A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Modified Tail Vein and Penile Vein Puncture for Blood Sampling in the Rat Model
* These authors contributed equally
In This Article
Summary
Here, we present a protocol to offer rapid, easy, and reliable blood collection alternatives for the rat model. We describe three different blood sampling methods according to the context: tail vein puncture under anesthesia or on a conscious animal, and dorsal penile vein puncture under anesthesia.
Abstract
Blood samples are required in most experimental animal designs to assess various hematological parameters. This paper presents two procedures for blood collection in rats: the lateral tail vein puncture and the dorsal penile vein puncture, which offer significant advantages over other previously described techniques. This study shows that these two procedures allow for fast sampling (under 10 min) and yield sufficient blood volumes for most assays (202 μL ± 67.7 μL). The dorsal penile vein puncture must be done under anesthesia, whereas the lateral tail vein puncture can be done on a conscious, restrained animal.
Alternating these two techniques, therefore, enables blood draw in any situation. While it is always recommended for an operator to be assisted during a procedure to ensure animal welfare, these techniques require only a single operator, unlike most blood sampling methods that require two. Moreover, whereas these previously described methods (e.g., jugular stick, subclavian vein blood draw) require extensive prior training to avoid harm to or death of the animal, tail vein and dorsal penile vein puncture are rarely fatal. For all these reasons, and according to the context (e.g., for studies including male rats, during the perioperative or immediate postoperative period, for animals with thin tail veins), both techniques can be used alternately to enable repeated blood draws.
Introduction
Blood sampling is necessary for most animal studies, both in vivo and in vitro. In rats, as the frequency and amount of blood sampling can be significant, it is helpful to have different alternatives for collection. Various methods have been described in previous studies.
The most commonly used techniques are tail vein puncture and saphenous vein blood draw. Tail vein sampling is suitable for all rat strains. With proper training, the procedure is simple to perform and causes minimal distress to the animal1. Similarly, the saphenous vein blood draw, provided it is done properly, is also a quick and simple collection method. Neither method requires anesthesia, and both allow for repeated draws of small amounts of blood. However, the saphenous vein puncture usually yields a lower blood volume1 and requires the presence of two people to leave one hindlimb exposed for puncture2.
If large amounts of blood need to be collected from a single animal, cardiac puncture or puncture of the vena cava may be used (up to 10 mL of blood can be drawn from a 150 g rat with cardiac puncture2). These techniques require anesthesia and are terminal procedures. The animal must be euthanized after any of these two techniques2. The jugular stick is an alternative that can be used if large amounts of blood need to be collected in a study that has not yet reached its endpoint. However, this technique also requires significant technical skills to avoid harm to the animal; hence, its use should be limited3.
Other techniques, such as the subclavian vein blood draw, do not need the use of anesthetics before blood collection and allow for repeated sampling of small volumes of blood. However, restrained handling and appropriate needle incision are required for this technique. An improper operation may result in animal pain or even mortality, and the training for this method may be fastidious4.
Other anecdotal procedures include the orbital puncture and the sublingual vein puncture, both of which require an anesthetic, and neither are recommended nor widely used. Though previous studies have shown faster blood collection by orbital puncture than by tail vein puncture, it was found that orbital puncture under diethyl-ether anesthesia was less well tolerated than the latter method (based on the animals' excitation scores and urine production)5. Moreover, this method is highly influenced by the skill of the person who performs the procedure and is mainly performed by experienced veterinarians. Comparably, the sublingual vein puncture is less distressing and is recommended for repeated blood sampling6. However, this technique presents severe adverse effects such as reduced food and water intake, which can lead to the animal's death7.
This study describes two methods used in our laboratory for repeated blood sampling. Tail vein puncture can be performed on a conscious animal, and the tissue damage and adverse effects are minimal. The modification of this technique in this study includes stabilizing the tail with the index and middle finger, which allows a single operator to perform the blood collection. The dorsal penile vein puncture has already been described for simple intravenous injections. This technique is performed under anesthesia and allows for a reliable blood source in case of difficulties with other methods (e.g., during the immediate postoperative period, with a small animal, when performing perioperative blood draw under anesthesia). Similar to tail vein sampling, the injury at the puncture site will have a minor overall effect on the animal compared to the techniques mentioned above8. The aim of this methods paper is to offer inexperienced researchers simple and reliable blood sampling alternatives according to the context (e.g., for procedures done under anesthesia, for studies including male rats, for animals with thin tail veins).
Protocol
The procedures were performed on 3 month old male Lewis rats, each weighing 300-400 g. A total of 24 animals were included, with three puncture conditions: 12 rats underwent tail vein puncture without anesthesia (group TV without anesthesia), and another 12 rats were anesthetized to undergo both tail vein puncture (group TV with anesthesia) and penile vein puncture (group PV with anesthesia). All the procedures were approved and respected the Institutional Animal Care and Use Committee (IACUC) guidelines. All the animals were euthanized at the end of the study (after a 1 month follow-up) by carbon dioxide overdose. See the Table of Materials for details related to all the materials and instruments used in this protocol.
1. General guidelines
- In line with the IACUC guidelines, ensure that the maximum blood volume drawn is no more than 10% of the total blood volume every 2 weeks9. For example, a rat of 300 g should have a total blood volume of approximately 19.2 mL. In the case of a protocol requiring four blood draws in the first week alone (day 0, day 1, day 3, day 7), limit the collection to a maximum of 250 μL of blood per sample.
- For procedures performed under anesthesia, administer isoflurane via a precision vaporizer to anesthetize the animal. Induce anesthesia in a chamber with a dose of 3%-5% isoflurane for 5 min, and maintain using a dose of 1%-3% isoflurane through a nose cone during the procedure. Adjust the isoflurane level based on continuous monitoring of the respiratory rate. Verify whether sedation is sufficient by toe pinch before starting the procedure.
- Do not leave the animal unattended during the procedure or until it has regained sufficient consciousness to maintain sternal recumbency.
- After the blood collection, monitor the animal until full recovery before returning it to its cage, and do not introduce it to the company of other animals until fully recovered.
NOTE: In agreement with veterinary services, no post-procedure pain medication was necessary after tail vein or penile vein puncture.
2. Blood draw from the penile vein
- Preparation
- Prepare the following equipment: sterile gauze, gloves, alcohol wipes, a microcapillary blood collection EDTA tube (purple cap), and a 30 G insulin syringe (30 U or 50 U).
- Take the rat out of its cage, and put it in a chamber for induction with isoflurane via a precision vaporizer (dose: 3%-5%). Once the animal is sedated, transfer it to the procedure table, and lay the animal on its back with its nose placed in the nose cone to maintain the anesthesia. Monitor the respiratory rate, and adjust the isoflurane level accordingly (maintenance dose: 1%-3%). Verify that the animal is sufficiently sedated by toe pinch before starting the procedure.
- Blood sampling
- Move the plunger back and forth in the syringe several times to smoothen the withdrawal. Create negative pressure in the syringe by pulling on the plunger to remove a couple of microliters.
- With the help of the non-dominant hand, retract the foreskin from the end of the penis, and hold the glans between the index and thumb, pulling gently. The dorsal penile vein will appear as a superficial blue cord. See Figure 1 and Figure 2.
- With the eye of the needle pointing upward, insert the insulin syringe into the vein at an angle of 35°. Once the needle has entered the vein, blood will flow into the syringe.
- Slowly withdraw the plunger of the syringe at a slow and steady rate to collect the desired volume.
NOTE: Do not withdraw the plunger too fast, as this will cause the vein to collapse and stop the blood flow. - If the blood flow decreases, rotate the needle slightly clockwise or counterclockwise.
- Remove the syringe. A blood drop will form on the puncture site, the aspiration of which will allow the collection of a few more microliters of blood in the case of a non-sterile procedure.
- If the first puncture fails, reinsert the needle more proximally on the vein.
NOTE: Unlike for tail vein sampling, the iterative puncture of the dorsal penile vein is usually unsuccessful. - Apply light pressure to the puncture site to stop the bleeding, and wipe the area with a new alcohol wipe.
- Place the penis back in its neutral position.
- Turn the isoflurane off, and monitor the rat until complete recovery. Return the rat to its cage.
3. Tail vein puncture
- Preparation
- Prepare the following equipment: a plastic restraining holder, sterile gauze, gloves, alcohol wipes, a microcapillary blood collection EDTA tube (purple cap), and a 28 G 1/2 insulin syringe (30 U or 50 U).
- Take the rat out of its cage, and quickly secure it in a plastic restraining cone. Close the large end of the cone around the base of the tail. Ensure that the animal is comfortable and that breathing is unrestricted throughout the whole procedure.
- Dip the tail in warm water (37 °C) for about 1 min to dilate the vein. Dry the tail with a paper towel. Place the animal (in its restrainer) face down, with the tail lying on a heating pad.
- Select the right or left tail vein (blue line) for sampling by rotating the entire animal to either side (this avoids the twisting of the tail). Use the terminal third of the tail for blood vessel puncturing since the vessels become more superficial in this zone. The artery is ventral, and the two veins are lateral10.
- Wipe the tail with 70% ethanol wipes at the puncture site.
- Place the tail on the edge of the heating pad to create an angle in the terminal third of the tail. This brings the vein to the surface and creates more space for taking the sample.
- Blood sampling
- Move the plunger back and forth in the syringe several times to smoothen the withdrawal. Create negative pressure in the syringe by pulling on the plunger to remove a couple of microliters.
- With the help of the non-dominant index and middle finger, secure the tail flat on the heating pad. Place the middle finger proximally and the index finger distally, with the puncture site between these two fingers. Apply more pressure on the middle finger than on the index to secure the tail, occluding the vessel only proximally and allowing the blood to pool. See Figure 1 and Figure 3.
- With the eye of the needle pointing upward, slide the insulin syringe against the index finger until it is inserted into the vein (this creates an angle of 35° between the needle and the tail). Once the needle has entered the vein, blood will flow into the syringe. At this point, release the pressure on the index and middle finger to ensure that the blood flow is not occluded.
- Slowly withdraw the plunger of the syringe at a steady rate to collect the desired volume.
NOTE: Do not withdraw the plunger too fast; this will cause the vein to collapse and stop the blood flow. - If the blood flow decreases, rotate the needle slightly in either direction.
- Remove the syringe from the tail. A blood drop will form on the tail's puncture site. The aspiration of this blood allows for the collection of a few more microliters of blood in the case of a non-sterile procedure.
- If the first puncture fails, reinsert the needle more proximally on the vein.
NOTE: The vein becomes progressively more profound as it approaches the tail base. If there is no blood flow in the syringe, increase the angle between the syringe and the tail, or rotate the needle. - Apply pressure to the puncture site to stop the bleeding, and wipe the area with a new alcohol wipe. Remove the rat from the plastic cone, and return it to its cage.
- Tail vein puncture under anesthesia
- Perform step 2.1.1 and step 2.1.2 for inducing and maintaining anesthesia.
- Perform steps 3.1.3-3.2.7 for blood collection; see Figure 1.
- Perform step 2.2.10 for animal recovery.
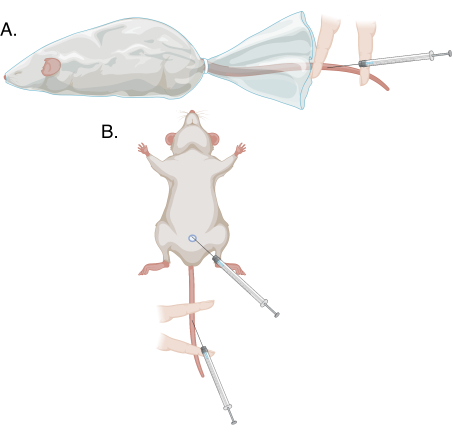
Figure 1: Schematics of the different puncture methods in this protocol. (A) Modified tail vein puncture on a conscious, restrained animal; (B) modified tail vein puncture and penile vein puncture under anesthesia. Please click here to view a larger version of this figure.
Results
Success was defined as a blood draw yielding at least 100 µL of blood in under 10 min (from the puncture time to the end of the blood collection), and failure was defined as a blood draw yielding less than 100 µL of blood or taking more than 10 min to retrieve the required blood volume. A maximum of 250 µL of blood per sample was allowed. The statistical analyses were conducted using a one-way ANOVA test for multiple comparisons and the chi-squared test. The data were presented as mean value ± standar...
Discussion
The tail vein puncture is an efficient method to obtain blood from a conscious rat. However, when an animal is under anesthesia, the effect of isoflurane can lead to vessel spasms and make tail vein puncture unsuitable11. As shown in this study, an alternative in this situation is to collect blood from the penile vein, which is more successful and yields a significantly greater volume of blood in less time. It is important to remember that in the case of failure with this method on the first try, ...
Disclosures
None of the authors have any conflicts of interest to declare.
Acknowledgements
This work was funded by Shriners Children's Boston (B. E. U., K.U., C.L.C.). L.C. is funded by "La Bourse des Gueules Cassées", "La Bourse Année Recherche", and "La Bourse de l'Amicale des Anciens Internes des Hôpitaux de Paris". Y.B. is funded by "La Bourse des Gueules Cassées". Y.B. and I.F.v.R. are funded by the Shriners Hospitals for Children (Fellowships ID is #84308-BOS-22 #84302-BOS-21 respectively). This material is partially based upon work supported by the National Science Foundation under Grant No. EEC 1941543. Partial support from the US National Institutes of Health (R01EB028782, R56AI171958, and R01DK114506) is gratefully acknowledged. Figure 1 was created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 0.5 mL | 28 G ½ Insulin Syringes | BD | 329424 | for tail vein puncture |
| 0.5 mL | 30 G x 5/16 Insulin Syringes | BD | 320468 | for penile vein puncture |
| 250 L Microtainer blood collection tubes with K2EDTA | BD | 365974 | |
| Gauze Sponges | Curity | 6939 | |
| Isoflurane Auto-Flow Anesthesia Machine | E-Z Systems | EZ-190F | for penile vein puncture |
| Isoflurane, USP | Patterson Veterinary | 1403-704-06 | for penile vein puncture |
| Nosecone for Anesthesia | World Precision Instruments | EZ-112 | for penile vein puncture |
| Rodent Restraint Cone | Harvard Apparatus | ST2 52-95-86 | for tail vein puncture |
| Small Animal Heated Operating Table (Adjustable) | Peco Services Ltd | 69023 | |
| Webcol Alcohol prep pads | Simply Medical | 5110 |
References
- Lee, G., Goosens, K. A. Sampling blood from the lateral tail vein of the rat. Journal of Visualized Experiments. (99), e52766 (2015).
- Beeton, C., Garcia, A., Chandy, K. G. Drawing blood from rats through the saphenous vein and by cardiac puncture. Journal of Visualized Experiments. (7), 266 (2007).
- Luzzi, M., et al. Collecting blood from rodents: A discussion by the Laboratory Animal Refinement and Enrichment Forum. Animal Technology and Welfare. 4 (2), 99-102 (2005).
- Wang, L., et al. Repetitive blood sampling from the subclavian vein of conscious rat. Journal of Visualized Experiments. (180), e63439 (2022).
- Van Herck, H., et al. Blood sampling from the retro-orbital plexus, the saphenous vein and the tail vein in rats: Comparative effects on selected behavioural and blood variables. Laboratory Animals. 35 (2), 131-139 (2001).
- Harikrishnan, V. S., Hansen, A. K., Abelson, K. S., Sørensen, D. B. A comparison of various methods of blood sampling in mice and rats: Effects on animal welfare. Laboratory Animals. 52 (3), 253-264 (2018).
- Zeller, W., Weber, H., Panoussis, B., Bürge, T., Bergmann, R. Refinement of blood sampling from the sublingual vein of rats. Laboratory Animals. 32 (4), 369-376 (1998).
- Nightingale, C. H., Mouravieff, M. Reliable and simple method of intravenous injection into the laboratory rat. Journal of Pharmaceutical Sciences. 62 (5), 860-861 (1973).
- Blood collection: The rat. IACUC Guideline. UCSF Office of Research Institutional Animal Care and Use Program Available from: https://iacuc.ucsf.edu/sites/g/files/tkssra751/f/wysiwyg/guidelines%20-%20Blood%20Collection%20-%20Rat.pdf (2022)
- Staszyk, C., Bohnet, W., Gasse, H., Hackbarth, H. Blood vessels of the rat tail: A histological re-examination with respect to blood vessel puncture methods. Laboratory Animals. 37 (2), 121-125 (2003).
- Constantinides, C., Mean, R., Janssen, B. J. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR Journal. 52 (3), e21-e31 (2011).
- Hernaningsih, Y., Akualing, J. S. The effects of hemolysis on plasma prothrombin time and activated partial thromboplastin time tests using photo-optical method. Medicine. 96 (38), 7976 (2017).
- Powles-Glover, N., Kirk, S., Jardine, L., Clubb, S., Stewart, J. Assessment of haematological and clinical pathology effects of blood microsampling in suckling and weaned juvenile rats. Regulatory Toxicology and Pharmacology. 69 (3), 425-433 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved