Method Article
使用急性海马脑片啮齿动物突触标记/捕获和交叉捕获的研究
摘要
This video article describes experimental procedures to study long-term plasticity and its associative processes such as synaptic tagging, capture and cross-tagging in the CA1 pyramidal neurons using acute hippocampal slices from rodents.
摘要
Synaptic tagging and capture (STC) and cross-tagging are two important mechanisms at cellular level that explain how synapse-specificity and associativity is achieved in neurons within a specific time frame. These long-term plasticity-related processes are the leading candidate models to study the basis of memory formation and persistence at the cellular level. Both STC and cross-tagging involve two serial processes: (1) setting of the synaptic tag as triggered by a specific pattern of stimulation, and (2) synaptic capture, whereby the synaptic tag interacts with newly synthesized plasticity-related proteins (PRPs). Much of the understanding about the concepts of STC and cross-tagging arises from the studies done in CA1 region of the hippocampus and because of the technical complexity many of the laboratories are still unable to study these processes. Experimental conditions for the preparation of hippocampal slices and the recording of stable late-LTP/LTD are extremely important to study synaptic tagging/cross-tagging. This video article describes the experimental procedures to study long-term plasticity processes such as STC and cross-tagging in the CA1 pyramidal neurons using stable, long-term field-potential recordings from acute hippocampal slices of rats.
引言
The encoding and storage of information in the brain still remains the most significant and keenly pursued challenge in neuroscience. Over the years, long-term potentiation (LTP) and long-term depression (LTD) have emerged as the leading cellular correlates of memory1,2. These activity dependent changes, which exhibit input specificity and associativity, result in the stabilization of memory traces in the neuronal networks 1,3,4. The maintenance of the two forms of synaptic plasticity requires the synthesis of plasticity-related products (PRPs)5-10. Synapse specificity that involves the interaction of newly synthesized protein only with specific activated synapses expressing LTP or LTD, is critical to memory. This specificity is explained by the concept of ‘Synaptic Tagging and Capture’ (STC), where the PRPs interact with recently active, ‘tagged’ synapses11,12. The STC process offers a framework for associative properties of memories at the cellular level. It provides us with a conceptual basis of how short-term forms of plasticity are transformed into long-lasting forms of plasticity in an associative and time-dependent manner13.
During the process of STC, a strong tetanization in one input that leads to protein synthesis dependent late-LTP, results in the reinforcement of a protein synthesis independent early-LTP induced in another independent input on to the same population of neurons into a persistent one13. The setting of a local synaptic tag by a transient neural activity and the synthesis of the diffusible PRPs by the strong neural activity are the two key events during STC13,14. The capture of the PRPs by the recently potentiated ‘tagged’ synapses is fundamental to the maintenance of long-term potentiation. Many studies have been done to confirm the existence of STC phenomenon15-17 and identify the candidate ‘tags’18 and ‘PRPs’19. Calcium/calmodulin-dependent protein kinase II (CaMKII) and extracellular signal-regulated kinase1/2 (ERK1/2); CaMKIV, Protein Kinase M (PKM) and brain-derived neurotrophic factor (BDNF) are some of the candidate molecules for ‘tag’ and ‘PRP’ respectively19-21. The synaptic tagging model has further been expanded to include the positive associative interactions between LTP and LTD - the “synaptic cross-tagging”22. In synaptic cross-tagging, a late LTP/ LTD in one synaptic input transforms the opposite protein synthesis-independent early-LTD/LTP in an independent input into its long-lasting form or vice versa22.
The hippocampal slice preparation is the most widely used model in the studies of long-term synaptic plasticity23,24. Much of the understanding about the concepts of synaptic tagging and cross- tagging arises from the studies done in CA1 region of the hippocampus and because of the technical complexity many of the labs are still unable to study these processes. Experimental conditions for the preparation of rat hippocampal slices and the recording of stable late-LTP/LTD for extended hours are extremely important to study synaptic tagging/cross-tagging23,25,26. This article describes the detailed experimental procedures for studying long-term plasticity processes such as STC and cross-tagging in the CA1 pyramidal neurons using stable, long-term field-potential recordings from acute hippocampal slices of rats.
研究方案
所有动物的程序批准了新加坡国立大学的机构动物护理和使用委员会(IACUC)。
1.准备与人工脑脊液(学联)
- 制备ACSF由(以mM计)124 NaCl的,3.7氯化钾,1.0 硫酸镁 ·7H 2 O,2.5氯化钙 2·2H 2 O,1.2 KH 2 PO 4,24.6碳酸氢钠 ,和10 D-葡萄糖。确保ACSF的pH为7.2-7.4之间时鼓泡在电生理记录到饱和用95% 的 O 2和5%CO 2的混合物(卡波金)。21使用该人工脑脊液两个解剖,切片准备和用于灌注。
注:使用干净的设备,用于测量和保持学联。使用不洁的设备可能会导致混浊的解决方案或形成沉淀。使用去离子水的各项准备工作。 - 准备一个2升10X学联股票不包括碳酸氢钠 和D-葡萄糖在容量瓶中。加入试剂以去离子水按照以下顺序:氯化钠(144.96克),氯化钾(5.52克), 硫酸镁 ·7H 2 O(4.92克), 氯化钙 2·2H 2 O(7.56克),KH 2 PO 4(3.28用磁力搅拌器以确保所有试剂溶解连续至少30分钟克)和顶部高达2 L.体积搅拌。存储股票在4℃,2周内使用。
- 在此之前的解剖和实验中,稀释ACSF库存在容量瓶随着加入所需量的NaHCO 3和D-葡萄糖。对于1升溶液,加入2.07克碳酸氢钠和1.802克D-葡萄糖稀释后,将100毫升的股票,以1升。学联应无任何沉淀或溶解的颗粒的澄清溶液。
- 凉爽约200-300毫升的ACSF上的冰,以解剖过程中可以使用。确保用于解剖的学联介于2-4℃。使用的electrop剩余学联hysiological实验。泡所有的ACSF溶液饱和与卡波金(5%CO 2,95%O 2)的连续。在等待学联冷却,准备清扫面积和片室。
2.编制界面商会
注意:一个接口脑切片室,用于培养该切片并在电生理记录保持它们( 图2B),包括两个隔室。下部腔室包含蒸馏保持在32℃的水通过一个温度控制器和带卡波金连续鼓泡。
- 切换温度控制器上,并在32℃预设。通过流入管道运行蒸馏水洗10到15分钟,上腔室。确保上部室是放置在网前的清洁。检查在底部室中水位约70%填充有蒸馏水。
- 放置网中的上腔室,以提供一搁置表面为切片( 图2C)。调整流出管道,以确保该溶液水平被充分润湿网的整个区域。放置净上述盖子上腔室内保持加湿卡波金气氛。
- 调节流速为1毫升/分钟。保持整个切片潜伏期和实验这个流速。启动carbogenating新配制的1X学联沉浸流入管进入学联。允许20分钟的ACSF要与卡波金以及上腔室被填充有它的饱和。
3.准备急性海马脑片
注:解剖协议包括(1)除去大脑从动物到冷ACSF和(2)分离和海马的切片。为了让神经细胞保持活力,隔离和快速地将大脑在寒冷学联和完成整个过程包括在3-5分钟切片。
- 切除脑入冷的ACSF
- 铺陈清扫工具,可在图 1A中所示的方式。根据使用的为了便于解剖过程安排的工具。在开始之前,确保所有的清扫工具都准备好了。
- 安装一个刀片,用乙酸乙酯萃取,无水乙醇和蒸馏水清洗,放置到手动组织斩波器( 图1B),牢固地固定,并确保该切削刃被均匀地排列。试验斩波一张滤纸,以确保刀片牢固地固定。设置的滑动游标微米至其起始位置。
- 安乐死在利用动物的二氧化碳(CO 2)在感应腔和斩首用绷带剪刀 或断头台。使用虹膜剪,去掉皮和毛皮的头骨上面。做一个切口穿过后删除brainstem.Make沿个一个小切口Ë右侧颅骨和左侧较长的切口。
注意!使只有一个小的切口,以被用于实验,以避免损坏它的一侧。当插入剪刀,确保施加的力向上,以避免损坏到大脑。 - 小心地用一个骨咬骨钳从左边开始向右侧颅骨以显示皮质除去头骨。薄层硬膜也可见到。小心取出正面板的咬骨钳。除去大部分硬脑膜连同正面板上。
注意!要小心,硬脑膜不经过大脑组织切片。 - 如果任何除去残留的硬膜,特别是在用抹刀的平坦端皮质和小脑之间的交界处。对于步骤3.1.5和3.1.6,有保有压,即向上,远离大脑,避免损坏。用锅铲轻轻舀大脑进入一个培养皿中充满了寒冷和carbogenated学联(2-4℃),PLA土木工程署铝制散热块上。
- 海马隔离
- 用手术刀,使直切以除去小脑和另一个切以除去脑(约四分之一)的前部。使沿中线浅伤口。
- 小心地用镰刀洁牙机去除皮质,从中线开始显露背海马。除去皮质海马以上的层。用手指或镊子角度来支持大脑。做一个小切口,以海马连合。轻轻地用镰刀定标器使用滚动运动背海马开始取出海马。
注意!要轻柔,避免拉伸和撕裂海马。 - 卸下所有皮质和周围的隔离海马结缔组织与镰刀定标器。
- 切片海马组织和转移切片到接口室
- 将一块ACSF-的浸泡滤纸(1级,30mm)的在手动切片机切片阶段。舀并将海马组织到过滤纸上。移动滤纸对齐海马在相对 于限幅器的叶片的适当的取向,使得海马切片在约70度到伞的角度。
- 吸干,围绕着一个折叠滤纸的海马组织(85 mm粒级1)离开海马微湿多余的解决方案。启动横向切片海马。切片和丢弃的组织从海马的末端,其中所述片的形态是不明确的。
- 切片剩余组织成400微米厚的片。从与用温和刷卡动作软刷毛的刷子刮板拾取海马切片轻轻并将切片入一个小烧杯装满冷carbogenated ACSF。执行的步骤3.3.1-3.3.3尽快由于海马组织暴露于空气中。
注:通常三分之二的海马切片,和4-6片,用清晰的形态可制得。 - 转移片轻轻地放在网上堰板腔中使用干净的塑料巴斯德吸管具有宽尖端(通过切除2-3厘米的尖端的制造)。仔细调整片上用小注射器弯曲尖端的净位置。定位片以有利于电极的位置和记录的方式。检查,以确保片由ACSF充分包围但不被水淹没或浮动(图2C-D)。盖上室和孵化切片2-3小时。
注:在健康切片的锥体细胞层应该表现出一些透明度。
4.录音的CA3-CA1突触反应
注:电设置用于场势记录示于图2A。一法拉天笼强烈建议,如果电磁干扰超出电设置正确的接地后控制。是市售的许多不同类型的淹没和接口腔室。然而,接口室优选作为他们切片表现出更强大的突触反应。
- 电极定位
- 打开电气设备中使用(刺激剂和放大器)。安装并固定刺激和记录电极的显微的有机玻璃持有人。
注:我们使用单极,5MΩ电阻漆涂层,不锈钢焊条为刺激和录音的目的。 - 使用前,将拉玻璃毛细管内这些电极与环氧胶暴露的电极头( 图2E)的一小部分固定。这给力的,否则细长电极,并帮助确保它们的稳固的ELEctrode持有人。
- 在显微镜下的指导下,在海马CA1区域的辐射层放置刺激电极(多个)刺激谢弗侧支纤维和记录电极在CA1区的顶端树突区域来记录场EPSP(fEPSP)的反应。
注:接近液体表面与电极片上面给出了一个声音,有助于快速定位片(提供,放大器被连接到一个扬声器)的表面上。 - 在突触标记和捕获实验,根据实验的需要,在记录电极的任一侧的位置两个或三个刺激电极(S1,S2或S3)刺激两个或多个独立的,但重叠的输入。放置约200微米相距刺激和记录电极。
- 如果有必要,找到地层中的锥体层的另一个记录电极来记录人口峰值( 图3A)。当这两个电极已经触及切片,用采集软件,对测试的刺激,以确保正确fEPSP信号。
注意:我们使用双相,恒流脉冲(脉冲持续时间的0.1毫秒/半波),用于测试刺激。 - 一旦获得适当的fEPSP信号,小心地约200μm深使用操纵器的微动旋钮的电极。允许20分钟的片恢复。测试通路的独立性与配对的脉冲便利化的协议27,28。
- 打开电气设备中使用(刺激剂和放大器)。安装并固定刺激和记录电极的显微的有机玻璃持有人。
- 输入输出关系
- 通过测量在一定范围的电流强度的斜率值确定每个输入的输入 - 输出关系(传入刺激VS fEPSP斜率)。 20μA之间执行此为100μA。然后将刺激强度对于每个输入以获得最大的fEPSP斜率的40%。保持整个实验此常数。
- 后15-20分钟,开始记录基线。监视的FEPSP斜率密切在此期间和重置刺激强度如果斜率波动从设定值的10%以上,并开始一个新的基线。在继续之前得到至少30分钟或1小时稳定的基线。
注:对于测试或基线的刺激,我们用四次扫描的0.2赫兹双相,恒定电流脉冲(每个极性0.1毫秒)给予每5分钟。这四种反应的平均斜率则认为是一个重复。的信号进行滤波和放大的差分放大器,数字化使用模拟 - 数字转换器,并与定制软件在线监测。
- 使用刺激方案的LTP / LTD诱导
注:两个LTP和LTD被归类为早期和晚期-LTP / LTD基于蛋白质合成的要求;后者需要翻译和/或转录为它的后期维修[综述见4]。各种电刺激范式可以specificallŸ诱导不同形式的LTP和有限公司。- WTET:100赫兹,21双相恒定电流脉冲(每相0.2毫秒)。
- STET:三连发的100个脉冲一秒(100赫兹),每10分钟(每脉冲相位宽度0.2毫秒)。
- 900脉冲串在15分钟的持续时间。 1一阵由3个脉冲(0.2毫秒宽度)为50毫秒(20赫兹)的脉冲间隔。间脉冲串间隔是1秒(2700脉冲的总数)。
5.清洗切片商会和灌注系统
- 在记录结束后,收集海马做进一步生化分析,否则丢弃适当。关闭卡波电源和温度控制器。洗蒸馏水卡波起泡。
- 用刷子和蒸馏水彻底清洗净。用蒸馏水在一个较高的流速洗钻机15-20分钟。一旦在3-4天,更改蒸馏水腔室的下部隔室和也干净腔室经常用3%过氧化氢溶液,以避免真菌生长。
结果
所描述的方法已被用于研究的LTP / LTD及其缔相互作用持久形式如突触标记和横捕获从成年大鼠的急性海马切片。23这种技术已被证明是有效的与两个大鼠实验(只Wistar)和各种小鼠株30,31。该方法已经成功地用于高达8-12小时稳定的LTP的录音。32
"标签"由一个输入(S1)的弱强直电刺激设置捕获"前提方案"因另一独立,但重叠的输入强强直电刺激(S2; 图3B,实心圆 ),从而转化否则腐烂的LTP的形式(早期-ltp)在S1中成长效酮( 图3B中,空心圆)(对于早期-LTP诱导WTET比较见20,33)。由弱捕获的前提方案强直电刺激集标记不一定来自STET诱导的后期的LTP,但也可以由SLFS诱导后期LTD。提供的。这种类型的LTP和LTD之间正缔相互作用的被称为"交叉标记/捕获"。该WTET诱导早期LTP的S1被捕获在S2提供的SLFS诱导后期LTD。的前提方案( 图3C,实心圆)加强后期-LTP( 图3C,空心圆)。统计学显著增强或抑郁保持在S1和S2在这两种情况下,当相对于它自己的基线(Wilcoxon检验; P <0.05)。
对于发生的标签PRP互动,这两个事件的时间顺序(弱前强/强前弱),是不是只要关键,因为这两个事件之间的时间窗口保持在30-60的范围内分钟。这将是明智的,包括在第三,独立的,但重叠的突触放,并使用它作为基准控制来监视记录的稳定性。用于诱导长时程增强/ LTD早期和晚期形式的电刺激的协议必须在单输入实验的一致性和可靠性,将它们用在STC实验前进行验证。我们还要强调在协议中所述切片准备方法的重要性,因为这些实验的成功在很大程度上依赖于片的质量。
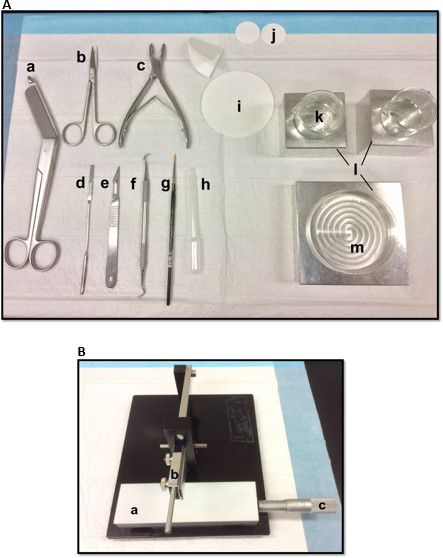
在海马的解剖用图1(A)工具:(一)绷带剪刀 (二)虹膜剪刀(三)骨咬骨钳(D)薄锅铲,(五)手术刀11号(六)镰定标器(G)软-bristle油漆刷(H)塑料巴斯德吸管(一)滤纸(85毫米)(J)滤纸(30毫米)(k)的玻璃烧杯(L)铝散热模块,以适应培养皿和烧杯(M)培养皿中。(B)手动组织菜刀。 (一)平台(b)切割臂刀座(C)游标千分尺,分辨率为10微米。 请点击此处查看该图的放大版本。
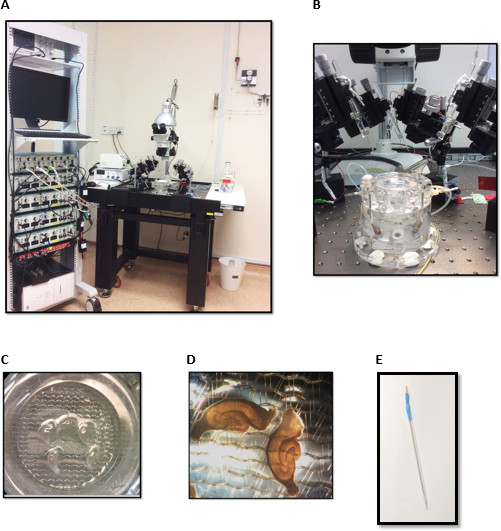
图2.电设置为场电位记录组成的(A)的刺激(b)一种差分放大器(c)一种模拟-数字转换器(D)示波器(五)计算机与采集软件(F)无振动耐台式(G)显微镜>放大4倍(H)接口脑切片室(i)就学联和卡波供应(J)温度控制器(K)灌注系统的照明光源(L)机器人与电极持有人。 (B)的界面脑切片室。(C)(D)的界面室海马切片的玻璃毛细管密封。(E)不锈钢焊条。 请点击此处查看该图的放大版本。
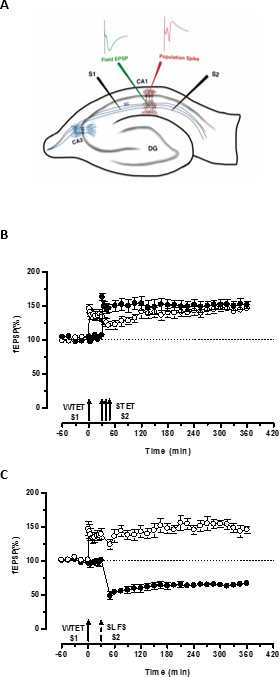
图3.(A)中为场电位记录横向海马切片和电极位置的示意图:在这种表示,2刺激电极(S1和S2)定位在CA1区的辐射层,以刺激有两个独立但重叠突触投入到CA1锥体神经元。两外记录电极,一个从顶端树突隔室录制场EPSP(兴奋性突触后电位),另一个来自锥体细胞记录体细胞群体峰机构,分别位于辐射层与层锥体。 CA1- CORNU ammonis区域1,CA3-大角ammonis区域3,DG-齿状回,SC-谢弗侧支纤维,强大的范式来研究STC前S1-刺激电极1,S2刺激电极2(B)弱:弱强直电刺激(WTET)施加到S1(空心圆)用于诱导早期-LTP接着S2的强强直电刺激(STET)(实心圆)在30分钟时以诱导后期LTP。早期-LTP在S1得到加强,后期LTP显示标记和捕捉交互(N = 6),(C)强孰弱范式前研究跨标签:早期LTP由WTET在S1(空心圆),随后引起通过使用SLFS 30分钟后诱导的后期LTD。在S2中(实心圆)。在S1中,早期LTP转化为后期LTP持续6小时呈现交叉标记和捕捉(N = 6)。单箭头表示适用于诱导早期LTP弱强直电刺激。箭头的三重代表强大的强直电刺激诱导后期LTP。虚线箭头表示在该SLFS施加到代表突触输入以诱导后期LTD。的时间点。误差线表示SEM。 请点击此处查看该图的放大版本。
讨论
急性海马切片是LTP和其他功能的可塑性过程,如STC和交叉采集的研究一个很好的模型系统。它保留了大部分海马电路的层状结构的网络,可以精确电极的位置,并提供沿着,开放平台的快速神经药理学操作无血 - 脑屏障。
本文介绍了从年轻的成年大鼠编制可行的急性海马切片的方法,并利用它们来研究STC和交叉标记。先前的研究已经强调性别的动物和年龄是考虑在电生理研究中使用的重要因素。27,28因此,年轻的成年动物的完全表示成年受体功能(年龄在5-7周雄性Wistar大鼠)被使用。23不对称在左,右海马之间的连接已注意到在啮齿类29和在NMDA受体表达的主要区别有报道以及34。我们已经使用了权利海马,以便与我们以前的LTP研究一致。23,32然而,无论是海马可以只要一致性被保持被使用。
正如在任何协议,这是非常重要的执行隔离和快速切割程序,但照顾该组织没有被拉伸,损坏,呈现干或缺氧。在pH,温度和溶液的离子组成的变化可以对切片和结果的生存能力深远的影响。因此应该避免这样的变化。已经观察到,在准备步骤发生谷氨酸受体依赖性钙释放可以不可逆地影响蛋白质合成的神经组织35,36,37。使用手动组织切片机可以帮助通过允许过程中尽量减少这是非常快速地完成相比,六braslicers。然而,许多实验室也有效地使用vibraslicers有必要的预防措施,以保护片的可行性。要考虑的另一个重要因素是在开始实验前的长潜伏期。这已被注意到是真关键实现稳定在后扰动制备23中产生的切片代谢状态和激酶活化的水平。这种稳定是必要的长期记录一致性。我们这一观察再次强调,并提出约3小时的潜伏期长小时。
多种刺激参数被已知诱导LTP的,但引起在每种情况下的分子机制可能不是相同的(综述见38)。这会影响耐用性和其他特性的LTP,反过来,可影响突触标记和捕获实验的结果。因此,它验证的刺激模式和特点是非常重要的被诱发的LTP下进行实验室的条件和保持一致性。
我们一般不考虑实验中具有非常大的突触前纤维截击和最大fEPSPs小于0.5毫伏,涉及在纤维凌空实质性的变化过程中的录音也拒绝了实验。另外,虽然进行了两通路或三途径的实验中,以确保该途径独立性是重要的。此,可以进行与配对的脉冲易协议28。
接口记录系统的一个缺点是在电极上结露的液滴的过程中,由于腔室和周围环境之间的温度和湿度的差异长记录小时的形成。这些液滴需要仔细印迹不时。否则液滴滴到切片和引起干扰甚至丧失的信号。我们通常解决这家BŸ巧妙地印迹用纤细的滤纸灯芯在显微镜下引导液滴,不接触电极。然而,最好的解决办法是使用集中式加热系统,诸如由爱丁堡大学的研究人员开发了ETC系统。
在总结笔记,存在于世界各地的用于海马针对不同的实验目的,编制实验室多种方法的。每个程序的提供一些优于其它。人们需要仔细优化了协议的微小细节,以适应实验的目的。我们希望这篇文章有助于改善方法的某些方面进行研究后期联想过程,如STC和交叉捕获。
披露声明
此视频文章开放获取是由食益补太平洋有限公司赞助。
致谢
This video article is sponsored by Cerebos Pacific Limited. This work is supported by National Medical Research Council Collaborative Research Grant (NMRC-CBRG-0041/2013) and Ministry of Education Academic Research Funding (MOE AcRF- Tier 1 - T1-2012 Oct -02).
材料
| Name | Company | Catalog Number | Comments |
| I. Dissection Tools | |||
| 1. Bandage scissors | KLS Martin, Germany | 21-195-23-07 | |
| B-Braun/Aesculap, Germany | LX553R | ||
| 2. Iris scissors | B-Braun/Aesculap, Germany | BC140R, | |
| BC100R | |||
| 3. Bone rongeur | World Precision Instruments (WPI), Germany | 14089-G | |
| 4. Scalpel | World Precision Instruments (WPI), Germany; | 500236-G | |
| B-Braun/Aesculap, Germany | |||
| BB73 | |||
| 5. Scalpel blade#11 | B-Braun/Aesculap, Germany | BB511 | |
| 6. Sickle scaler | KLS Martin, Germany | 38-685-00 | |
| 7. Angled forceps | B-Braun/Aesculap, Germany | BD321R | |
| 8. Anesthetizing/Induction chamber | MIP Anesthesia Technologies (Now, Patterson Scientific), Oregon | AS-01-0530-LG | |
| II. ACSF component chemicals | |||
| 1. Sodium chloride (NaCl) | Sigma-Aldrich | S5886 | |
| 2. Potassium chloride (KCl) | Sigma-Aldrich | P9541 | |
| 3. Magnesium sulphate heptahydrate (MgSO4.7H20) | Sigma-Aldrich | M1880 | |
| 4. Calcium chloride dihydrate (CaCl2.2H2O) | Sigma-Aldrich | C3881 | |
| 5. Potassium phosphate monobasic (KH2PO4) | Sigma-Aldrich | P9791 | |
| 6. Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | S5761 | |
| 7. D-Glucose anhydrous (C6H12O6) | Sigma-Aldrich | G7021 | |
| III. Electrophysiology Instruments | |||
| 1. Microscope | Olympus, Japan | Model SZ61 | |
| 2. Temperature Controller | Scientific Systems Design Inc. Canada | PTC03 | |
| 3. Differential AC Amplifier | AM Systems, USA | Model 1700 | |
| 4. Isolated Pulse Stimulator | AM Systems, USA | Model 2100 | |
| 5. Oscilloscope | Rhode & Schwarz | HM0722 | |
| 6. Digital-Analog Converter | Cambridge Electronic Design Ltd. Cambridge, UK | CED-Power 1401-3 | |
| 7. Interface Brain Slice Chamber | Scientific Systems Design Inc. Canada | BSC01 | |
| 8. Tubing Pump | Ismatec, Idex Health & Science, Germany | REGLO-Analog | |
| 9. Carbogen Flowmeter | Cole-Parmer | 03220-44 | |
| 10. Fiber Light Illuminator | Dolan-Jenner Industries | Fiber Lite MI-150 | |
| 11. Micromanipulators | Marzhauser Wetzlar, Germany | 00-42-101-0000 (MM-33) | |
| 00-42-102-0000 (MM-32) |
参考文献
- Bliss, T. V. P., Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361, 31-39 (1993).
- Siegelbaum, S. A., Kandel, E. R. Learning-related synaptic plasticity: LTP and LTD.. Current Opinion in Neurobiology. 1, 113-120 (1991).
- Martin, S. J., Grimwood, P. D., Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annual review of neuroscience. 23, 649-711 (2000).
- Malenka, R. C., Bear, M. F. LTP and LTD: An Embarrassment of Riches. Neuron. 44, 5-21 (2004).
- Krug, M., Lossner, B., Ott, T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain research bulletin. 13, 39-42 (1984).
- Frey, U., Krug, M., Reymann, K. G., Matthies, H. Anisomycin blocks an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain research. 452, 57-65 (1988).
- Otani, S., Marshall, C. J., Tate, W. P., Goddard, G. V., Abraham, W. C. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience. 28, 519-526 (1989).
- Frey, U., Frey, S., Schollmeier, F., Krug, M. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. The Journal of physiology. 490 (3), 703-711 (1996).
- Nguyen, P. V., Kandel, E. R. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices). The Journal of neuroscience : the official journal of the Society for Neuroscience. 16, 3189-3198 (1996).
- Manahan-Vaughan, D., Kulla, A., Frey, J. U. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 20, 8572-8576 (2000).
- Frey, U., Morris, R. G. M. Synaptic tagging and long-term potentiation. Nature. 385, 533-536 (1997).
- Frey, U., Morris, R. G. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 37, 545-552 (1998).
- Redondo, R. L., Morris, R. G. Making memories last: the synaptic tagging and capture hypothesis. Nature reviews. Neuroscience. 12, 17-30 (2011).
- Martin, K. C., Kosik, K. S. Synaptic tagging -- who's it?. Nature reviews. Neuroscience. 3, 813-820 (2002).
- Frey, U., Morris, R. G. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends in neurosciences. 21, 181-188 (1998).
- Martin, K. C. Synaptic tagging during synapse-specific long-term facilitation of Aplysia sensory-motor neurons. Neurobiology of learning and memory. 78, 489-497 (2002).
- Shires, K. L., Da Silva, B. M., Hawthorne, J. P., Morris, R. G., Martin, S. J. Synaptic tagging and capture in the living rat. Nature communications. 3, (2012).
- Ramachandran, B., Frey, J. U. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29, 12167-12173 (2009).
- Sajikumar, S., Navakkode, S., Frey, J. U. Identification of compartment- and process-specific molecules required for 'synaptic tagging' during long-term potentiation and long-term depression in hippocampal CA1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 27, 5068-5080 (2007).
- Sajikumar, S., Navakkode, S., Sacktor, T. C., Frey, J. U. Synaptic tagging and cross-tagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 25, 5750-5756 (2005).
- Redondo, R. L., et al. Synaptic Tagging and Capture: Differential Role of Distinct Calcium/Calmodulin Kinases in Protein Synthesis-Dependent Long-Term Potentiation. The Journal of Neuroscience. 30, 4981-4989 (2010).
- Sajikumar, S., Frey, J. U. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiology of learning and memory. 82, 12-25 (2004).
- Sajikumar, S., Navakkode, S., Frey, J. U. Protein synthesis-dependent long-term functional plasticity: methods and techniques. Current Opinion in Neurobiology. 15, 607-613 (2005).
- Villers, A., Ris, L. Improved preparation and preservation of hippocampal mouse slices for a very stable and reproducible recording of long-term potentiation. Journal of visualized experiments : JoVE. , (2013).
- Pohle, W., Reymann, K., Jork, R., Malisch, R. The influence of experimental conditions on the morphological preservation of hippocampal slices in vitro. Biomedica biochimica acta. 45, 1145-1152 (1985).
- Reymann, K. G., et al. The duration of long-term potentiation in the CA1 region of the hippocampal slice preparation. Brain research bulletin. 15, 249-255 (1985).
- Sajikumar, S., Korte, M. Different compartments of apical CA1 dendrites have different plasticity thresholds for expressing synaptic tagging and capture. Learning & Memory. 18, 327-331 (2011).
- Li, Q., et al. Making Synapses Strong: Metaplasticity Prolongs Associativity of Long-Term Memory by Switching Synaptic Tag Mechanisms. Cerebral Cortex. 24, 353-363 (2014).
- Sajikumar, S., Frey, J. U. Anisomycin inhibits the late maintenance of long-term depression in rat hippocampal slices in vitro. Neuroscience Letters. 338, 147-150 (2003).
- Ishikawa, Y., Tamura, H., Shiosaka, S. Diversity of neuropsin (KLK8)-dependent synaptic associativity in the hippocampal pyramidal neuron. The Journal of physiology. 589, 3559-3573 (2011).
- Ishikawa, Y., Horii, Y., Tamura, H., Shiosaka, S. Neuropsin (KLK8)-dependent and -independent synaptic tagging in the Schaffer-collateral pathway of mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 28, 843-849 (2008).
- Sajikumar, S., Morris, R. G. M., Korte, M. Competition between recently potentiated synaptic inputs reveals a winner-take-all phase of synaptic tagging and capture. Proceedings of the National Academy of Sciences. 111, 12217-12221 (2014).
- Navakkode, S., Sajikumar, S., Frey, J. U. The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 24, 7740-7744 (2004).
- Kohl, M. M., et al. Hemisphere-specific optogenetic stimulation reveals left-right asymmetry of hippocampal plasticity. Nature. 14, 1413-1415 (2011).
- Frey, U., Huang, Y. Y., Kandel, E. R. Effects of cAMP Simulate a Late Stage of LTP in Hippocampal CA1 Neurons. Science. 260, 1661-1664 (1993).
- Erdogdu, G., Uto, A., Hossmann, K. -. A. The effect of global ischemia and recirculation of rat brain on protein synthesis in vitro. Metab Brain Dis. 8, 199-206 (1993).
- Djuricic, B., Berger, R., Paschen, W. Protein synthesis and energy metabolism in hippocampal slices during extended (24 hours) recovery following different periods of ischemia. Metab Brain Dis. 9, 377-389 (1994).
- Park, P., et al. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philosophical Transactions of the Royal Society B: Biological Sciences. 369, 20130131 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。