughout the American continent. They are highly socially organized and inhabit elaborated underground nests composed of a variety of chambers. Their maintenance in a controlled environment depends on a daily routine of several procedures and frequent care that are described here. It starts with the collection of queens during the reproductive season (i.e., nuptial flight), which are then individually transferred to plastic containers. Due to the high mortality rate of queens, a second collection can be carried out about 6 months after the nuptial flight, when incipient nests with developed fungus wad are excavated, hand-picked, and placed in plastic containers. In the laboratory, leaves are daily provided to established colonies, and ant-produced waste is weekly removed along with remaining dry plant material. As the fungus garden keeps growing, colonies are transferred to different types of containers according to the experimental purpose. Leaf-cutting ant colonies are placed in interconnected containers, representing the organizational system with functional chambers built by those insects in nature. This setup is ideal to monitor factors such as waste amount, fungus garden health, and the behavior of workers and queen. Facilitated data collection and more detailed observations are considered the greatest advantage of keeping ant colonies in controlled conditions.
Introduction
Ants compose a diverse group of individuals that exert an influence on most terrestrial environments1. They act as efficient dispersers2,3,4, predators5 and ecosystem engineers6,7,8,9,10, highlighting their importance and ecological success on natural ecosystems. All ant species are classified as eusocial insects; however, their social organization varies greatly among different species groups, i.e., labor division systems, functional groups, communication among individuals, forage organization, colony foundation, and reproduction process11. As a highly diversified group, they resort to several food resources and specialized feeding behaviors. As a matter of fact, agriculture was not only a huge step for human civilization, but also for ant species. Approximately 55 to 65 Ma ago12, attine ants began to culture fungi and incorporate them into an almost exclusive diet. They became so specialized that they developed strict, dependent, and obligatory interactions classified as symbiosis, where one individual does not survive without the other.
Lower fungus-growing ants collect and process dead organic matter, such as fragments of rotting leaf, to grow their mutualistic fungus; while higher fungus-growing ants harvest fresh plant material, composing one of the most successful symbiotic natural systems13. This highly specialized agriculture technique allowed them to seize a new niche. The higher attine ants comprise the leaf-cutting ants, a monophyletic group that arouse between 19 Ma (15-24 Ma) and 18 Ma (14-22 Ma)14,15,16 consisting of four valid genera: Atta Fabricius, Acromyrmex Mayr, Amoimyrmex Cristiano, and Pseudoatta Gallardo. The leaf-cutter agriculture system performed by the leaf-cutting ants, evolved from derived agriculture systems17. Most of these species exclusively exploit the mutualistic fungus species Leucoagaricus gongylophorus Singer18 (also called Leucocoprinus gongylophorus Heim19), marking a significant evolutionary transition11. The fungal cultivars are transmitted vertically, from original nests to offsprings, suggesting that they are clonally propagated20.
Remarkably, Atta societies developed a complex organizational structure of enormous importance in their environment and of great interest to myrmecologists. Their population can be composed of millions of individuals, most of them sterile female workers that display an accentuated polymorphism, i.e., distinct size and anatomical morphology. The population is distinguished by castes according to age, physiological state, morphological type, behaviors, and specialized activities in the colony21. Workers can be discriminated into gardeners and nurses, within-nest generalists, foragers and excavators, and defenders or soldiers21. This organization allows the performance of tasks in cooperation and a self-organizing system that can produce highly structured collective behaviors, allowing them to respond efficiently to environmental disturbances22.
The role of population renewal is played by a single queen (i.e., monogynous), for as long as she lives, constituting the permanent reproductive caste22. Atta queens are known to live for more than 20 years, laying eggs throughout their lifespan23. As the queen is irreplaceable, its endurance is crucial for the survival of the colony13,20,23,24. Yet, thousands of winged reproductive females and males can be found in the nest during breeding seasons, but none stays in the original nest, forming a temporary caste22. In Atta sexdens colonies, nearly 3,000 reproductive females and 14,000 reproductive males are produced25. It occurs when a colony reaches sexual maturity, approximately 38 months from its implementation, and is repeated annually ever since until it is extinguished23,25. New Atta colonies are established through haplometrosis, where one queen commences a new nest.
When environmental conditions are favorable, the reproducers leave the underground nest to begin the nuptial flight. The period of its occurrence differs by region, ranging along the year throughout the brazilian territory depending on the species. However, the event seems to be preceded by rainfalls and humidity elevation26, which can be related to excavation facilitation due to soil moisture22. Frequently, 1-5 weeks before the nuptial flight, nest entrances and channels are widened to facilitate the reproductive individuals to depart. Before leaving their mother colonies, the winged females collect and store, in an infrabuccal cavity, a portion of the mutualistic fungus20,27. Multiple copulations are performed mid-flight, and it is calculated that one queen can be inseminated by three to eight males (i.e., polyandry) in some species28, ensuring genetic variability29. Afterward, the queens proceed to the soil, giving preference to locations with no or few vegetation25, where they remove their wings and excavate their first nest chamber. This is the only period where queens can be seen outside the nest. Although individuals of the temporary caste were seen in artificial nests, it is unknown whether any successful copulation (i.e., nuptial flight) was performed in laboratory conditions24.
The initial nest construction corresponds to the most crucial period of the colony, which can last from 6 h to 8 h23,25. At this moment, the queen cloisters herself in the initial chamber, and in a matter of days, oviposition begins. The first eggs are fed to the mycelial that the queen regurgitates, marking the start of the colony's fungus garden. The first larvae appear in approximately 25 days22, and nearly at the end of the first month, the colony consists of a mat of proliferating fungus, where immatures (eggs, larvae, and pupae) are nested, and the queen, who raises her initial offspring in isolation23. Eggs are also the food resource of the first larvae and highly consumed by the queen13. Additionally, the queen sustains herself with fat-body reserves and catabolizing wing muscles that are no longer of use13. The initial fungus culture is not consumed as the colony survival depends on its development, and during this period, the queen fertilizes it with fecal fluid13. Days after emerging, the first workers open the nest entrance and begin a foraging activity in the immediate area of the nest13. They incorporate the material collected as the substrate of the fungus garden, which is now serving as food for the workers13,22. Before being added to the fungus culture, the plant material carried in by the workers is cut into tiny pieces and moistened with fecal liquid13. The ants manipulate fungus inoculum to increase and control its growth, which will serve for partitioning big soil excavated chambers, specialized in conditioning the garden13,22,25.
About 6 months after the nuptial flight, A. sexdens nests contain a fungus chamber and a few channels. The great specialization in the construction of leaf-cutting ant nests works as a defense mechanism against natural enemies and unfavorable environmental factors22. Leaf-cutting ants are known to fragment the fungus garden and transpose it to chambers with high humidity when chambers start to dry out13. Thus, despite the excavation of the nest having a considerable energy cost, the energy invested is reversed in benefits for the colony itself22. With a few exceptions, Atta species also make specialized chambers for the colony's waste, made mostly of depleted fungus substrate and bodies of dead ants, isolating it from the rest of the nest, and establishing an important social immunity strategy30. In addition, a distinct group of workers manipulate the refuse directly, to avoid contamination of other individuals. Workers constantly forage to nurture the fungus, which is the main nutritional resource of the colony. However, they can feed on plant sap as well while cutting fragments. Plant material is carefully selected for the fungus garden maintenance and influenced by many factors such as leaf traits and properties of the ecosystem13.
The foraging strategy of leaf-cutting ants to obtain fresh material is highly complex, and combined with the high harvest demand of established colonies, result in considerable economic loss to agricultural producers and jeopardizes forest restoration areas22,31. Therefore, these ants can be categorized as pests in most areas where they may be encountered, ranging from southern United States to north-eastern Argentina11,13,22,32. The extinguishing of problematic colonies is challenging due to the series of adaptations inherent in the biology of these insects (i.e., social organization, foraging, fungus-cultivation, hygiene, and complex nest structures)33. Hence, the population control strategies are distinct from those generally applied to other insect pests, and mainly resort to attractive contaminated bait offerings33,34. However, as these ants can reject harmful substances for both the fungus and the colony individuals, and compromise cultivated fields33, new natural compounds and alternatives of control are constantly being tested33,35,36. As experiment results can hardly be monitored on field-tested colonies, preliminary essays are conducted in a controlled environment.
Thus, experimental protocols must be adapted to groups of interest considering the heterogeneous lifestyle of ants, supporting studies on a species level, and accounting for colonies as operational units, where one ant is an element of a complex superorganism11. The reports gathered until now concerning the Atta genus made it attainable to successfully collect and maintain colonies in laboratory conditions and acknowledge their basic needs and general functioning. Based on their natural processes such as reproduction, colony founding, and feeding behaviors, a routine of practices has been developed that permits the long-term establishment of colonies in different types of nests. Here, a procedural protocol to maintain leaf-cutting ants in the laboratory is described and highlights possible general research with distinct experimentation purposes and science outreach.
Protocol
1. Collection of queens
- Search in the literature for the period of Atta reproductive season in the region of interest. The reproductive season occurrence, frequency, and day time of nuptial flights, varies according to regional climate conditions (Table 1). Although it generally takes place in spring, this information must be gathered for the location where the collection is intended to occur37,38,39,40,41,42,43,44,45,46.
- Identify and mark locations with Atta nests considered as possible areas for collecting queens and young colonies. During nuptial flights, queens are dispersed around nest locations; therefore, areas with a greater number of colonies have higher chances to have queen-landing spots where they initiate new nest excavations.
- Check the areas selected previously for signs of nuptial flight during the reproductive season of Atta ants. Keep track of the environmental conditions of nuptial flight days, such as hot and rainfall weather.
- Identify leaf-cutting ant nests in the areas selected previously and look for external features that indicate the upcoming departure of winged-reproductive ants. Nest features include tunnel entrances widened (Figure 1), increased flow of workers showing more aggressive behavior toward possible predators and winged-reproductive ants appearing at tunnel entrances (Figure 1). Beware to high humidity days succeeding rainfall, as they generally precedes nuptial flights.
- Prepare plastic lidded containers with a bottom plaster layer to retain the queens individually. Ensure that the container volume is approximately 200 mL, and the plaster layer at the bottom is around 1 cm in height and is highly absorbent for humidity control.
NOTE: To prepare the plaster base, follow the manufacturer's instructions. - Prepare an environment with a constant temperature of 23 ± 1 °C and approximately 70% ± 10% of relative humidity. Choose a location without intense activities and high flow of people to avoid vibrations and disturbance. Use cleaning products of neutral fragrance to prevent any interference in ant behavior.
NOTE: Fluctuations in specified environmental conditions can cause water condensation or moisture loss, and compromise the fungus garden. - After the nuptial flight, collect wingless queens that have initiated nest excavation, and carefully place them in the plastic containers prepared with a plaster layer, individually. Avoid touching the queens with bare hands and use latex gloves or entomological tweezers.
NOTE: Wing removal and soil excavation behavior indicate reproductive females that have already copulated, and therefore, are able to start a new colony. Queens collection is also treated as the first collection in this work. - Move the containers bearing the queens to the location with a controlled environment previously selected. Perform queens' transportation with utmost caution, avoiding too much disturbance and maintaining a minimum temperature constancy.
- Do not manipulate or move the queens for approximately 3 days after collection to avoid stress.

Figure 1. Nest entrance widened with winged ant reproducers and workers. Widened tunnel entrances are one of nests features that indicates Atta nuptial flights occurrence. Please click here to view a larger version of this figure.
2. Queens' maintenance
- Initially, add 2.5 mL of water to the plaster layer of the recipient every 2 days with the help of a needle syringe.
- Instead of opening the recipient, carefully puncture the container lids with the needle to avoid disturbance due to manipulation. The same hole can be used during this period. Ensure that the water added does not soak the plaster layer. Avoid directly watering the queen, the initial fungus sponge, and any immatures. For as long as the fungus garden exhibits a dry aspect with water absence, irrigate the plaster layer.
- Two weeks after the collection, check whether the fungus has been regurgitated by the queens. If there is no fungus, transfer approximately 2 g of fungus obtained from an established colony. Also, perform this step if the fungus does not develop.
NOTE: For fungus transfer, it is necessary to collect healthy fungus from an established colony and remove all the ants that might be on it. Use a tablespoon, entomological tweezers, and latex gloves to manipulate the fungus. - After the appearance of the first workers, begin offering fragments of young and thin leaves regularly, according to the cut activity of the colony. Ensure that the offered leaves are healthy, and the plants have not been treated with insecticides or other chemical substances. At early stages, ensure that the leaf fragments are not over 4 cm.
NOTE: As the first workers initiate leaf foraging, plant material must be offered after their appearance. The offering frequency depends on the agility with which the workers incorporate the plant material on the fungus but can range to 2-3 days a week. Oat flakes and corn flakes can also be offered but should be alternated with leaves to avoid fungus dryness. - When offering new leaves, remove colony waste and dry leaf fragments. Avoid the use of perfumes, moisturizers, creams, or any substance with a strong odor when manipulating the queens. Additionally, use latex gloves during all the processes.
- Follow the development of the colony, and when the fungus garden reaches at least half of the container volume, transfer the colony to an artificial perdurable nest.
NOTE: As the development rate is inherent for each colony, there is not an estimated time for colony transfer. Usually, colonies from the first collection are transfered to nests with fungus garden chamber of 1L volume maximum, due to the small fungus garden.
3. Collection of young colonies
- Acquire plastic containers of approximately 500 mL volume.
- About 6 months after the nuptial flight, identify indicative tower-shaped mounds with granulated soil particles (Figure 1) of incipient Atta nests (Figure 2) on the locations with leaf-cutting ants occurrence previously marked.
NOTE: Six months after the nuptial flight, the nests of young colonies are estimated to be up to 1 m deep in the soil. A new collection is indicated at this period to achieve higher chances of successful and enduring colonies in large quantities. - With a garden hoe, excavate the nest entrance until you reach the chamber holding the young colony. Collect the queen, fungus garden, immatures, and young workers, and place them in the plastic container. Perform the collection process as gently as possible.
NOTE: Naturally, a great amount of soil will be collected too and ought to be removed gradually in future maintenance procedures in the laboratory. - Move the plastic lidded containers holding the colonies to the designated controlled environment. Perform the transportation of young colonies with ultimate caution, avoiding too much disturbance and maintaining a minimum temperature constancy. Refrain manipulating or moving the colonies for approximately 3 days to avoid stress. If the room has an active routine, a dark cloth can be put over the colonies.

Figure 2. Tower-shaped soil mound. The characteristic tower-shaped mound indicates the presence of incipient colonies of Atta sexdens and Atta laevigata. Please click here to view a larger version of this figure.
4. Maintenance of young colonies
- Provide thin young leaves 3 times a week.
- Ensure that the leaves offered are healthy and the plants have not been treated with insecticides or other chemical substances. At this stage, ensure the leaf fragments are at least 7 cm in length.
NOTE: Oat flakes and corn flakes can also be offered but should be alternated with leaves to avoid fungus dryness. - The offering frequency depends on the agility with which the workers incorporate the plant material on the fungus. With the cut activity being intense, increase the offering two times a day three times a week or 5 days a week.
- Ensure that the leaves offered are healthy and the plants have not been treated with insecticides or other chemical substances. At this stage, ensure the leaf fragments are at least 7 cm in length.
- When offering new leaves, remove colony waste, including soil remnants, with the help of a spoon. Use latex gloves during all the processes. When manipulating the young colonies avoid the use of perfumes, moisturizers, creams, or any substance with a strong odor.
NOTE: The workers themselves will separate the soil and the waste from the fungus. - Follow the development of the colony, and when the fungus garden reaches at least half of the container volume, transfer the colony to an artificial perdurable nest.
NOTE: As the development rate is inherent for each colony, there is not an estimated time for colony transfer.
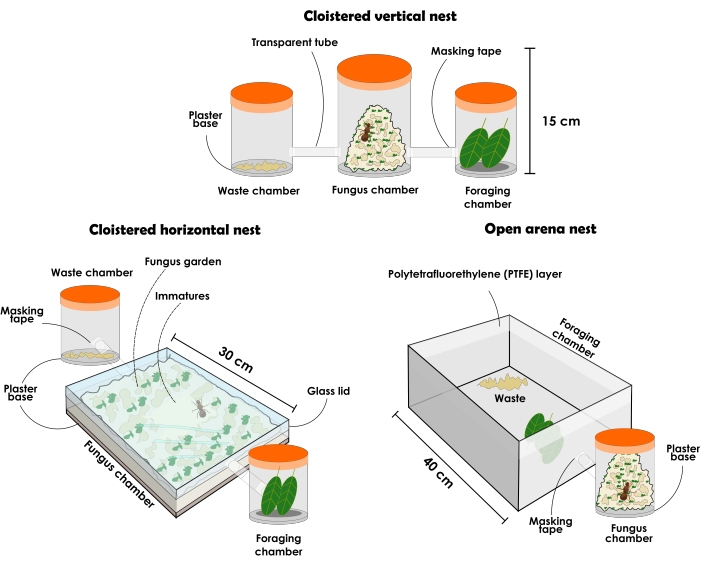
Figure 3: Types of artificial nests to hold Atta sexdens and Atta laevigata colonies. Illustration of perdurable artificial nests of leaf-cutting ants: cloistered vertical nest setup, cloistered horizontal nest setup, and open arena nest setup. Please click here to view a larger version of this figure.
5. Perdurable artificial nests
- Prepare a cloistered vertical nest setup as described below (Figure 3 and Figure 4).
NOTE: Cloistered nest configurations should always have different recipients to separately pose as (1) fungus garden chamber, (2) waste disposal chamber and (3) foraging chamber. Initially, it should start with three containers, but more recipients can be added to increase the fungus garden. The nests may vary in size and material according to their experimental purpose, although here it is described and recommended the use of see-through materials. The containers must be without openings, or else the ants will escape. The type of nest as described below can be used for general research, but it is not well recommended for assertive ant collection due to the disturbance caused when removing the lid, resulting in great agitation among the individuals. Nonetheless, due to the material transparency, it is possible to locate the queen and different ant castes even when the fungus garden has filled the entire container. As it is a tendency, it is always assumed that the immatures are in the center of the fungus garden when it has occupied most of the space available in vertical containers.- Select a transparent lidded container of approximately 1 L and add a 1 cm layer of highly absorbent plaster base. This will be the fungus garden chamber. Select two lidded see-through containers of approximately 500 mL each to be waste disposal and foraging chambers. The ants will choose which chamber will be each, and after that, ensure that they are not interchanged.
NOTE: To prepare the plaster base, follow the manufacturer's instructions. - Perforate and connect the three containers with a transparent tube or a hose. If necessary, apply masking tape on the tubes border to guarantee a thigh connection with the containers and avoid ants escaping. Place the plaster base container in the middle and the other containers on opposite sides.
- Carefully transfer the fungus sponge of selected colonies (see step 2.5 and step 4.3) along with the queen, workers, and immatures to the plaster base container. Before the transference, make sure the plaster base is watered. Use latex gloves.
- Select a transparent lidded container of approximately 1 L and add a 1 cm layer of highly absorbent plaster base. This will be the fungus garden chamber. Select two lidded see-through containers of approximately 500 mL each to be waste disposal and foraging chambers. The ants will choose which chamber will be each, and after that, ensure that they are not interchanged.
- Prepare a cloistered horizontal nest setup as described below (Figure 3 and Figure 4).
NOTE: Nests with horizontal configuration allow close observation of the fungus garden and workers' activities toward it. As younger portions of the fungus garden are at the top, it is possible to observe recently offered substrates being incorporated by the workers. New portions of the fungus can be spotted by its color, which will be similar to the color of the last resource offered, whereas the older portions usually bear a beige color. Offsprings and the queen can also be easily located, as in horizontal containers they are usually at the top of the fungus garden, even when it has occupied most of the space. This configuration can be used for behavior-focused research, focal sampling and science outreach purposes, as it gives perception of the organization inside the nest.- Acquire a transparent lidded container with approximately 31 cm x 21 cm x 4.5 cm dimensions, and add a 1 cm layer of highly absorbent plaster base. This will be the fungus garden chamber. Select two lidded see-through containers of approximately 500 mL each to be waste disposal and foraging chambers. The ants will choose which chamber will be each, and after that, ensure that they are not interchanged.
NOTE: To prepare the plaster base, follow the manufacturer's instructions. If necessary, close the little space between the lid and the container with masking tape to prevent the ants from escaping. - Perforate and connect the containers with a transparent tube or a hose. If necessary, apply masking tape on the tubes border to guarantee a thigh connection with the containers and avoid ants escaping. Place the plaster base container in the middle and the other containers on opposite sides.
- Carefully transfer the fungus sponge of selected colonies (see step 2.5 and step 4.3) along with the queen, workers, and immatures to the plaster base container. Before the transference, make sure the plaster base is watered. Use latex gloves.
- Acquire a transparent lidded container with approximately 31 cm x 21 cm x 4.5 cm dimensions, and add a 1 cm layer of highly absorbent plaster base. This will be the fungus garden chamber. Select two lidded see-through containers of approximately 500 mL each to be waste disposal and foraging chambers. The ants will choose which chamber will be each, and after that, ensure that they are not interchanged.
- Prepare an open arena nest setup as described below (Figure 3 and Figure 5).
NOTE: Open arena nests allow the collection of ants without great disturbance and foraging behavior analysis. They can also provide a reliable representation of a colony found in nature for science outreach purposes.- Select a transparent lidded container of approximately 1 L and add a 1 cm layer of highly absorbent plaster layer base. This will be the fungus garden chamber.
NOTE: It is recommended to start with 1 L containers and gradually pass on to containers with higher volumes for a bigger fungus garden. However, the containers should not overcome a 5 L volume. Add as many containers as necessary. - Select an open arena. The size of the arena can vary according to study purposes. If a large arena is selected, place the containers holding the fungus garden in its interior (Figure 5). In case of a small arena, connect it to the fungus garden container with a transparent hose or tube (Figure 3). The arena will serve as a foraging and waste disposal chamber, so, ensure that it is not too small.
- Apply one layer of polytetrafluoroethylene liquid in a single movement to the arena border to contain the ants. Use a cotton soaked with the liquid and a nitrile glove.
CAUTION: Avoid inhalation and skin touching while using polytetrafluoroethylene liquid. - Carefully transfer the fungus sponge of selected colonies (see step 2.5 and step 4.3) along with the queen, workers, and immatures to the plaster base container. Before the transference, make sure the plaster base is watered. Use latex gloves.
- Select a transparent lidded container of approximately 1 L and add a 1 cm layer of highly absorbent plaster layer base. This will be the fungus garden chamber.

Figure 4: Artificial cloistered nests of the leaf-cutting ants Atta sexdens and Atta laevigata. Cloistered vertical nest setup top (A) and side view (B); cloistered horizontal nest setup top (C) and side view (D). Please click here to view a larger version of this figure.

Figure 5: Artificial open arena nest of the leaf-cutting ants Atta sexdens and Atta laevigata. Open arena nest setup of Atta sexdens top (A) and side view (B). 1) Fungus garden chambers; 2) Waste; 3) Orange slices; 4) Glass with polytetrafluorethylene (PTFE) layer. Please click here to view a larger version of this figure.
6. Maintenance of developed colonies
- Daily offer at least one large leaf into the foraging chamber per colony with 1 L of fungus garden. If the cut activity of the ants is intense, increase the number of leaves. If the fungus is dry, pre-moisten the leaves to provide extra humidity. In cloistered nests, perform the offering quickly to avoid the ants from escaping the foraging chamber.
NOTE: Here, leaves are collected from plant species such as mulberry (Morus nigra), mango (Mangifera indica), eucalyptus (Eucalyptus sp.), jambolan (Syzygium cumini), hibiscus (Hibiscus sp.), acalypha (Acalypha wilkesiana), and ligustrum (Ligustrum lucidum).- Offer fruits such as orange and apple, and oat and corn flakes to diversify and complement the diet. To big colonies with intense foraging activity, offer oat flakes and corn flakes daily, and fruits once a week. If it is not the case, offer flakes alternately with leaves, but not more than three times a week, and fruits once or twice a month. Adjust the amount and frequency of each food according to the foraging activity of the ants.
- If the options described above are not available, identify the ants' foraging preference among leaves, flowers, and fruits of regional trees and shrubs, or even commercialized vegetables, grains, and other flakes. Avoid offering resources with defensive chemical compounds and pesticides.
- Remove all the content of the waste chamber every 2 weeks from all colonies. Remove workers as well for population control purposes. If the workers transfer healthy fungus to the waste chamber, ensure that the queen is not on it and remove it. If the amount of waste disposed is high or it is too humid, remove it once per week.
- Remove the material not taken by the ants from the foraging chamber whenever offering new ones and make sure it is always clean.
- If the workers transfer healthy fungus to the foraging chamber, disturb it, leave the container lid open and apply neutral talcum powder to the chamber margin surface. Perform this procedure only if there is still some space on the fungus chamber, this way the workers will transfer the fungus back to the container without losing it or any immatures.
- If more fungus garden is wanted, add another plastered container, and move a portion of the fungus sponge into it. Until the fungus reaches half of the container, add leaves in the fungus chamber. The growth of the fungus garden should happen gradually to not compromise the colony balance. If a bigger container is wanted, make sure to let the fungus occupy the whole space of the smallest containers before transferring it. Colony waste and dry leaves should not be let to accumulate into the fungus garden chamber.
- Check the plaster base from the containers, as with time, it may acquire a dark brown color and become ineffective due to ant excretion, waste matter transportation, and high humidity concentration. Also, some colonies may cut the layer and dispose of it. In these cases, transfer the fungus garden to a new plastered container.
Results
A flowchart depicting the process of ant collection is shown in Figure 6. Here, some results obtained employing the protocol of collection, maintenance, and nest setups described above are shown.
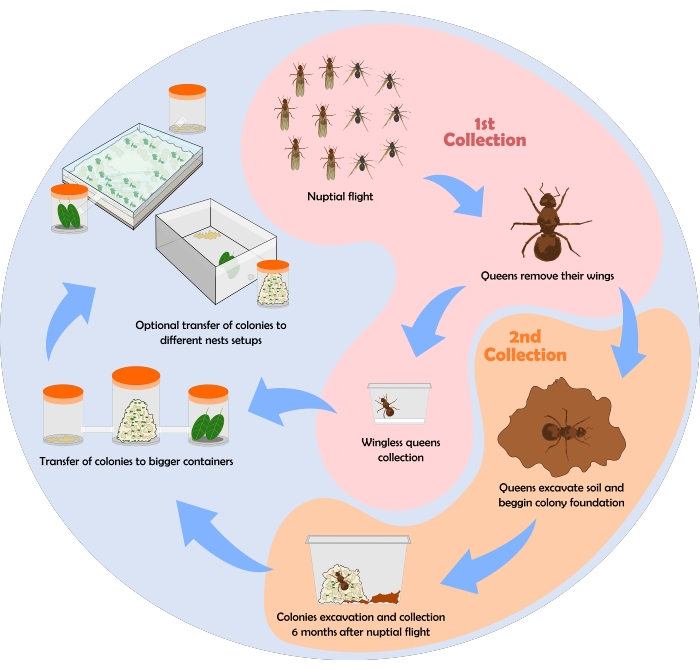
Figure 6: Flowchart for collection of leaf-cutting ants' colonies. Following the protoc...
Discussion
The protocol described here to maintain leaf-cutting ant colonies has been developed and applied for over three decades in an assertive and replicable way. It allowed the development of research that would be limited by field conditions. Thereby, healthy ants and colonies became available for research in several areas such as comparative morphology, toxicology51,52, histology53, and microbiology54,
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
Dedicated to Mario Autuori (in memoriam) and Walter Hugo de Andrade Cunha who greatly contributed to the leaf-cutting ant studies. We acknowledge the support of São Paulo State University and the Institute of Biosciences. This study was in part financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) - Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Fundação para o Desenvolvimento da UNESP (Fundunesp).
Materials
| Name | Company | Catalog Number | Comments |
| Entomologic forceps | N/A | N/A | N/A |
| Glass tank | N/A | N/A | Tempered glass, custom made |
| Hose | N/A | N/A | Transparent, PVC 1/2 Inch x 2,0 mm |
| Latex gloves | Descarpack | 550301 | N/A |
| Nitrile gloves | Descarpack | 433301 | N/A |
| Open arena | N/A | N/A | Polypropylene crate |
| Plaster pouder | N/A | N/A | Plaster pouder used in construction, must be absorbant |
| Plastic Containers for collection | Prafesta | Natural Cód.: 8231/Natural Cód.: 8262 | Lidded, transparent , polypropylene |
| Plastic containers for nests | Prafesta | Discontinued | Polystyrene, hermetic |
| Teflon | Dupont | N/A | Polytetrafluoroethylene liquid (PTFE Dispertion 30) |
References
- Wilson, E. O. . The Insect Societies. , (1971).
- Ortiz, D. P., Elizalde, L., Pirk, G. I. Role of ants as dispersers of native and exotic seeds in an understudied dryland. Ecological Entomology. 46 (3), 626-636 (2021).
- Christianini, A. V., Oliveira, P. S. Birds and ants provide complementary seed dispersal in a neotropical savanna. Journal of Ecology. 98 (3), 573-582 (2010).
- Camargo, P. H. S. A., Martins, M. M., Feitosa, R. M., Christianini, A. V. Bird and ant synergy increases the seed dispersal effectiveness of an ornithochoric shrub. Oecologia. 181 (2), 507-518 (2016).
- Sanders, D., van Veen, F. J. F. Ecosystem engineering and predation: the multi-trophic impact of two ant species. Journal of Animal Ecology. 80 (3), 569-576 (2011).
- Swanson, A. C., et al. Welcome to the Atta world: A framework for understanding the effects of leaf-cutter ants on ecosystem functions. Functional Ecology. 33 (8), 1386-1399 (2019).
- Meyer, S. T., et al. Leaf-cutting ants as ecosystem engineers: topsoil and perturbations around Atta cephalotes nests reduce nutrient availability. Ecological Entomology. 38 (5), 497-504 (2013).
- Sosa, B., Brazeiro, A. Positive ecosystem engineering effects of the ant Atta vollenweideri on the shrub Grabowskia duplicata. Journal of Vegetation Science. 21 (3), 597-605 (2010).
- De Almeida, T., et al. Above- and below-ground effects of an ecosystem engineer ant in Mediterranean dry grasslands. Proceedings of the Royal Society B: Biological Sciences. 287 (1935), 20201840 (2020).
- Folgarait, P. J. Ant biodiversity and its relationship to ecosystem functioning: a review. Biodiversity & Conservation. 7 (9), 1221-1244 (1998).
- Hölldobler, B., Wilson, E. O. . The Ants. , (1990).
- Barrera, C. A., Sosa-Calvo, J., Schultz, T. R., Rabeling, C., Bacci, M. Phylogenomic reconstruction reveals new insights into the evolution and biogeography of Atta leaf-cutting ants (Hymenoptera: Formicidae). Systematic Entomology. 47 (1), 13-35 (2021).
- Hölldobler, B., Wilson, E. O. . The Leafcutter Ants: Civilization By Instinct. , (2011).
- Branstetter, M. G., et al. Dry habitats were crucibles of domestication in the evolution of agriculture in ants. Proceedings of the Royal Society B: Biological Sciences. 284 (1852), 20170095 (2017).
- Solomon, S. E., et al. The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of ‘higher-attine’ ant agriculture. Systematic Entomology. 44 (4), 939-956 (2019).
- Cristiano, M. P., Cardoso, D. C., Sandoval-Gómez, V. E., Simões-Gomes, F. C. Amoimyrmex Cristiano, Cardoso & Sandoval, gen. nov. (Hymenoptera: Formicidae): a new genus of leaf-cutting ants revealed by multilocus molecular phylogenetic and morphological analyses. Austral Entomology. 59 (4), 643-676 (2020).
- Schultz, T. R., Brady, S. G. Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences of the United States of America. 105 (14), 5435-5440 (2008).
- Mueller, U. G., et al. Phylogenetic patterns of ant–fungus associations indicate that farming strategies, not only a superior fungal cultivar, explain the ecological success of leafcutter ants. Molecular Ecology. 27 (10), 2414-2434 (2018).
- Mueller, U. G., et al. Biogeography of mutualistic fungi cultivated by leafcutter ants. Molecular Ecology. 26 (24), 6921-6937 (2017).
- Weber, N. A. The fungus-culturing behavior of ants. American Zoologist. 12 (3), 577-587 (1972).
- Wilson, E. O. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behavioral Ecology and Sociobiology. 7 (2), 157-165 (1980).
- Della Lucia, T. M. C. Formigas cortadeiras: da bioecologia ao manejo. Viçosa Editora UFV. 421, (2011).
- Autuori, M. Contribuição para o conhecimento da saúva (Atta spp). I. Evolução do sauveiro (Atta sex dens rubolpilosa Forel, 1908). Arquivos do Instituto Biologico Saul Paulo. 12, 197-228 (1941).
- Bueno, O. C., Hebling, M. J. A., Schneider, M. O., Pagnocca, F. C. Ocorrência de formas aladas de Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae) em colônias de laboratório. Neotropical Entomology. 31 (3), 469-473 (2002).
- Mariconi, F. A. M. Biologo. As Saúvas. , (2021).
- Bento, J. M. S. . Condições climáticas para o vôo nupcial e reconhecimento dos indivíduos em Atta sexdens rubropilosa (Hymenoptera: Formicidae). , (1993).
- Little, A. E. F., Murakami, T., Mueller, U. G., Currie, C. R. The infrabuccal pellet piles of fungus-growing ants. Naturwissenschaften. 90 (12), 558-562 (2003).
- Kerr, W. E. Acasalamento de rainhas com vários machos em duas espécies da tribo Attini. Revista Brasileira de Biologia. 21, 45-48 (1961).
- Kerr, W. E. Tendências evolutivas na reprodução dos himenópteros sociais. Arquivos do Museu Nacional. 52, (1962).
- Cremer, S., Armitage, S. A. O., Schmid-Hempel, P. Social immunity. Current Biology. 17 (16), 693-702 (2007).
- Hernández, J. V., Jaffé, K. Dano econômico causado por populações de formigas Atta laevigata (F. Smith) em plantações de Pinus caribaea (Mor.) e elementos para o manejo da praga. Anais da Sociedade Entomológica do Brasil. 24 (2), 287-298 (1995).
- Kempf, W. W. Catálogo abreviado das formigas da Região Neotropical Studia Entomologica. antbase.org. 15, 3 (1972).
- Della Lucia, T. M. C., Gandra, L. C., Guedes, R. N. C. Managing leaf-cutting ants: peculiarities, trends and challenges. Pest Management Science. 70 (1), 14-23 (2013).
- Boaretto, M. A. C., Forti, L. C. Perspectivas no controle de formigas-cortadeiras. Série Técnica IPEF. 11 (30), 31-46 (1997).
- Folgarait, P. J., Goffré, D. Conidiobolus lunulus, a newly discovered entomophthoralean species, pathogenic and specific to leaf-cutter ants. Journal of Invertebrate Pathology. 186, 107685 (2021).
- Cardoso, S. R. S., Rodrigues, A., Forti, L. C., Nagamoto, N. S. Pathogenicity of filamentous fungi towards Atta sexdens rubropilosa (Hymenoptera: Formicidae). International Journal of Tropical Insect Science. 42 (2), 1215-1223 (2022).
- Ichinose, K., Rinaldi, I., Forti, L. C. Winged leaf-cutting ants on nuptial flights used as transport by Attacobius spiders for dispersal. Ecological Entomology. 29 (5), 628-631 (2004).
- Pagnocca, F. C., Rodrigues, A., Nagamoto, N. S., Bacci, M. Yeasts and filamentous fungi carried by the gynes of leaf-cutting ants. Antonie Van Leeuwenhoek. 94 (4), 517-526 (2008).
- Attili-Angelis, D., et al. Novel Phialophora species from leaf-cutting ants (tribe Attini). Fungal Diversity. 65 (1), 65-75 (2014).
- Delabie, J. H. C., do Nascimento, I. C., Mariano, C. S. F. Estratégias de reprodução e dispersão em formigas attines, com exemplos do sul da Bahia. XIX Congresso Brasileiro de Entomologia. , 16-21 (2002).
- Fjerdingstad, E. J., Boomsma, J. J. Variation in size and sperm content of sexuals in the leafcutter ant Atta colombica. Insectes Sociaux. 44 (3), 209-218 (1997).
- Currie, C. R., Mueller, U. G., Malloch, D. The agricultural pathology of ant fungus gardens. Proceedings of the National Academy of Sciences of the United States of America. 96 (14), 7998-8002 (1999).
- Moser, J. C., et al. Eye size and behaviour of day-and night-flying leafcutting ant alates. Journal of Zoology. 264 (1), 69-75 (2004).
- Moreira, S. M., Rodrigues, A., Forti, L. C., Nagamoto, N. S. Absence of the parasite Escovopsis in fungus garden pellets carried by gynes of Atta sexdens. Sociobiology. 62 (1), 34-38 (2015).
- Arcuri, S. L., et al. Yeasts found on an ephemeral reproductive caste of the leaf-cutting ant Atta sexdens rubropilosa. Antonie Van Leeuwenhoek. 106 (3), 475-487 (2014).
- Staab, M., Kleineidam, C. J. Initiation of swarming behavior and synchronization of mating flights in the leaf-cutting ant Atta vollenweideri FOREL, 1893 (Hymenoptera: Formicidae). Myrmecol. News. 19, 93-102 (2014).
- Gálvez, D., Chapuisat, M. Immune priming and pathogen resistance in ant queens. Ecology and Evolution. 4 (10), 1761-1767 (2014).
- Baer, B., Armitage, S. A. O., Boomsma, J. J. Sperm storage induces an immunity cost in ants. Nature. 441 (7095), 872-875 (2006).
- Carlos, A. A. . Semioquímicos e comunicação sonora em formigas cortadeiras (Hymenoptera: Formicidae). , (2013).
- Veja um FORMIGUEIRO por DENTRO. Boravê Available from: https://youtu.be/sN99x_Rjf90 (2021)
- Ortiz, G., Vieira, A. S., Bueno, O. C. Toxicological and morphological comparative studies of insecticides action in leaf-cutting ants. International Journal of Agriculture Innovations and Research. 6 (3), 516-522 (2017).
- Decio, P., Silva-Zacarin, E. C. M., Bueno, F. C., Bueno, O. C. Toxicological and histopathological effects of hydramethylnon on Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers. Micron. 45, 22-31 (2013).
- Vieira, A. S., Morgan, E. D., Drijfhout, F. P., Camargo-Mathias, M. I. Chemical composition of metapleural gland secretions of fungus-growing and non-fungus-growing ants. Journal of Chemical Ecology. 38 (10), 1289-1297 (2012).
- Vieira, A. S., Ramalho, M. O., Martins, C., Martins, V. G., Bueno, O. C. Microbial communities in different tissues of Atta sexdens rubropilosa leaf-cutting ants. Current Microbiology. 74 (10), 1216-1225 (2017).
- Ramalho, M. d. e. O., Martins, C., Morini, M. S. C., Bueno, O. C. What can the bacterial community of Atta sexdens (Linnaeus, 1758) tell us about the habitats in which this ant species evolves. Insects. 11 (6), 332 (2020).
- Machado, L. M., et al. Attractivity or repellence: relation between the endophytic fungi of Acalypha, Colocasia and the leaf-cutting ants—Atta sexdens. Advances in Entomology. 9 (2), 85-99 (2021).
- Moreira, A., Forti, L. C., Andrade, A. P., Boaretto, M. A., Lopes, J. Nest architecture of Atta laevigata (F. Smith, 1858) (Hymenoptera: Formicidae). Studies on Neotropical Fauna and Environment. 39 (2), 109-116 (2004).
- Della Lucia, T. M. C., Moreira, D. D. O., Oliveira, M. A., Araújo, M. S. Perda de peso de rainhas de Atta durante a fundação e o estabelecimento das colônias. Revista Brasileira de Biologia. 55 (4), 533-536 (1995).
- Fujihara, R. T., Camargo, R. d. a. S., Forti, L. C. Lipid and energy contents in the bodies of queens of Atta sexdens rubropilosa Forel (Hymenoptera, Formicidae): pre-and post-nuptial flight. Revista Brasileira de Entomologia. 56 (1), 73-75 (2012).
- da Silva Camargo, R., Forti, L. C. Queen lipid content and nest growth in the leaf cutting ant (Atta sexdens rubropilosa) (Hymenoptera: Formicidae). Journal of Natural History. 47, 65-73 (2013).
- Camargo, R. S., Forti, L. C., Fujihara, R. T., Roces, F. Digging effort in leaf-cutting ant queens (Atta sexdens rubropilosa) and its effects on survival and colony growth during the claustral phase. Insectes Sociaux. 58 (1), 17-22 (2011).
- Mota Filho, T. M. M., Garcia, R. D. M., Camargo, R. S., Stefanelli, L. E. P., Forti, L. C. Observations about founding queens (Atta sexdens) and their unusual behavior. International Journal of Agriculture Innovations and Research. 9, 352-357 (2021).
- Barcoto, M. O., Pedrosa, F., Bueno, O. C., Rodrigues, A. Pathogenic nature of Syncephalastrum in Atta sexdens rubropilosa fungus gardens. Pest Management Science. 73 (5), 999-1009 (2017).
- Silva, A., Bacci, M., Pagnocca, F. C., Bueno, O. C., Hebling, M. J. A. Production of polysaccharidases in different carbon sources by Leucoagaricus gongylophorus Möller (Singer), the symbiotic fungus of the leaf-cutting ant Atta sexdens Linnaeus. Curr. Microbiology. 53 (1), 68-71 (2006).
- Majoe, M., Libbrecht, R., Foitzik, S., Nehring, V. Queen loss increases worker survival in leaf-cutting ants under paraquat-induced oxidative stress. Philosophical Transactions of the Royal Society B. 376 (1823), 20190735 (2021).
- Della Lucia, T. M. C., Peternelli, E. F. O., Lacerda, F. G., Peternelli, L. A., Moreira, D. D. O. Colony behavior of Atta sexdens rubropilosa (Hymenoptera: Formicidae) in the absence of the queen under laboratory conditions. Behavioural Processes. 64 (1), 49-55 (2003).
- Sales, T. A., Toledo, A. M. O., Zimerer, A., Lopes, J. F. S. Foraging for the fungus: why do Acromyrmex subterraneus (Formicidae) queens need to forage during the nest foundation phase. Ecological Entomology. 46 (6), 1364-1372 (2021).
- Forti, L. C., et al. Do workers from subspecies Acromyrmex subterraneus prepare leaves and toxic baits in similar ways for their fungus garden. Neotropical Entomology. 49 (1), 12-23 (2020).
- Dorigo, A. S., et al. Projeto Primeiros Passos na Ciência: rompendo barreiras sociais e estreitando laços entre a comunidade acadêmica e o ensino médio público. Revista Brasileira de Extensão Universitária. 11 (1), 47-59 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved