Method Article
Localizing Function-specific Targets for Transcranial Magnetic Stimulation in the Absence of Navigation Equipment
In This Article
Summary
This paper describes how to localize function-specific targets for repetitive transcranial magnetic stimulation interventions or treatments when navigation equipment is unavailable.
Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique that modulates neural activity in the brain. Studies have shown that rTMS can regulate neural plasticity, promote neural network reorganization, and has been widely applied to neuropsychiatric disorders such as stroke. Although some studies suggest that rTMS can aid in stroke rehabilitation, its efficacy remains uncertain, possibly because of limitations in the traditional localization of the hand motor hotspot.
The hand motor hotspot is determined by motor evoked potentials (MEPs), which reflect the conductivity of the corticospinal or pyramidal tract, representing non-voluntary movement. In contrast, functional magnetic resonance imaging (fMRI) activation points from a motor task define function-specific targets, which involve both perception and motor execution, representing voluntary movement. Based on this, we propose the concept of function-specific targets -- targets identified through brain imaging techniques aimed at specific functions. Function-specific targets exhibit stronger and more extensive functional connectivity with brain regions related to motor cognition, potentially offering more effective regulatory effects than the hotspots.
We explored and validated the modulatory effects of function-specific targets in previous study. However, institutions without navigation equipment are unable to utilize these function-specific targets. Therefore, we have developed a non-navigated localization method for function-specific targets, specifically designed to define and localize rTMS targets in the post-stroke ipsilateral hemisphere, addressing the challenges faced by institutions lacking navigation equipment when applying function-specific targeted rTMS.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique that can regulate brain activity and has been widely used in the treatment of neuropsychiatric disorders, such as in the rehabilitation of hand motor dysfunction in stroke patients. Some studies have shown that rTMS has therapeutic effects on post-stroke sequelae1,2,3, but its efficacy remains uncertain. One key reason for this uncertainty is the difficulty in identifying precise stimulation targets. TMS studies targeting motor function often rely on the International 10-20 Electroencephalogram system for localization, using C3/C4 as the stimulation targets, or they employ individualized targets, such as the hand motor hotspot. However, these methods cannot accurately determine the cortical areas affected by TMS. Functional magnetic resonance imaging (fMRI)-guided, targeted rTMS has been widely used in the treatment of depression.
Our previous research also explored its application in treating Tourette syndrome by stimulating supplementary motor area4, but it has not yet been applied to the primary motor area (M1). For rTMS, M1 is distinct from other brain regions because it contains the hand motor hotspot. The muscle contractions induced by TMS represent involuntary movements, reflecting top-down conduction through the corticospinal or pyramidal tracts. In contrast, the activation peak voxels defined by fMRI during finger tapping tasks are more functionally connected to brain regions involved in motor cognition, representing voluntary movements5. Therefore, when treating movement disorders, using the task-related "activation" defined by fMRI as function-specific targets may lead to improved therapeutic outcomes5,6. In our previous work, we compared brain activation patterns between the visual-guided task and the self-initiated task using fMRI and determined that the self-initiated task more closely aligns with the requirements of active rehabilitation training6. We confirmed this finding by reanalyzing a subset of data from the original study (Figure 1).
Accurately targeting specific brain function areas requires precise navigation tools. However, current systems are not only cumbersome to operate and limited in functionality, but the head-mounted calibrators often fail to remain stable during procedures, are prone to shifting, and are expensive -- sometimes costing up to one million Chinese Yuan (CNY), approximately 140,000 United States Dollar (USD). According to a survey on the usage patterns among member institutions of the Precision Medicine Consortium for Imaging-Guided Transcranial Magnetic Stimulation Therapy (PRECISE), these drawbacks have led to navigation technologies being used in less than 5% of TMS research and clinical practice in China, despite their potential benefits. More importantly, however, is that these systems only focus on "locating" stimulation sites without addressing the critical issue of "defining" the target, i.e. selecting the most appropriate area for stimulation. Given the high costs, operational complexity, and time demands, this is why these devices have yet to achieve widespread clinical adoption.
To address the challenge of using function-specific targets without navigational devices, we explored the method of non-navigated, targeted rTMS. Using fMRI, we identified function-specific targets in the motor cortex and projected them onto the scalp surface, allowing for target definition and localization without the need for navigation equipment7. While non-navigated rTMS does not provide real-time monitoring throughout the entire process, it addresses the issues of precision in target localization under clinical conditions where navigational devices are unavailable. This paper elaborates on the overall study rationale and outlines the complete experimental process, with a particular focus on comparing the effects of function-specific targets on brain function under both navigated and non-navigated conditions. To verify the feasibility of function-specific targeted rTMS, the current study only included healthy individuals.
Protocol
This work has been approved by the Ethics Committee of Chengdu Sport University, and all participants provided written informed consent (Figure 2). This protocol describes non-navigated versus navigated function-specific targeted rTMS.
1. Participant recruitment
- Recruit 10 healthy right-handed adult participants (ages 22 to 29, with 5 females and 5 males; mean age 24 ± 2 years). Exclude one participant due to head motion exceeding 2.5 mm in translation or 2.5 ° in rotation. Finally, include 9 participants in the statistical analysis.
- Inclusion criteria
- Recruit participants aged 18 to 30 years, who are right-handed, pass both MRI and TMS safety screenings, and have no history of epilepsy or other neurological or psychiatric disorders.
- Ensure that the participants meet additional criteria such as no contraindications for MRI scanning, no history of brain injury or severe heart disease, and that they are not currently taking antiepileptic or anticoagulant medications.
- Recruit participants who have no consciousness disorders, no metal objects in their body (such as pacemakers, metal dental implants, or intrauterine device), no severe claustrophobia or pregnancy, and whose vision is either normal or corrected to normal.
- Inclusion criteria
- Postexperiment data exclusion criteria
- Exclude data from participants who are unable to complete the experiment or whose head motion during fMRI scanning exceeds 2.5 mm of translation or 2.5° of rotation.
- Preexperiment participant guidelines
- Ensure that all participants have signed the informed consent form, which explains the purpose of the research, the experimental procedures, and potential side effects and risks involved.
- Conduct safety screenings for the participants.
- Explain the experimental procedures and precautions to ensure smooth execution of the experiment.
- Advise participants to avoid alcohol, coffee, or vigorous exercise before the experiment.
- Remind participants to get sufficient sleep and avoid staying up late.
2. fMRI data acquisition
NOTE: All participants undergo MRI scanning at the Magnetic Resonance Brain Imaging Center on the Qingshuihe Campus of the University of Electronic Science and Technology of China, using a 3T GE MR750 scanner. Each scanning session includes a T1-weighted structural image, an 8 min resting-state fMRI (RS-fMRI), and a 4 min Task-fMRI. Participants receive two rTMS interventions: one with navigation and one without, with a 1 week interval between sessions to eliminate residual effects. Conduct MRI scans before and after each intervention, totaling four scans.
NOTE: Counterbalance the sequence of the navigated and non-navigated conditions across participants.
- Scan procedures
- Prior to entering the MRI room, fit participants with earplugs to reduce noise and ensure all metal objects are removed.
- Explain the tasks that participants need to perform during the scan.
- Ensure the participants lie supine on the scanning bed, with their heads securely fixed using foam pads to minimize head movement.
- During the RS-fMRI scan, instruct participants to close their eyes, avoid deliberate thinking, and remain awake to prevent falling asleep.
- Manually export the images to the designated network drive or external storage device.
- Scanning parameters
- Use the following RS-fMRI scanning parameters: repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 220 mm × 220 mm, matrix = 64 x 64, slice thickness/gap = 3.4 mm/0 mm, with 41 slices in total, covering the whole brain, and 240 time points collected.
- Use the following T1-weighted structural images scanning parameters: Spoiled Gradient Recalled Echo (SPRG) sequence, sagittal scan TR/TE = 8.2 ms/2.98 ms, FA = 8°, FOV = 256 mm x 256 mm, matrix = 256 x 256, slice thickness/gap = 1 mm/0 mm, with 166 slices covering the whole brain.
- Use task-fMRI scanning parameters that are identical to those of the RS-fMRI, except that only 120 time points are collected.
- Task execution details
- Position participants with their palms facing up and holding a button box.
- Place towels between the participants' heads and the coil to stabilize their heads and minimize motion.
- Block design, self-initiated task (4 min): When an image of a "+" appears on the screen, ask the participants to rest. When an image of a clock appears on the screen, instruct the participant to press the button with their right thumb every 2 s, timing it themselves (Supplemental Figure S1).
3. Resting motor threshold (RMT) measurement
NOTE: Use surface electromyography (EMG) to record the amplitude of the motor-evoked potential (MEP) from the right abductor pollicis brevis (APB) muscle, using a 70 mm figure-eight coil attached to the Magstim Super Rapid2 stimulator to measure RMT with single-pulse stimulation.
- Remove all metal objects before testing to avoid interference and ensure safety.
- Have participants sit in a chair and relax completely.
- Apply exfoliating scrub and 75% alcohol to the participants' hands.
- Place silver/silver chloride (Ag/AgCl) surface electrodes on the muscle belly.
- Position the reference electrode on the metacarpophalangeal joint, ensuring the inter-electrode distance is between 20 mm and 30 mm.
NOTE: Relevant parameters: Use electrodes with a diameter of 9 mm for the measurements. The EMG signal from the APB muscle is amplified 1,000 times, band-pass filtered between 20 Hz and 2.5 kHz, then digitized via a micro-digital interface at a sampling rate of 5 kHz. The data is then stored on a computer and displayed on a screen. - Load the individual's T1 structural image. Place the coil over the contralateral primary motor area, specifically at the "middle knee" of the central sulcus, also known as the "hand knob", which represents the hand area in the primary motor cortex.
NOTE: Confirm muscle relaxation both visually and via EMG monitoring. - Move the coil around the "hand knob" in 0.5 cm increments.
- Position the handle at a 45° angle to the mid-sagittal plane to measure the MEP.
- Start at subthreshold stimulation intensity, increasing it by 5% of the maximum stimulus output each time. When the peak-to-peak amplitude of the MEP exceeds 50 µV, decrease the stimulation intensity stepwise by 1% of the maximum output.
- Record the minimum stimulation intensity that evokes at least five MEPs greater than or equal to 50 µV in 10 consecutive single-pulse stimulations as the RMT, with this location identified as the hotspot. If a hotspot cannot be determined after six stimulations, move the coil to the next location.
4. Individualized function-specific targeted rTMS
- Define the individualized function-specific target.
- After opening preprocessing software, click on DPARSF 5.4, then select DPARSF Advanced Edition to preprocess the task-state data using the specific parameters shown in Supplemental File 1. Perform slice timing and head motion corrections. Coregister the functional images to structural images and apply spatial smoothing with a full width at half maximum (FWHM) of 6 mm.
NOTE: Adjust the specific parameters according to the machine model or scanning task. - Open SPM12 and click on Coregister Estimate. For the Reference Image, select the file named "sub*crop_1.nii" from the T1Img folder. For the Source Image, choose the "mean*.nii" file from the RealignParameter folder. For the Other Image, select the "ra*.nii" file from the FunImgAR folder.
NOTE: Use the functional image file generated after motion correction and slice timing correction as the "Other Image". Alternative files may be selected depending on the research objective. - Click on Segment | Volumes and select the file named "sub*crop_1.nii" from the T1Img folder. For Deformation Fields, select Inverse + Forward, then click Run. Repeat this process to segment the "sub*.nii" file from the T1Img folder.
NOTE: Segment "sub*crop_1.nii" to calculate the individual task activation point. Segment "sub*.nii" to transform the standard space mask into individual space. - Click on Smooth, select the "ra*.nii" files from the FunImgAR folder for the Image to Smooth option, and enter 6 6 6 in the FWHM field.
- Perform first-level analysis to obtain individual activation maps and identify the peak voxel of activation as the stimulation target. Include the following three steps:
- Create a new folder named "indiv_act" and click on Specify 1st-level. In the Directory field, select the "indiv_act" folder, click on Units for design, choose Scans, and enter 2 for the Interscan interval. In the Data & Design section, select the "sra*.nii" files under Scans; in the Condition section, set the Name to tap (custom name), enter 0 30 60 90 for the Onset, and set the Durations to 15. Click on Multiple regressors and select the "rp_a*.txt" file from the RealignParameters.
NOTE: Fill in the Onset and Duration information according to the actual experimental design. - Estimate: In "Select SPM.mat", choose the "SPM.mat" file from the "indiv_act" folder and generate the individual task activation map, "spmT_0001".
- Click on Results, select the "SPM.mat" file from the "indiv_act" folder, check t-contrast, and click on Define new contrast. Enter a custom name in the name field, input 1 0 in the contrast field, click on Submit | OK | Done. In Apply masking, select None; under p value adjustment to control, choose None, with a value of 0.001; set the & extend threshold value to 0.
- Create a new folder named "indiv_act" and click on Specify 1st-level. In the Directory field, select the "indiv_act" folder, click on Units for design, choose Scans, and enter 2 for the Interscan interval. In the Data & Design section, select the "sra*.nii" files under Scans; in the Condition section, set the Name to tap (custom name), enter 0 30 60 90 for the Onset, and set the Durations to 15. Click on Multiple regressors and select the "rp_a*.txt" file from the RealignParameters.
- Click on Normalise (Write) | Data. In Deformation Fields, select the "iy_Crop_1" file from the T1Img folder. For Image to write, choose the M1 brain region mask. Enter the individual Bounding Box and Voxel sizes.
NOTE: Enter the Bounding Box and Voxel sizes based on the specific characteristics of the data. - Click on Coregister (Reslice), then select spmT_0001 from the "indiv_act" folder for Image Defining Space. For Image to Reslice, choose the "w*.nii" file generated in the step 4.1.6.
- Compute the individual task activation peak: In MATLAB, run the sort positive code. For InputName1, select the path of the "rw*.nii" file generated in step 4.1.7; for InputName2, select the path of the "spmT_0001" file from the "indiv_act" folder; for InputName3, select the output folder path. The first X-coordinate with a negative value (the left hemisphere) in the sorted results is the individual task activation peak; record the coordinates of this point.
- After opening preprocessing software, click on DPARSF 5.4, then select DPARSF Advanced Edition to preprocess the task-state data using the specific parameters shown in Supplemental File 1. Perform slice timing and head motion corrections. Coregister the functional images to structural images and apply spatial smoothing with a full width at half maximum (FWHM) of 6 mm.
- Locate the individualized function-specific target (navigated).
- Determine the output intensity of the stimulator based on the participant's RMT.
- Employ a frameless stereotactic optical tracking neuronavigation system, with the participant seated comfortably and wearing a head-mounted calibrator.
- Click on the anatomical option: Import the participant's T1-weighted structural images into the navigation system for head modeling.
- Click on the Reconstruction option: Reconstruct the skin on the image.
- Click on the Landmarks option: Use the localizer tool to mark four landmarks (the nasion, tip of the nose, and preauricular points on both sides) on the head.
- Click on the Target option: Identify and establish the target trajectory in the brain region. Locate the stimulation target on the participant's individual images. After positioning, move the target to align with the crosshairs. Complete TMS localization.
NOTE: Make the coil tangent to the scalp and align the stimulation focus with the target.
- Locate the individualized function-specific target (non-navigated).
NOTE: All the code for scalp target localization is provided in Supplemental File 2.- Use SPM12 to segment the Montreal Neurological Institute (MNI) standard brain template (mni_icbm152_t1_tal_nlin_asym_09c.nii, located in the Templates folder of DPABI) to obtain the standard scalp mask. The specific steps are as follows:
- Open SPM12, click on fMRI, and then select Segment from the pop-up menu. In the parameters interface, click on the Volumes button, select the standard brain template file (i.e., the MNI brain template) from the Volumes option, and then click on Deformation Fields to select Inverse + Forward.
- Outline the inner and outer edges of the standard scalp: In MATLAB, run the code edges. In the pop-up window, select the c5.nii image, click on Done, and generate the "c5_edges.nii" file.
- Outline the outermost edge image of the standard scalp: In MATLAB, run the outer_edge code. In the pop-up interface, select the c5_edges.nii file and click on Done to generate the "c5_outer_edge.nii" file, which represents the scalp boundary in standard space.
- Use SPM12 to transform the standard scalp edge back into individual space. In the menu interface, click Normalise (Write), then, in the parameters interface, click on Data. In Deformation Fields, select the iy_sub*.nii file from the T1Img folder. Choose c5_outer_edge.nii for Images to Write, and input the individual bounding box and voxel sizes.
- Convert cortical coordinates to scalp coordinates: Open the TransCortex2Scalp code in MATLAB and execute the first line. In the pop-up interface, enter the individual task activation point coordinates and select the wc5_outer_edge.nii file. Record the scalp coordinates.
- Open DPABI_Viewer, click on Underlay, and select the individual T1 structural image. Locate and record the coordinates of the four landmark points: the left and right auricular peaks, the nasion, and the inion.
- Define the scalp origin: Open the intersection code in MATLAB. In the editor, input the coordinates of the four landmark points at their specified positions. Run the code to calculate the intersection coordinates of the line connecting the left and right ear tips with the line connecting the nasion and inion, then record the coordinates.
- Move the intersection point along the Z-axis to the scalp: Open the origin code in MATLAB. Enter the intersection point coordinates at the Define point H position in the editor. Run the code, then select the wc5_outer_edge.nii file in the pop-up window to obtain the scalp origin coordinates O.
- Draw a line connecting the two ear tips to define the X-axis, and a line connecting the nasion and the external occipital protuberance to define the Y-axis. The axis perpendicular to both defines the Z-axis. The two-dimensional plane formed by the XY axis is the XY plane.
- Calculate the actual distance from the scalp origin O to each point: Run the distance code, select the wc5_outer_edge file in the popup interface, and in the Command Window, follow the prompts to input the scalp origin, scalp target, and the four landmark points.
NOTE: "Each point" refers to the four scalp landmark points in step 4.3.4 and the scalp target. This code can only calculate the arc distance between one point and another at a time. To calculate the distance between a different pair of points, you need to run the code again. - Calculate the angle between the line connecting the scalp target and the scalp origin and the X-axis in the XY plane: Open the code calculate_angle_X_axis and run the first line. In the Command Window, input the coordinates of the scalp origin and the stimulation target as prompted.
- Use the targeting ruler (as shown in Figure 3) to fix the corresponding soft ruler position based on the distance and angle calculated in the previous steps. Mark the scalp with a washable pen. Complete the localization of the scalp stimulation target (Figure 4).
- Use SPM12 to segment the Montreal Neurological Institute (MNI) standard brain template (mni_icbm152_t1_tal_nlin_asym_09c.nii, located in the Templates folder of DPABI) to obtain the standard scalp mask. The specific steps are as follows:
- rTMS
- Select the repetitive mode to set the stimulation parameters, including stimulation intensity, frequency (10 Hz), duration (3 s), number of pulses in each train (30 pulses), wait time (12 s), number of trains (60 trains), and total number of pulses delivered per day (1,800 pulses).
- Save the session and press the Run button to initiate the stimulation.
NOTE: The stimulation intensity is set according to the participant's RMT, which in this study is set at 100% RMT. - Within half an hour after the stimulation ends, have the participant undergo another MRI scan, using the same scanning sequence as that used before the stimulation.
5. rTMS modulatory effect detection (MRI data processing and analysis)
NOTE: Use preprocessing software to perform RS-fMRI data preprocessing, which includes the following specific steps:
- Remove the first 10 time points to achieve signal equilibrium and allow participants to adapt to the scanner noise.
- Correct the acquisition time delay between slices.
- Perform head motion correction.
NOTE: Different head motion limits may be set based on specific study requirements. - Normalize the functional images to MNI space using the EPI template.
- Regress out nuisance signals, including those from white matter, cerebrospinal fluid, and six head motion parameters.
- Remove linear trends.
- Apply bandpass filtering (0.01-0.1 Hz).
- Conduct spatial smoothing using a Gaussian kernel with a FWHM of 6 mm.
- Calculate brain activity metrics after preprocessing, including amplitude of low-frequency fluctuation (ALFF) and functional connectivity (FC). Calculate the differences in local brain activity metrics (ALFF and FC) between pre-rTMS and post-rTMS under both navigated and non-navigated conditions, and conduct paired t-tests on the difference maps (GRF correction, voxel p < 0.001, cluster p < 0.05).
Results
The paired t-test and two-way ANOVA results indicated that there were no significant differences in the changes in ALFF or FC before and after rTMS under both navigated and non-navigated conditions (GRF correction, voxel p < 0.001, cluster p < 0.05). No significant differences were observed between the navigation and non-navigation conditions. This result aligns with our expectations, indicating that our non-navigation method does not have a significant disadvantage compared to the navigation method. To avoid making unsupported claims of no significant differences, we present the one-sample t-test maps for both rTMS conditions here (uncorrected, voxel p < 0.05) (Figure 5). These results do not survive any type of multiple comparison adjustment, such as FDR or GRF correction. To assess the equivalence of brain function changes induced by the non-navigated and navigated methods, a power analysis was conducted using Cohen's d. The results indicated that the Cohen's d value for ALFF was 0.22, while the Cohen's d value for FC was 0.56.
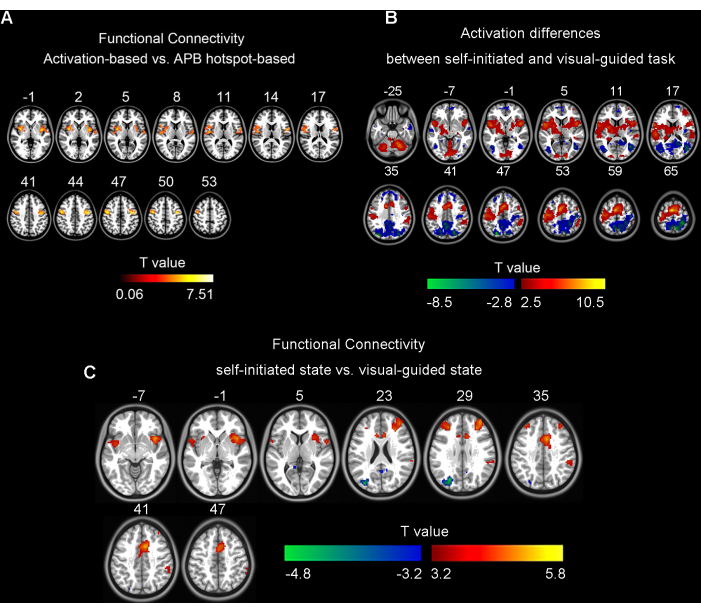
Figure 1: Results for paired t-tests. (A) The differences between activation-based and APB hotspot-based functional connectivity (GRF correction, single voxel p < 0.001, cluster level p < 0.05). (B) The differences in brain activation between self-initiated and visual-guided finger tapping tasks in 25 participants (FDR correction, q < 0.05). (C) The differences between self-initiated and visual-guided state activation-based functional connectivity in 35 participants (GRF correction, single voxel p < 0.001, cluster p < 0.05). Figure 1A was adapted from Wang et al. (2020)5; Figure 1B,C were prepared by extracting a different subset of data from Wang et al. (2023)6. Abbreviations: APB = Abductor Pollicis Brevis; GRF = Gaussian Random Field; FDR = False Discovery Rate. Please click here to view a larger version of this figure.
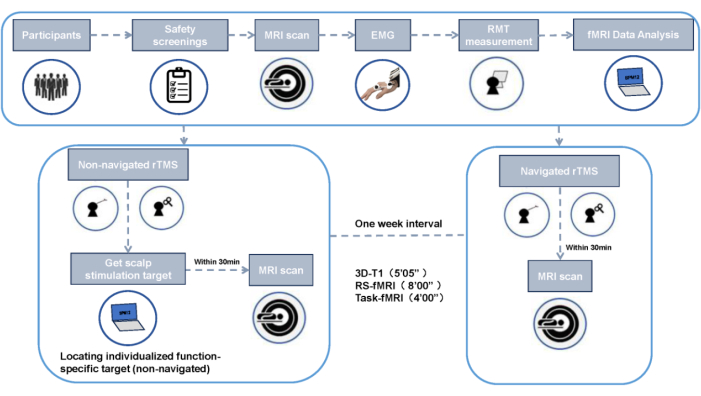
Figure 2: Experimental design flowchart. Please click here to view a larger version of this figure.
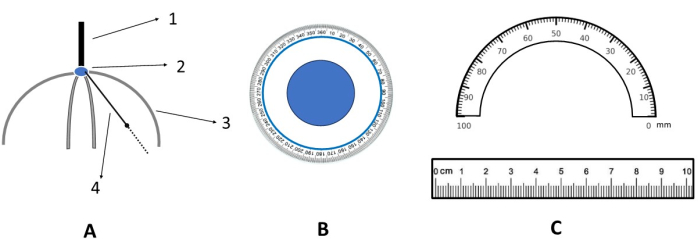
Figure 3: Schematic diagram of the targeting ruler. (A) Front view of the targeting ruler. 1. Handle; 2. Scalp anchor point (i.e., the scalp origin in the XY plane); 3. Rigid measuring ruler (acrylic material); 4. Rotatable and flexible measuring ruler (silicone material). (B) Magnified view of the scalp anchor point (i.e., an enlarged view of 2 in A). (C) Magnified view of the flexible measuring ruler (i.e., enlarged views of 3 and 4 in A). Please click here to view a larger version of this figure.

Figure 4: Conversion of the function-specific cortical target to the function-specific scalp target. The red dot represents the function-specific cortical target, the green dot represents the function-specific scalp target, and the blue dot indicates the origin of the 2D coordinate system on the scalp. Please click here to view a larger version of this figure.
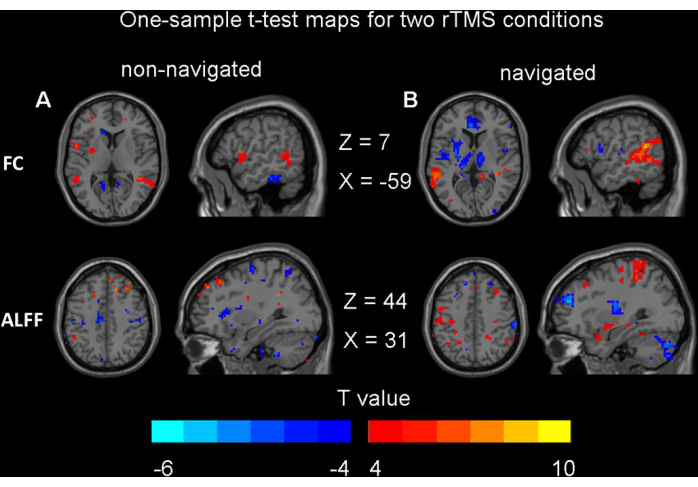
Figure 5: Results for one-sample t-tests. (A) Non-navigated rTMS modulatory effects on brain function (p < 0.05, uncorrected). (B) Navigated rTMS modulatory effects on brain function (p < 0.05, uncorrected). Abbreviations: FC = functional connectivity; ALFF = amplitude of low-frequency fluctuation; rTMS = repetitive transcranial magnetic stimulation. Please click here to view a larger version of this figure.
Supplemental File 1: Parameters used in the DPARSF Advanced Edition, as mentioned in protocol section 4.1.1. Please click here to download this File.
Supplemental File 2: The zip folder containing the MATLAB code used in this study. Please click here to download this File.
Supplemental Figure S1: Self-initiated finger tapping task. The task consisted of eight blocks, each duration lasting 30 s, resulting in a total length of 4 min. Please click here to download this File.
Discussion
In this study, we propose the concept of function-specific targets, which are brain regions associated with specific functions identified through neuroimaging techniques. Inspired by previous studies8,9,10, we developed a new toolkit7,11,12 for locating scalp targets corresponding to function-specific cortical areas, enabling function-specific targeted rTMS without the need for navigation equipment. Compared to stimulation using navigation equipment, no significant differences in brain function effects were observed. This suggests that, in certain cases, our method can achieve individualized function-specific targeted rTMS without the need for expensive navigation equipment.
Essential steps in the experimental protocol
To ensure the accuracy of non-navigated rTMS localization, the operator must align the scale on the targeting ruler with the left and right ear landmarks, the nasion, and the inion. The scale should be firmly pressed against the scalp surface to minimize measurement errors caused by hair thickness. This process is crucial for improving localization accuracy and ensuring precise targeting of the stimulation site.
Improvements to experimental method and potential technical issues
Since this method is an advanced version of a previously developed technique11, no areas for improvement have been identified so far. Regarding potential technical issues, individual differences in skull shape may result in less prominent occipital protuberances in some participants, which could lead to localization errors. In such cases, the occipital protuberance can be omitted, and other landmarks (such as the left and right ear markers and the nasion) can be used for localization without compromising accuracy, as redundancy has already been factored into the development phase.
Limitations of the non-navigated rTMS method
The main difference compared to navigated rTMS is the inability to monitor the coil's relative distance and direction to the stimulation target in real-time. However, even with navigated rTMS, real-time monitoring still requires experienced operators to make manual adjustments.
Significance of the experimental method in relation to existing methods
Compared to navigation equipment, our method does not require lengthy positioning or equipment calibration. Instead, users simply input MRI data into the code script and then calculate the corresponding distances via code, after which positioning is quickly completed using a measuring tool. Based on our experience, this method saves at least 15 minutes compared to the complex procedures involved in navigation. Navigation equipment typically requires expensive hardware and specialized training, while our method only requires MRI images and standard calculations to achieve fast, convenient, and precise localization, significantly reducing both upfront costs and operational complexity.
In terms of cost, our measuring tool has been granted an invention patent (ZL202411874788.9)12., which helps protect the intellectual property but does not significantly increase production costs. 3D modeling is currently underway, and we will soon be able to 3D print the tool for our clinical collaborators. Cost considerations were integrated into the design phase from the outset. For non-collaborators wishing to purchase the tool, the price is only 500 CNY (approximately 70 USD), which remains affordable despite the patent protection.
Importance and potential applications of the method in specific research fields
rTMS intervention and treatment have gained increasing popularity in both research and clinical fields in recent years. Like all therapeutic techniques, the development is moving toward precise, individualized treatments targeting specific functions. However, navigation systems and equipment are expensive, and most hospitals in China currently do not have access to such devices. This method addresses the issue of individualized, function-specific targeted rTMS without the need for navigation. It projects cortical target coordinates onto the scalp and uses a tool to mark the coordinates on the scalp surface. The fMRI-based cortical targeting method used in this approach is identical to the fMRI target coordinates employed by navigation systems and equipment internationally. Although it cannot monitor the real-time relative distance and direction between the coil and the stimulation target, it still offers advantages over current clinical "blind targeting" methods (such as using anatomical landmarks on the skin surface or selecting the hand motor hotspot). This method serves as a transitional approach between precise real-time navigation and "blind targeting". For clinical institutions without navigation systems and equipment, it can solve practical clinical problems. This method will significantly promote fMRI-guided TMS precision treatment, leading to the discovery of more effective stimulation targets and improving the efficacy of treatments for various neurological and psychiatric disorders.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by the Sichuan Province Science and Technology Support Program (No. 2024ZYD0189). The authors would like to thank the PREcision medicine Consortium for Imaging-guided transcranial magnetic Stimulation thErapy (PRECISE) for their professional guidance.
Materials
| Name | Company | Catalog Number | Comments |
| Brainsight Neuronavigation system | Rogue Research Inc. | KITBSF0104 | |
| DPABI_V7.0 toolkit | DeepBrain | for RS-fMRI and task-based fMRI data analysis | |
| Magstim Rapid2 | The MAGSTIM Company Limited | 3012-00 | |
| SPM12 (7771) | Wellcome Centre for Human Neuroimaging | for RS-fMRI and task-based fMRI data analysis | |
| The Brainsight 2 channel electromyography acquisition device | Rogue Research Inc. | NTBX001001 |
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved