A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Efficacy of Fu's Subcutaneous Needling on Sciatic Nerve Pain: Behavioral and Electrophysiological Changes in a Chronic Constriction Injury Rat Model
* These authors contributed equally
In This Article
Summary
We present a protocol for using Fu's subcutaneous needling in a chronic constriction injury model to induce sciatic nerve pain in rats.
Abstract
Fu's subcutaneous needling (FSN), an invented acupuncture technique from traditional Chinese medicine, is used worldwide for pain relief. However, the mechanisms of action are still not fully understood. During FSN treatment, the FSN needle is inserted and retained in the subcutaneous tissues for a long duration with a swaying movement. However, challenges arise from maintaining a posture while manipulating FSN in animal models (e.g., rats) for researchers. Uncomfortable treatment can lead to fear and resistance to FSN needles, increasing the risk of injury and may even affect research data. Anesthesia may also affect the study results too. Hence, there is a need for strategies in FSN therapy on animals that minimize injury during the intervention. This study employs a chronic constriction injury model in Sprague-Dawley rats to induce neuropathic pain. This model replicates the pain induced by nerve injury observed in humans through surgical constriction of a peripheral nerve, mimicking the compression or entrapment seen in conditions such as nerve compression syndromes and peripheral neuropathies. We introduce an appropriate manipulation for easily inserting an FSN needle into the subcutaneous layer of the animal's body, including needle insertion and direction, needle retention, and swaying movement. Minimizing the rat's discomfort prevents the rat from being tense, which causes the muscle to contract and hinder the entry of the needle and improves the study efficiency.
Introduction
Neuropathic pain, defined as pain caused by nerve damage, is estimated to affect 6.9%-10% of the world's population, and the reported lifetime prevalence is 49%-70%1,2. It is also considered to be one of the most difficult pain syndromes to manage. The use of pharmacological agents to manage neuropathic pain has yielded limited success as commonly prescribed pain medications like non-steroidal anti-inflammatory drugs and opioids have shown little efficacy in relieving this type of pain3,4. There is therefore a great need to explore new treatment options, especially non-pharmacological treatments. Acupuncture, as a non-pharmacological intervention, potentially alleviate neuropathic pain by exerting analgesic effects on the somatosensory system. Both clinical and preclinical studies have indicated that acupuncture is effective in relieving neuropathic pain symptoms without significant side effects5,6,7. However, the central mechanism of acupuncture treatment for pain relief in neuropathic pain remains to be further investigated.
In recent years, Fu's subcutaneous needling (FSN) has gained popularity for treating pain-related neurological disorders8. FSN originated from traditional Chinese acupuncture and was first described by traditional Chinese physician Zhonghua Fu in 19969,10. While originating from traditional acupuncture, FSN differs significantly in its techniques and theories from meridian-based acupuncture, yin and yang principles, and acupuncture point concepts. FSN places greater emphasis on neurophysiological and anatomical approaches to effectively address myofascial pain11. FSN therapy is applied in clinical practice to address various painful muscular disorders, targeting the connective tissues closely associated with the muscles, particularly focusing on the treatment of tightened muscles (TMs)12. As a complementary therapy for pain relief, there is also clinical evidence that FSN is effective in treating soft tissue injuries in addition to providing rapid pain management and significant improvement in soft tissue spasms13,14. FSN therapy involves specific techniques tailored to address the underlying myofascial trigger points (MTrPs) associated with the condition. The FSN needle insertion position is carefully chosen based on the location of these trigger points, allowing precise targeting of affected areas. During the procedure, the FSN needle is inserted into the subcutaneous layer, where it is intentionally stopped to optimize therapeutic effects. A distinctive technique known as the swaying movement is then employed, involving a gentle oscillating motion of the needle to stimulate the tissues and promote the therapeutic responses10. The development of MTrPs is associated with the energy crisis theory, which explains that factors such as chronic muscle overload, excessive exercise, improper exertional postures, muscle atrophy, and degeneration can contribute to the onset of muscle tissue ischemia and hypoxia. This oxygen and energy deficiency within the muscle tissue is believed to play a key role in the formation of MTrPs15,16. Previous animal studies have found that FSN treatment for chronic pain in rats improves the morphological structure and function of mitochondria in TMs to some extent, validating the potential of FSN therapy to promote the recovery of damaged nerves and muscles17.
Sciatica has been classified as neuropathic pain18. The origin of neuropathic pain is thought to lie anywhere between the motor endplate and the outer fibrous layer of the muscle, involving the microvascular system and neurotransmitters at the cellular level. Loss of muscle innervation and apoptosis of innervated nerve cells occurs when nerve damage occurs19, leading to pain-related gait in the affected limb. Additionally, chronic compression or irritation of the nerve can lead to a variety of changes in the way of nerve functions, which can further exacerbate the symptoms of sciatica20. However, the complexity of the nervous system makes it difficult to replicate it in vitro, thus necessitating the use of animal models for such studies. In the investigation of neuropathic pain disorders, model organisms are commonly employed, involving various methods of direct peripheral nerve injury, such as sciatic nerve ligature, transection, or compression21,22. The chronic constriction injury (CCI) model in Sprague-Dawley rats has been used to induce neuropathic pain. This model replicates the pain induced by nerve injury observed in humans through surgical constriction of a peripheral nerve, mimicking the compression or entrapment seen in conditions such as nerve compression syndromes and peripheral neuropathies.
In this study, we evaluated the analgesic effects of FSN therapy and low-frequency electrotherapy (transcutaneous electrical nerve stimulator, TENS) in rats with chronic constriction injury and neuropathic pain. As anesthesia slows or blocks nerve impulses and affects synaptic transmission and neuronal function23, animals cannot be anesthetized under all needling procedures and swaying movements. Therefore, an appropriate needle technique is required to reduce discomfort in rats. The steps for establishing a rat CCI model, the way the rats were treated with FSN combined swaying movement without anesthesia, feasible animal behavioral pattern tests, and electrophysiological investigations are described in detail.
Protocol
All procedures involving animal subjects were approved by the Institutional Animal Care and Use Committee (IACUC) of the Chang Bing Show Chwan Memorial Hospital, Changhua, Taiwan (111031) on October 2022 (Figure 1).
1. Preparation of animals
- Purchase 48 maleSprague-Dawley (SD) rats (age: 8-10 weeks, weight: 250-300 g).
- House rats individually in ventilated cages at 24 ± 2 °C and a 12-h dark and light cycle.
- Feed rats a standard pellet diet with sterile drinking water ready to use, and provide soft bedding.
2. Grouping of animals
- Randomly divide 48 SD rats into six groups (n = 8 per group): the sham group, CCI group, CCI+FSN group, CCI+TENS group, FSN alone group, and TENS alone group as in the previous study conducted by Chan et al.24.
NOTE: The details of six groups: (1) Sham group: no surgery and no treatment; (2) CCI group: prepared for surgery with no treatment; (3) CCI+FSN treatment group: FSN treatment after successful CCI modeling; (4) CCI+TENS treatment group: TENS treatment after successful CCI modeling; (5) FSN treatment alone group: only FSN treatment without surgery; (6) TENS treatment alone group: only TENS treatment without surgery.
3. Establishment of a CCI rat model
NOTE: The CCI surgery model in rats was modified according to Bennett and Xie conducted in 198825.
- Ensure the operator wears a surgical mask, disposable operating cap, and sterile gloves.
- Disinfect the surface of the surgical table with 70% ethanol. Sterilize instruments (e.g., scissors, forceps, and retractors), gauze, staples, and cotton swabs by autoclaving.
NOTE: Aseptic techniques are used throughout the surgical procedure. - Anesthetize the rats with 4% isoflurane after standard skin preparation (shaving) and maintain with 2% isoflurane (Figure 2A).
- Confirm the appropriate depth of anesthesia by observing the lack of response after pinching the hind paw and monitoring the anesthetized rats throughout the procedure.
- Apply enough veterinary ophthalmic ointment over the eyes for protection against drying.
- Place the rat in the prone position on the operating table and shave the hair on the side of the right hind leg, then disinfect the skin with povidone-iodine solution and 75% ethanol three times. Provide thermal support throughout the procedure and use sterile drapes to cover the surgical site.
- Make a parallel incision in the skin 3-4 mm below the femur of about 20-50 mm.
- Prioritize the identification of the positions of gluteus maximus and biceps femoris. Separate the subcutaneous fat and superficial fascia layer by layer using surgical scissors, cutting through the surrounding connective tissue to expose the muscle (Figure 2B).
NOTE: To distinguish the subcutaneous fat and superficial fascia layers, observe the texture and color. The subcutaneous fat layer should appear soft and pliable with a yellowish or whitish appearance. The superficial fascia is a thin fibrous layer situated directly beneath the subcutaneous fat. Differentiate between the layers by gently palpating or probing with a blunt instrument, noting that the subcutaneous fat offers more resistance to pressure compared to the superficial fascia.
- Using blunt scissors, cut the connective tissue between the superficial gluteus and biceps femoris muscles.
- Widen the gap between these two muscles using a retractor to expose the sciatic nerve (Figure 2C).
NOTE: To visually identify the sciatic nerve in a rat, focus on the thigh region. Locate the midpoint of the thigh region to visualize the sciatic nerve. Typically, the nerve runs along the posterior aspect of the thigh, starting from the hip region and extending toward the knee. - Without altering the nerve morphology, pick the sciatic nerve with a microneedle through a good light source. Ligate the sciatic nerve twice using 3-0 chromic gut ligatures, positioning the ligature points approximately 1 mm apart between the two sutures.
- Starting with a loose loop for each ligature, grasp the ends of the ligature close to the loop and tighten until the loop is just snug, ensuring that the ligature does not slip along the nerve. Stop when slight twitching of the limb is observed during ligation (Figure 2D).
- Widen the gap between these two muscles using a retractor to expose the sciatic nerve (Figure 2C).
- Close the muscle and skin layer by layer with 4-0 suture lines. Finally, disinfect the wound with iodine (Figure 2E).
- Closely monitor the rats' vital signs during anesthesia and place them in individual recovery cages until they are awake before placing them back in their cages. Line the cages with flat paper bedding to prevent asphyxiation in unconscious animals. A brief twitch in the post-operative limb indicates a successful operation (Figure 2F).
- Perform pain hypersensitivity testing several times before CCI (baseline) and at different time points after CCI.
- Observe for spontaneous pain and behavioral changes on days 1, 3, 5, and 7 following model construction.
NOTE: Observe the gait and posture of the right hind limb and the presence of licking and biting on the limb.- Identify the presence of neuropathic pain to determine the successful establishment of the model and exclude unsuccessful rats.
NOTE: Assess the success of the model by observing signs such as weakness in lower limb walking, toes of the right limb held together with mild valgus, frequent dangling, and reluctance to land. Observe the rat standing with the left hind limb supporting the weight, while the right hind limb is raised and close to the abdomen.
- Identify the presence of neuropathic pain to determine the successful establishment of the model and exclude unsuccessful rats.
4. Administration of FSN manipulation
- Fix the rat of the FSN treatment group (including CCI+FSN and FSN alone group) in the rodent restraint with the affected limb exposed laterally. Provide thermal support throughout the procedure. Both groups were treated with FSN disposable needles (Figure 3A).
- Without anesthesia, extend the rat's hind limbs gradually and slowly until they were stretched tight (Figure 3B).
NOTE: The rat head is covered with a surgical drape to keep the animal calm and stable. Do not overextend the leg to cause injury to the rat. Observe the rat's response closely for any signs of distress or discomfort. If the rat shows signs of pain or discomfort, stop the extension and provide a break before attempting again. - Remove the protecting sheath of the FSN needle.
- Insert the tip of the FSN needle toward the TMs (muscles with MTrPs), approximately close to the gluteus maximus muscle, located on the lower back and rear.
- Place the FSN needle flat and enter the skin at an angle of approximately 15°.
- Push it carefully and quickly through the skin and into the subcutaneous space to prevent stress in the rat until fully inserted. Ensure that the needle is inserted sufficiently to completely bury the soft tube under the skin.
- When pushing forward, raise the needle tip slightly to observe if the skin bulge moves along the needle tip (Figure 3C).
- Perform the swaying movement by smoothly and softly fanning the FSN needle tip with the thumb as the fulcrum while keeping the index finger, middle finger, and ring finger aligned in a straight line.
- Hold the FSN needle between the middle finger and thumb in a face-to-face position, and alternate the movement back and forth using the index and ring fingers.
- Set the frequency to 100 strokes per minute and perform the operation for approximately 1 min (Figure 3D).
- After completing the manipulation, quickly withdraw the FSN needle.
NOTE: The operation was performed every 2 days for a total of four sessions (days 1, 3, 5, and 7 after the CCI model was created). Disposable FSN needles must be used once. Repeated use will blunt the needle and cause increased pain in rats.
5. Administration of TENS manipulation
- Fix the rat of the TENS treatment group (including CCI+TENS and TENS alone group) in the rodent restraint with the affected limb exposed laterally. Provide thermal support throughout the procedure. Ensure that the fur is shaved before being treated.
NOTE: Electrodes were cut to 45 mm (length) by 5 mm (width) (Figure 4A). - Choose Zusanli point (ST36) and Sanyinjiao point (SP6) as the locations for TENS. This is based on the theory for treating neuropathic pain26,27.
- Locate the Zusanli point (ST36) approximately 5 mm lateral to the anterior tubercle of the tibia between the tibia and fibula just below the knee28.
- Locate the Sanyinjiao point (SP6) at the posterior border of the tibia, 3 mm proximal to the medial malleolus28.
NOTE: Both of these two acupuncture points are located by manual inspection as described by Stux and Pomeranz and in the animal acupuncture atlas28,29 (Figure 4B).
- Deliver a low-frequency electrical stimulation (2 Hz continuous sine wave, 3 mA) for 10 min using the TENS device with the electrode applied on the leg around the nerve. Cover the head of the rat head with a surgical drape to keep it calm and stable.
NOTE: This procedure is performed every 2 days for a total of four sessions (days 1, 3, 5, and 7 after the CCI model was created).
6. Physiological measurements performing the animal behavioral test
NOTE: Sciatic function index (SFI)30 is a widely used index by researchers studying pathology and potential treatment of nerve injuries, determined by comparing the geometry of the affected hind paw in injured rats with that of the contralateral paw and comparing it with the opposite paw.
- Design rat walkways with clear plexiglass and tilt mirrors to capture the footprints and body orientation of rats during the walk.
NOTE: The walkway is a platform 10 cm long, 50 cm wide, and 15 cm high with a white paper lining at the bottom (Figure 5A). - Gently and freely place the rats in the box and allow them to acclimatize to their new environment for at least 5 min before recording.
NOTE: Special care is taken to minimize unnecessary stress on the animal to avoid its possible effect on postural muscle tension. - Dip the rat's paws in red ink and allow the rat to walk along the walkway strip, leaving traces on the backing paper. Record at least 2 s of continuous walking for each test. Make the rat walk at least 3 times in one direction (Figure 5B).
NOTE: Apply quick-drying, nontoxic, water-soluble red ink to both hind feet to make the hind footprints clearly visible. - At the end of the experiment, dry the walkway strips to measure the parameters. Measure their footprints with a ruler and round to the nearest 0.5 mm.
NOTE: Three clear footprints from each rat were selected from several footprints, and three different parameters were measured. Factors for SFI include print length (PL), toe spread (TS), and intermediate toe spread (ITS).
SFI values are calculated using the following formula31:
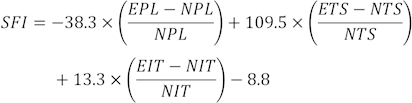
(EPL, experimental print length; NPL, normal print length; ETS, experimental toe spread; NTS, normal toe spread; EIT, experimental intermediate toe spread; NIT, intermediate toe spread.)
SFI = 0 and - 100 indicates normal and complete dysfunction. Rats that dragged their toes were arbitrarily assigned a value of -100. For normal neurological function, the SFI oscillates around 0, while around -100 SFI represents complete dysfunction32.
7. Neurophysiological assessment by electrophysiological measurement33
NOTE: Electromyography was used to record the electrophysiological activity in this study. The compound muscle action potential (CMAP) is caused by the activation of muscle fibers in the target muscle supplied by the nerve. CMAP amplitude and latency are investigated. The CMAP amplitude is measured from baseline to negative peak.The latency of CMAPs is determined by measuring the time between the application of the stimulus and the onset of the compound action potential, which is influenced by the distance between the stimulation site and the recording site. Electrophysiology provides an objective assessment of peripheral nerve function in rats.
- Administer Zoletil 50 (40 mg/kg, ip) to anesthetize the rats. Prepare the skin according to standard protocols (shaving).
- Place disposable adhesive surface electrodes (20 mm outer diameter) on the designated areas. Fix the recording electrodes to the lateral and dorsal surfaces of the gastrocnemius muscle (Figure 6A).
- Apply electrical stimulation (intensity 1.2 mA) to the right proximal sciatic nerve stem. Record a compound muscle action potential (CMAP) on the belly of the gastrocnemius muscle (Figure 6B).
NOTE: Be careful when inserting the electrodes to avoid the muscle tissue. - Record the effect of three repeated measurements for each rat.
NOTE: CMAP is expressed as the mean ± SD of each group. The signal was amplified by an amplifier, filtered (0.3-3 kHz). After integration (time constant = 0.05 s), both the original signal and the integrated signal are input. The original signal and the integrated signal are then digitized in the PowerLab system and stored on the computer hard disk. - After completing the electrophysiology procedures, move the rat to a different cage and monitor it until it regains enough consciousness to maintain a sternal recumbent position. Once the rat has fully recovered from the anesthetic, transfer it back to its original cage.
8. Statistics:
- Evaluate differences in SFI and CMAPs between groups using repeated measures analysis of variance (ANOVA).
- Quantify the data by assistants who are blind to the experimental conditions. Express the data as mean ± standard deviation.
- Compare the data, when appropriate, using Student's two-tailed paired and unpaired t-test. Establish statistical significance as p < 0.05.
Results
Footprints and determination of the SFI
We examined the development of SFI in the CCI alone, CCI+FSN, and CCI+TENS groups (Figure 7). After 4 sessions of FSN and TENS treatments on day 7 for CCI surgery, the SFI in the CCI+FSN (-15.85 ± 3.46) and CCI+TENS (-29.58 ± 9.19) groups improved significantly compared to the CCI alone group (-87.40 ± 14.22). The improvement was significant in the CCI+FSN group compared to the CCI+TENS group (
Discussion
This study observes the effect of FSN treatment on neuropathic pain in rat CCI models. This study presents a protocol for SFI and electrophysiological testing to evaluate the therapeutic effects after FSN or TENS treatment. Additionally, it illustrates how to evaluate the functional recovery of the injured nerve using noninvasive behavioral tests and physiological measurements. Results showed that the FSN treatment after CCI-induced sciatic nerve pain showed significantly better improvement in all prognostic indicators t...
Disclosures
The authors declare that no competing conflicts of interest exist.
Acknowledgements
This study was supported by a grant from the animal center of Chang Bing Show Chwan Memorial Hospital, Changhua, Taiwan. The authors would like to thank Show Chwan Memorial Hospital IRCAD TAIWAN for their invaluable support and assistance throughout this research project.
Materials
| Name | Company | Catalog Number | Comments |
| Forceps | World Precision Instruments | 14098 | |
| Fu’s subcutaneous needling | Nanjing Paifu Medical Science and Technology Co. | FSN needles are designed for single use. The FSN needle is made up of three parts: a solid steel needle core (bottom), a soft casing pipe (middle), and a protecting sheath (top). | |
| Medelec Synergy electromyography | Oxford Instrument Medical Ltd. | 034W003 | Electromyogram (EMG) are used to help in the diagnosis and management of disorders such as neuropathies. Contains a portable two-channel electromyography/nerve conduction velocity system. |
| Normal saline (0.9%) 20 mL | Taiwan Biotech Co.,Ltd. | 4711916010323 | Lot: 1TKB2022 |
| POLYSORB 4-0 VIOLET 30" CV-25 | UNITED STATES SURGICAL, A DIVISION OF TYCO HEALTHC | GL-181 | |
| Retractor | COOPERSURGICAL, INC.(USA) | 3311-8G | |
| Rompun | Elanco Animal Health Korea Co. Ltd. | 27668 | |
| SCISSORS CVD 90MM | BBRUAN | XG-LBB-BC101R | |
| Transcutaneous Electrical Nerve Stimulation | Well-Life Healthcare Co. | Model Number 2205A | Digital unit which offers TENS. Supplied complete with patient leads, self-adhesive electrodes, 3 AAA batteries and instructions in a soft carry bag. Interval ON time 1–30 s. Interval OFF time 1–30 s. |
| Zoletil | VIRRBAC | 8V8HA |
References
- van Hecke, O., Austin, S. K., Khan, R. A., Smith, B. H., Torrance, N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 155 (4), 654-662 (2014).
- Younes, M., et al. Prevalence and risk factors of disk-related sciatica in an urban population in Tunisia. Joint Bone Spine. 73 (5), 538-542 (2006).
- Woolf, C. J., Mannion, R. J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 353 (9168), 1959-1964 (1999).
- Baron, R., et al. Neuropathic low back pain in clinical practice. European Journal of Pain. 20 (6), 861-873 (2016).
- Ma, X., et al. Potential mechanisms of acupuncture for neuropathic pain based on somatosensory system. Frontiers in Neuroscience. 16, 940343 (2022).
- Jang, J. H., et al. Acupuncture alleviates chronic pain and comorbid conditions in a mouse model of neuropathic pain: the involvement of DNA methylation in the prefrontal cortex. Pain. 162 (2), 514-530 (2021).
- He, K., et al. Effects of acupuncture on neuropathic pain induced by spinal cord injury: A systematic review and meta-analysis. Evidence Based Complement and Alternative Medicine. 2022, 6297484 (2022).
- Fu, Z., Lu, D. Fu's Subcutaneous Needling: A Novel Therapeutic Proposal. Acupuncture - Resolving Old Controversies and Pointing New Pathways. IntechOpen. , (2019).
- Fu, Z. H. . The Foundation of Fu's Subcutaneous Needling. , (2016).
- Fu, Z. H., Chou, L. W. . Fu's Subcutaneous Needling, Trigger Point Dry Needling: An Evidence and Clinical-Based Approach. 2nd Edition. , 255-274 (2018).
- Fu, Z., Shepher, R. Fu's Subcutaneous Needling, a Modern Style of Ancient Acupuncture? Acupuncture in Modern Medicine. IntechOpen. , (2013).
- Chiu, P. E., et al. Efficacy of Fu's subcutaneous needling in treating soft tissue pain of knee osteoarthritis: A randomized clinical trial. Journal of Clinical Medicine. 11 (23), 7184 (2022).
- Huang, C. H., Lin, C. Y., Sun, M. F., Fu, Z., Chou, L. W. Efficacy of Fu's Subcutaneous Needling on Myofascial Trigger Points for Lateral Epicondylalgia: A randomized control trial. Evidence Based Complement and Alternative Medicine. 2022, 5951327 (2022).
- Huang, C. H. Rapid improvement in neck disability, mobility, and sleep quality with chronic neck pain treated by Fu's subcutaneous needling: A randomized control study. Pain Research and Management. 2022, 7592873 (2022).
- Chou, L. W., Hsieh, Y. L., Kuan, T. S., Hong, C. Z. Needling therapy for myofascial pain: recommended technique with multiple rapid needle insertion. Biomedicine (Taipei). 4 (2), 13 (2014).
- Ye, L., et al. Depression of mitochondrial function in the rat skeletal muscle model of myofascial pain syndrome is through down-regulation of the AMPK-PGC-1α-SIRT3 axis. Journal of Pain Research. 13, 1747-1756 (2020).
- Li, Y., et al. Effects of Fu's subcutaneous needling on mitochondrial structure and function in rats with sciatica. Molecular Pain. 18, 17448069221108717 (2022).
- Perreault, T., Fernández-de-Las-Peñas, C., Cummings, M., Gendron, B. C. Needling interventions for sciatica: Choosing methods based on neuropathic pain mechanisms-A scoping review. Journal of Clinical Medicine. 10 (10), 2189 (2021).
- Weller, J. L., Comeau, D., Otis, J. A. D. Myofascial pain. Seminars in Neurology. 38 (6), 640-643 (2018).
- Grøvle, L., et al. The bothersomeness of sciatica: patients' self-report of paresthesia, weakness and leg pain. European Spine Journal. 19 (2), 263-269 (2010).
- Jaggi, A. S., Jain, V., Singh, N. Animal models of neuropathic pain. Fundament Clinical Pharmacology. 25 (1), 1-28 (2011).
- Burma, N. E., Leduc-Pessah, H., Fan, C. Y., Trang, T. Animal models of chronic pain: Advances and challenges for clinical translation. Journal of Neuroscience Research. 95 (6), 1242-1256 (2017).
- McCann, M. E., Soriano, S. G. Does general anesthesia affect neurodevelopment in infants and children. British Medical Journal. 367, 6459 (2019).
- Chan, K. Y., et al. Ameliorative potential of hot compress on sciatic nerve pain in chronic constriction injury-induced rat model. Frontiers in Synaptic Neuroscience. 14, 859278 (2022).
- Bennett, G. J., Xie, Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33 (1), 87-107 (1988).
- Somers, D. L., Clemente, F. R. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: The effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Physical Therapy. 86 (5), 698-709 (2006).
- Xing, G., Liu, F., Wan, Y., Yao, L., Han, J. Electroacupuncture of 2 Hz induces long-term depression of synaptic transmission in the spinal dorsal horn in rats with neuropathic pain. Beijing Da Xue Xue Bao Yi Xue Ban. 35 (5), 453-457 (2003).
- Schone, A. M. . Veterinary Acupuncture: Ancient Art to Modern Medicine. , (1999).
- Stux, G., Pomeranz, B. . Acupuncture: Textbook and Atlas. , (1987).
- de Medinaceli, L., Freed, W. J., Wyatt, R. J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experimental Neurology. 77 (3), 634-643 (1982).
- Bain, J. R., Mackinnon, S. E., Hunter, D. A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plastic and Reconstructive Surgery. 83 (1), 129-138 (1989).
- Kanaya, F., Firrell, J. C., Breidenbach, W. C. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plastic and Reconstructive Surgery. 98 (7), 1264-1271 (1996).
- Wild, B. M., et al. In vivo electrophysiological measurement of the rat ulnar nerve with axonal excitability testing. Journal of Visualized Experiments: JoVE. (132), e56102 (2018).
- Wong, J. Y., Rapson, L. M. Acupuncture in the management of pain of musculoskeletal and neurologic origin. Physical Medicine and Rehabilitation Clinics of North America. 10 (3), 531-545 (1999).
- Qin, Z., Liu, X., Yao, Q., Zhai, Y., Liu, Z. Acupuncture for treating sciatica: A systematic review protocol. BMJ Open. 5 (4), 007498 (2015).
- Zhi, M. J., et al. Application of the chronic constriction injury of the partial sciatic nerve model to assess acupuncture analgesia. Journal of Pain Research. 10, 2271-2280 (2017).
- Fu, Z. H., Xu, J. G. A brief introduction to Fu's subcutaneous needling. Pain Clinic. 17, 343-348 (2005).
- Peng, J., et al. The effect of Fu's subcutaneous needling combined with reperfusion approach on surface electromyography signals in patients with cervical spondylosis and neck pain: A clinical trial protocol. Biomed Research International. 2022, 1761434 (2022).
- Fu, Z. H., Wang, J. H., Sun, J. H., Chen, X. Y., Xu, J. G. Fu's subcutaneous needling: possible clinical evidence of the subcutaneous connective tissue in acupuncture. Journal Alternative and Complementary Medicine. 13 (1), 47-51 (2007).
- Harrison, T. M., Churgin, S. M. Acupuncture and traditional Chinese veterinary medicine in zoological and exotic animal medicine: A review and introduction of methods. Veterinary Science. 9 (2), 74 (2022).
- Gollub, R. L., Hui, K. K., Stefano, G. B. Acupuncture: pain management coupled to immune stimulation. Zhongguo Yao Li Xue Bao. 20 (9), 769-777 (1999).
- Simons, D. G., Travell, J., Simons, L. E. . Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd ed. , (1999).
- Gerwin, R. D., Dommerholt, J., Shah, J. P. An expansion of Simons' integrated hypothesis of trigger point formation. Current Pain and Headache Reports. 8 (6), 468-475 (2004).
- Hong, C. Z., Simons, D. G. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Archives of Physical Medicine and Rehabilitation. 79 (7), 863-872 (1998).
- Fu, Z., et al. Remote subcutaneous needling to suppress the irritability of myofascial trigger spots: an experimental study in rabbits. Evidence Based Complement and Alternative Medicine. 2012, 353916 (2012).
- Hsieh, Y. L., Yang, C. C., Liu, S. Y., Chou, L. W., Hong, C. Z. Remote dose-dependent effects of dry needling at distant myofascial trigger spots of rabbit skeletal muscles on reduction of substance P levels of proximal muscle and spinal cords. Biomed Research International. 2014, 982121 (2014).
- Ma, K., et al. Peripheral nerve adjustment for postherpetic neuralgia: a randomized, controlled clinical study. Pain Medicine. 14 (12), 1944-1953 (2013).
- Gao, Y., Sun, J., Fu, Z., Chiu, P. E., Chou, L. W. Treatment of postsurgical trigeminal neuralgia with Fu's subcutaneous needling therapy resulted in prompt complete relief: Two case reports. Medicine. 102 (9), e33126 (2023).
- Lucas, L. R., Wang, C. J., McCall, T. J., McEwen, B. S. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Research. 1155, 108-115 (2007).
- Yang, C. H., et al. Effect of electroacupuncture on response to immobilization stress. Pharmacology, Biochemistry, and Behavior. 72 (4), 847-855 (2002).
- Adams, S., Pacharinsak, C. Mouse anesthesia and analgesia. Current Protocols in Mouse Biology. 5 (1), 51-63 (2015).
- Cantwell, S. L. Traditional Chinese veterinary medicine: the mechanism and management of acupuncture for chronic pain. Topics in Companion Animal Medicine. 25 (1), 53-58 (2010).
- Liebano, R. E., Rakel, B., Vance, C. G. T., Walsh, D. M., Sluka, K. A. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 152 (2), 335-342 (2011).
- Khalil, Z., Merhi, M. Effects of aging on neurogenic vasodilator responses evoked by transcutaneous electrical nerve stimulation: relevance to wound healing. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 55 (6), B257-B263 (2000).
- Sato, K. L., Sanada, L. S., Silva, M. D. D., Okubo, R., Sluka, K. A. Transcutaneous electrical nerve stimulation, acupuncture, and spinal cord stimulation on neuropathic, inflammatory and, non-inflammatory pain in rat models. The Korean Journal of Pain. 33 (2), 121-130 (2020).
- Maeda, Y., Lisi, T. L., Vance, C. G., Sluka, K. A. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Research. 1136 (1), 43-50 (2007).
- Degrugillier, L., et al. A new model of chronic peripheral nerve compression for basic research and pharmaceutical drug testing. Regenerative Medicine. 16 (10), 931-947 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved