需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
在压缩的胶原组织糜:含细胞Biotransplant单上演重建修复
摘要
组织工程经常包括在体外扩增 ,以创建用于组织再生自体移植。在这项研究中用于组织扩张,再生, 和体内重建的方法,以便最小化在体外的细胞和生物材料的处理被开发。
摘要
Conventional techniques for cell expansion and transplantation of autologous cells for tissue engineering purposes can take place in specially equipped human cell culture facilities. These methods include isolation of cells in single cell suspension and several laborious and time-consuming events before transplantation back to the patient. Previous studies suggest that the body itself could be used as a bioreactor for cell expansion and regeneration of tissue in order to minimize ex vivo manipulations of tissues and cells before transplanting to the patient. The aim of this study was to demonstrate a method for tissue harvesting, isolation of continuous epithelium, mincing of the epithelium into small pieces and incorporating them into a three-layered biomaterial. The three-layered biomaterial then served as a delivery vehicle, to allow surgical handling, exchange of nutrition across the transplant, and a controlled degradation. The biomaterial consisted of two outer layers of collagen and a core of a mechanically stable and slowly degradable polymer. The minced epithelium was incorporated into one of the collagen layers before transplantation. By mincing the epithelial tissue into small pieces, the pieces could be spread and thereby the propagation of cells was stimulated. After the initial take of the transplants, cell expansion and reorganization would take place and extracellular matrix mature to allow ingrowth of capillaries and nerves and further maturation of the extracellular matrix. The technique minimizes ex vivo manipulations and allow cell harvesting, preparation of autograft, and transplantation to the patient as a simple one-stage intervention. In the future, tissue expansion could be initiated around a 3D mold inside the body itself, according to the specific needs of the patient. Additionally, the technique could be performed in an ordinary surgical setting without the need for sophisticated cell culturing facilities.
引言
上移植到皮肤和泌尿生殖道最组织工程研究包括来自于专门配备细胞培养设施1,2-健康组织和细胞扩张的自体细胞的收成。
细胞扩增后,将细胞通常存储供以后使用时制备的患者以接收自体移植。氮冷冻允许在-150℃或更低的低温长期贮存。冷冻的过程中一定要小心,为了不失去控制的细胞。细胞死亡的一个危险是在解冻过程中,这可导致细胞膜的破裂的细胞内的水的结晶。细胞冷冻通常通过缓慢和受控冷却(-1℃,每分钟)进行的,使用的细胞,胎牛血清和二甲亚砜的浓度高。解冻后,将细胞需要通过除去冷冻培养基并培养细胞培养塑料或再次处理移植前生物材料回给患者。
所有上述步骤是费时,费力,且昂贵3。此外,针对患者的移植细胞全部排出体外处理是高度管制的,需要训练有素和认可的人员和实验室4。总而言之,促使安全和可靠的制造工艺,这项技术只有在极少数技术先进中心成立,并在常见的外科疾病更广泛的使用是值得怀疑的。
为了克服在实验室环境的细胞培养的局限性,移栽组织糜用于体内细胞扩增的概念是通过使用体本身作为生物反应器引入的。为了这些目的,在自体移植将优先被上一个3D模具按照所需要对i的器官的最终重建的形状移植nterest 5-7。
原来,移栽剁碎上皮细胞的想法,在1958年提出由温顺,当他描述的上皮细胞从伤口的边缘如何生长。他表明,一小片皮肤的将增加其利润率并且由此其为细胞扩增潜力100%通过切割片两次在垂直的方向(图1)8。理论已被使用啮合部分厚度皮肤移植的皮肤移植9支撑 ,并在皮肤伤口愈合模型10。
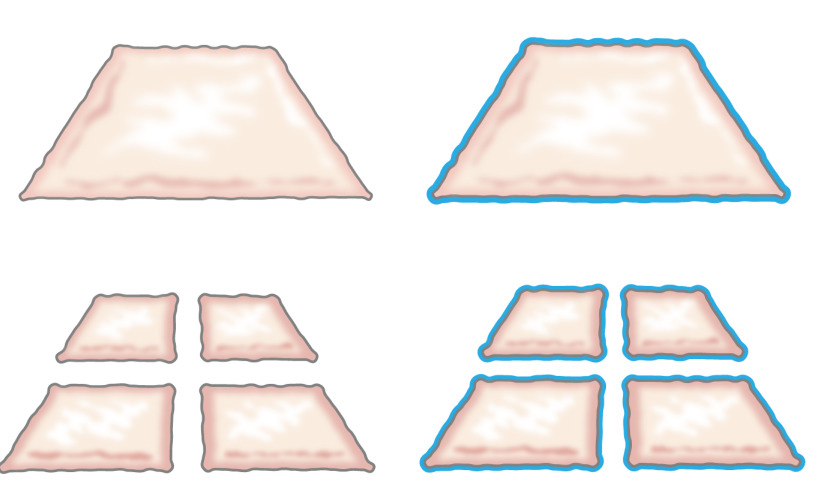
图1:米克理论根据温顺的理论,上皮生长从伤口的边缘。通过增加切碎技术的曝光区域,组织糜处上皮许多景点的伤口。
本研究是基于低论文,相同的原理可以通过将剁碎上皮围绕模具施加到皮下组织。上皮细胞会从剁碎移植(重组),以便形成连续neoepithelium从内部主体覆盖伤口区域,并分离异物(模具)动员,覆盖伤口区域(迁移)和除(展开)( 图2)。
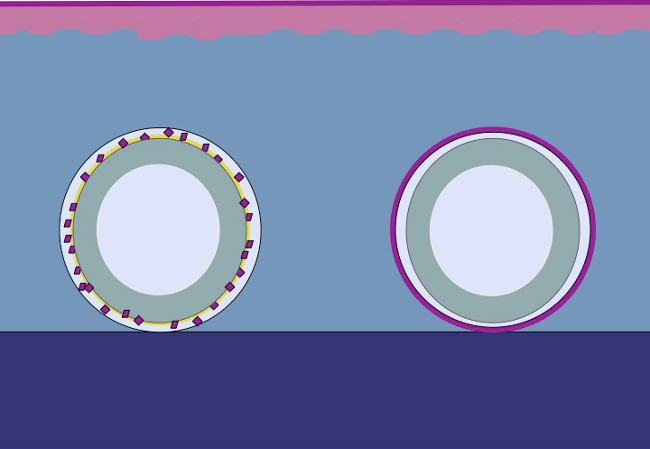
图2:根据米克理论三维模具剁碎上皮用于体内intracorporal组织扩张的卡通通过使用放置在模具中,然后移植到皮下组织组织糜,所述假设是,上皮细胞从迁移组织糜的边缘,重组,和扩展,以便形成一个连续的neoepithelium覆盖伤口区域和异物(模具)从内体中分离。
尽管以前的体内研究表明可喜的成果,进一步改善可以通过加强自体移植来实现,使再生上皮细胞可以抵御机械损伤更好7。为了这些目的,一个成功的生物材料的重要先决条件被确定,如:容易扩散营养素和废物的,可能性模具在三维方式和手术操作容易。结论所做的这些需要可以通过添加的复合生物材料的组织糜得到满足。
目前的研究旨在开发一种在组织糜构成的支架塑性压缩胶原含有可生物降解的织物的增强芯。通过这些手段,活细胞能够从组织糜粒子迁移,并与原来的上皮细胞(皮肤或上皮)的形态特征特性增殖。使用塑料压缩,脚手架是减少d在1厘米大小至约420微米的切碎的颗粒在上层胶原进行了包裹。该芯部织物可以是任何聚合物,但需要用亲水表面修饰,以便与覆盖胶原层 11相互连接起来。
该方法通过使用其作为支架用于培养切碎膀胱粘膜或猪碎肉皮肤掺入两个塑料压缩胶原凝胶内的编织网由聚(ε - 己内酯)(PCL)提供了一种增强的支架的完整性。该构建物维持在细胞培养条件为长达6周的体外 ,证明成功地形成一个良好的固结杂交构建体的顶部分层,多层上皮或鳞状皮肤上皮。该构建是易于处理和来代替膀胱增加目的或皮肤缺陷的覆盖物被缝合。组织支架的所有部件都FDA批准和技术可以通过组织收获,切碎,塑性压缩,并移栽回病人作为一个单一阶干预可用于单级的程序。该程序可以在任何普通外科单位在无菌条件下组织扩张和重建进行。
研究方案
所有动物方案都预先核准由斯德哥尔摩县委员会关于动物和所有程序符合动物用,以及相关的联邦法规的规定。
1.动物规程
- 准备手术的动物
- 与所需的无菌条件下的操作的所有材料和仪器制备手术台。只进行手术无菌条件下,以减少感染的风险,并优化在存活手术的条件。
- 禁食12小时动物手术前并测量它的重量。管理二甲苯胺噻嗪的对术前用药肌内注射(2毫克/千克)。麻醉与替来他明次氯酸钠(2.5毫克/千克),唑拉西泮次氯酸钠(2.5毫克/千克),美托咪定(25微克/千克)和阿托品(25微克/千克)肌内注射动物。
- 注入phenobarbiturate(15毫克/千克),以endotrache前人插管,并继续与0.8%-2%的异氟醚全身麻醉。插入外周静脉导管一只耳朵和静脉注射的过程中注入葡萄糖(25毫克/毫升),以保持动物的福祉。
- 放置监测在耳朵上或尾部设备检查体温,血压,饱和度,和外周脉搏。由耳或蹄的疼痛刺激控制麻醉。适用软膏眼睛,防止干燥时的麻醉下。
- 膀胱活检标本切除术
- 使用10法国硅氧烷导管通过引入窥器查看尿道和通过尿道插入导管并进入膀胱清空半无菌的条件下尿导尿膀胱。填用无菌生理盐水溶液膀胱20-25厘米的 H 2 0,约100-300毫升的压力,然后空至20厘米的 H 2 O(约8毫升/公斤体重)。
- 随着日Ë猪仔细转向侧的位置,与葡萄糖酸氯己定的应用程序执行皮肤的基本术前消毒。与剃剪去掉皮毛后,应用一个透热板的肩膀。
- 小心地将猪成仰卧位并删除使用剃剪腹毛色和做葡萄糖酸氯己定应用腹部皮肤的基本术前消毒。
- 软服装嵌入四肢以最小化过伸损坏在四肢关节的风险。消毒用葡萄糖酸洗必泰的连续应用动物的腹部皮肤,并放置无菌披盖周围的手术野。
- 之前切开皮肤,应用静脉镇痛在脐下中线选自丁丙诺啡(45微克/千克),卡洛芬(3毫克/千克)和利多卡因的局部注射。抓住SK检查疼痛反应在用钳子。通过使筋膜较低的正中切口,用透热为控制出血腹膜。本地化具有在猪完全腹膜内位置,并且可以通过手术伤口动员它被自由地暴露膀胱。
- 以保持与镊子膀胱,并用消毒卷尺测量,并标志着一个椭圆形活检标本纵向约两厘米见方,1厘米横向,或更小,使用杀菌笔(猪容忍膀胱大小的四分之一减少很好)。切除标记的区域,使用解剖刀,并放置在DMEM膀胱活检标本在无菌条件下。
- 与biotransplant执行自体移植或两层用5-0薇乔缝合它关闭膀胱。仔细2-0或3-0薇乔运行关闭腹部筋膜。关闭用3-0薇乔皮下组织和3-0 Ethilon皮肤。将包扎伤口,并仔细往往给动物,直到它已经从麻醉充分恢复和不表达的疼痛。
- 移动动物的动物护理设施,以确保对术后护理情况。把动物在一个笼子里有取暖灯和照顾动物,直到从麻醉中完全恢复,然后让动物成对停滞。
- 提供恢复顺利关于疼痛和福祉和日常管理丁丙诺啡(45微克/千克)肌注术后镇痛和甲氧苄氨嘧啶(4毫克/千克)和磺酰胺(20毫克/千克)的两倍了三天,每天一次五天以减少术后感染的风险。
- 皮肤活检标本切除术
- 与所有必要的材料准备手术台。麻醉如先前在1.1中描述的动物。用蜡取出皮毛,洗净,用聚维酮碘和70%的酒精消毒切口区域,然后将消毒铺巾不要蜘蛛d是切口区域。
- 使用皮收获为0.3毫米的厚度局部皮肤活检标本。放在DMEM皮肤样品切碎之前,如2.2所述。覆盖脂肪软膏和包扎伤口面积。
2.组织糜准备
- 膀胱粘膜
- 在DMEM洗膀胱穿刺活检的两倍。膀胱活检标本放置到朝上粘膜灭菌解剖板并用解剖针固定侧之一到板上。
- 分开使用细剪刀和镊子(图3)逼尿肌粘膜组织并保持粘膜通过滴盐水或DMEM在它湿润。
- 通过将其上的粘膜用切碎设备,然后从一端通过设备到另一垂直和水平,施加人工压力,以获得0.8毫米×0.8毫米切碎组织片(1.3毫米是rotatin之间的距离摹切割刀片)。
- 皮肤
- 如果皮肤biopsis厚:皮肤放置到无菌解剖板,并使用手术剪刀从皮下脂肪及真皮表皮分离。表皮较薄和半透明(约0.3 MM),当它准备好切碎。
- 通过将其上的表皮使用切碎设备。压力,从一端通过设备到另一垂直和水平,以获得0.8毫米×0.8毫米组织糜的碎片。
3.塑料压缩PCL /胶原自体移植的制备
- 广场上冰的所有成分,保持冷。所需用具:猎鹰管,10X DMEM,1X DMEM,1N NaOH和鼠尾型胶原1。
- 混合料2毫升10×DMEM(小心,以避免气泡)的12型胶原毫升1.添加1N的NaOH,逐滴,使pH值达到7.4-8(在介质中颜色应可通过从激烈改变显示pH黄引脚K)。此外,使用pH条。
- 小心加2ml 1X DMEM和混合解决方案。板约2毫升的钢制矩形模具(20x30x10毫米)的各孔的胶原蛋白,并在37孵化 °下在5%CO 2的10分钟。
注:胶原蛋白的浓度应为2.06毫克/毫升在0.6%乙酸和胶原以1ml /厘米2的量。 - 一旦胶原设置到模具,放置在胶原凝胶(20毫米×30mm)的顶部上的生物材料(PCL)和倒在它上面的其余胶原(约6 ml)中。孵育在37℃下在5%CO 2 20分钟。
- 放置组织糜(对于1:6扩展)对胶原凝胶的顶部。采用塑性压缩如下通过机械力按水从构建体(图3和4)。
- 地方纱布上厚厚一层无菌表面。放置一个不锈钢筛网(400微米厚)上gauz顶部Ë垫,然后尼龙网(110微米厚)的片材。小心地将胶原凝胶/肉末组织转移到尼龙网,小心地取出矩形模具钢。
- 放置尼龙网的一个新的层上的胶原凝胶/组织糜的顶部。放在尼龙网顶部上的第二钢丝网。在适当的位置放置压力或装载板称重最低为120克( 即 ,玻璃板)5分钟。
- 除去重量,尼龙和钢网格。自体移植的现在准备缝合到猪膀胱中的全层皮肤伤口或体外培养。
- 对于体外培养,切细构建成小片接头12孔板中。加入1 ml角质细胞培养基。放置在孵化所述板在37℃,5%CO 2和培养长达6周和改变介质每周3次。
4.自体移植的缝合
- 自体缝合剁碎膀胱黏膜的猪膀胱
- 保持自体的DMEM湿润时的等待时间。缝合用细单丝运行的缝线自体移植。使用非吸收5-0 Ethilon用于研究目的。
- 检查,如果通过经由留置导尿管填充盐水膀胱不漏水。如果可能的话,请用大网膜一层的自体移植。如1.2.6所述关闭腹壁,皮下组织和皮肤。应用伤口敷料。
- 用剁碎的皮肤表皮自体移植的缝合全层伤口
- 保持自体的DMEM湿润时的等待时间。
- 缝合移植到由在弯道间断缝合,并在自体移植的中间卷绕保持紧密地附着在底层表面上的自体移植的皮肤全层的底部。
- 盖上塑料敷料,保持伤口湿润的伤口。
5。终止
- 稳重与唑拉西泮次氯酸钠肌肉注射(2.5毫克/千克)和美托咪定(25微克/千克)之前终止,并应用监控设备的耳朵或尾巴动物检查脉搏和血压。
- 通过给予戊巴比妥钠(60〜140毫克/千克)静脉注射了致命剂量的安乐死的动物。检查脉搏和血压,直到发生死亡。
6.在 体外培养
注:评价组织学在体外在PCL /胶原构建体的组织糜的进展,胶原/ PCL /剁碎贴剂使用角化细胞培养基的12孔板中培养。
- 角质形成细胞培养基的制备:
- 消毒的500毫升玻璃瓶中。
- 混合400毫升DMEM中,用100ml含的F12(4:1的混合物)。用10%胎牛血清补充,5微克/毫升胰岛素,0.4微克/ ml氢化可的松,21微克/毫升腺嘌呤,10 -10 mol / L的霍乱毒素,2×10 -9 mol / L的碘甲状腺原氨酸,5微克/毫升转铁蛋白,10ng / ml的表皮生长因子,50U / ml青霉素和50μg/ ml链霉素。
- 通过一个0.2微米的过滤器过滤灭菌,并收集滤液在无菌一瓶500毫升。
7.免疫组化
注:免疫组化协议一般分为以下步骤:(1)固定,石蜡包埋,(2)的微切片至5μm切片,在载玻片上,deparaffination和补液安置,(3)抗原修复,染色和安装。在启动免疫过程中的最后步骤之前,准备清洗缓冲区和抗原修复液(参见单独的材料详细说明)。使用前准备ABC复杂的解决方案至少30分钟。
- 固定术
没有TE:在体外培养的结束,修复补丁程序如下:- 准备Eppendorf管1毫升4%的缓冲甲醛(PFA)的(注意:甲醛是有毒的,请与本化学品之前,请先阅读材料安全数据表戴上手套和防护眼镜,并准备在通风橱内的溶液)。
- 传送每个胶原补丁含有4%PFA的Eppendorf管中。在室温下固定过夜。
- 在70%的乙醇代替样品长期储存在4℃。样品现已准备好脱水和切片之前石蜡包埋块。
- 补液
- 放置载玻片在以X-TRA SOLV 15分钟染色罐子。通过使用X-TRA SOLV一个新的染色缸重复。放置载玻片与10分钟无水乙醇染色缸。通过使用无水乙醇新的染色缸重复。放置在用95%乙醇的染色缸幻灯片10分钟,此后与70%乙醇的染色缸10分钟。最后洗载玻片两次5分钟,用蒸馏水。
- 抗原修复
- 放幻灯片的科普林缸用TE溶液,并把罐子在水浴中煮沸20分钟。就拿瓶子经过严格水浴。冷却该滑动到室温30分钟,并在Tris缓冲液5分钟洗涤两次。放置幻灯片中,用3%的过氧化氢为10分钟的染色缸。洗载玻片两次在Tris缓冲液中5分钟。绘制使用防水记号笔样品一圈。
- 方框使用100-300微升的封闭溶液的抗体的非特异性结合。除去封闭溶液,并添加100-300微升溶解于Tris缓冲液中推荐的浓度初级抗体。孵育过夜。除去抗体溶液,并在Tris缓冲液洗部分两次5分钟。
- 与二级抗体孵育在室温TEMPERAT 1小时URE。在Tris缓冲液5分钟洗涤两次。孵育使用ABC精英套件(按照制造商的说明)30分钟。在Tris缓冲液洗两次。
- 发展,通过使用矢量贵宾套件,按照制造商的说明(1-7分钟温育通常产生明显的紫色强度)抗体反应。把载片于蒸馏水。染液用Mayer氏苏木30秒。
- 洗5分钟自来水。放置幻灯片中,用70%乙醇的染色缸1分钟。通过使用具有70%的乙醇新染色缸重复。放置载玻片在用95%乙醇的染色缸1分钟。通过使用具有95%的乙醇新染色缸重复。
- 放置在以X-TRA SOLV一个染色缸载玻片5分钟。除去,一次一个,以保持湿润。放置在每张幻灯片的顶部安装介质下降,并把顶部玻璃盖(请小心操作,避免气泡)。让幻灯片干通宵,并查看微下滑梯范围。
结果
本研究提出,显示了如何使用胶原蛋白和组织糜的塑料压缩生产用于移植的生物材料的方法。
膀胱粘膜和皮肤可以收获并然后机械剁碎成小颗粒( 图3)。通过塑性压缩,碎粒子一个居中设置可生物降解的聚合物,胶原凝胶(图4)的外层内机械强度高的组成的复合支架内并入本文。组织糜颗粒可以被分离,以允许一个1:6膨胀率。通过压出胶原的水含?...
讨论
这项研究提出了一种易于使用的方法,以产生具有用于移植自体组织膀胱壁修补在手术台。贴片通过在中间和胶原有和没有外表面与塑性压缩组合组织糜可生物降解的聚合物针织的组合形成。塑性压缩是以前由其他作者描述的方法,并且可以被定义为从胶原凝胶12,13流体的迅速排出。膀胱粘膜或皮肤的组织糜接种到该支架和膀胱或皮肤上皮的形成可能在6周被遵循。免疫组织化学分析表明?...
披露声明
The authors have nothing to disclose.
致谢
The authors thank the Swedish Society for Medical Research, the Promobilia Foundation, the Crown Princess Lovisa Foundation, the Freemason Foundation for Children’s Welfare, the Swedish Society of Medicine, the Solstickan Foundation, Karolinska Institutet, and the Stockholm City Council for financial support.
材料
| Name | Company | Catalog Number | Comments |
| Silicone catheter 10-French | Preparing the animal for surgery, Section 1 | ||
| DMEM 10x | Gibco | 31885-023 | Plastic compression section 4 |
| 24 well plates | Falcon | 08-772-1 | Plastic compression section 4 |
| 3',3',5-Triiodothyronine | Sigma-Aldrich | IRMM469 | In vitro culture; Section 5 |
| 4% PFA | Labmed Solutions | 200-001-8 | Immunocytochemistry; Section 6 |
| 70% ethanol | Histolab | Immunocytochemistry; Section 6 | |
| ABC Elite kit: Biotin-Streptavidin detection kit | Vector | PK6102 | Immunocytochemistry; Section 6 |
| Absolute ethanol | Histolab | 1399.01 | Immunocytochemistry; Section 6 |
| Adenine | Sigma-Aldrich | A8626 | In vitro culture; Section 5 |
| Atropine 25 μg/kg | Temgesic, RB Pharmaceuticals, Great Britain | Preparing the animal for surgery, Section 1 | |
| Azaperone 2 mg/kg | Stresnil, Janssen-Cilag, Pharma, Austria | Preparing the animal for surgery, Section 1 | |
| Biosafety Level 2 hood | Plastic compression; Section 4 | ||
| Blocking solution: Normal serum from the same species as the secondary secondary antibody was generated in. | Vector | The blocking solution depends of the origin of first antibody | Immunocytochemistry; Section 6 |
| Buprenorphine 45 μg/kg | Atropin, Mylan Inc, Canonsburg, PA | Preparing the animal for surgery, Section 1 | |
| Carprofen 3 mg/kg | Rimadyl, Orion Pharma, Sweden | Preparing the animal for surgery, Section 1 | |
| Chlorhexidine gluconate | Hibiscrub 40 mg/mL, Regent Medical, England | Preparing the animal for surgery, Section 1 | |
| Cholera toxin | Sigma-Aldrich | C8052 | In vitro culture; Section 5 |
| Coplin jar: staining jar for boiling | Histolab | 6150 | Immunocytochemistry; Section 6 |
| Stainless mold (33 mm x 22 mm x 10 mm) custom made | Plastic compression; Section 4 | ||
| DMEM | Gibco | 3188-5023 | Plastic compression section 4. Keep on ice when using it in plastic compression |
| Epidermal growth factor | Sigma-Aldrich | E9644 | In vitro culture; Section 5 |
| Ethilon (non-absorbable monofilament for skin sutures) | Ethicon | Surgery, Section 1 | |
| Fetal bovine serum (FBS) | Gibco | 10437-036 | Plastic compression section 4 |
| Forceps (Adison with tooth) | Preparing the animal for surgery, Section 1 | ||
| Gauze (Gazin Mullkompresse) | Preparing the animal for surgery, Section 1 | ||
| Ham's F12 | Gibco | 31765-027 | Plastic compression section 4 |
| Hematoxylin | Histolab | 1820 | Immunocytochemistry; Section 6 |
| Humidity chamber | DALAB | Immunocytochemistry; Section 6 | |
| Hydrocortisone | Sigma-Aldrich | H0888 | In vitro culture; Section 5 |
| Hydrogen peroxide Solution 30% | Sigma-Aldrich | H1009 | Immunocytochemistry; Section 6 |
| Insulin | Sigma-Aldrich | I3536 | In vitro culture; Section 5 |
| Isoflurane | Isoflurane, Baxter, Deerfield, IL | Preparing the animal for surgery, Section 1 | |
| Lidocaine 5 mg/ml | Xylocaine, AstraZeneca, Sweden | Preparing the animal for surgery, Section 1 | |
| Lucose 25 mg/ml | Baxter, Deerfield, IL | Preparing the animal for surgery, Section 1 | |
| Marker pen pap pen | Sigma-Aldrich | Z377821-1EA | Immunocytochemistry; Section 6 |
| Medetomidine 25 μg/kg | Domitor, Orion Pharma, Sweden | Preparing the animal for surgery, Section 1 | |
| Mincing device | Applied Tissue Technologies LLC | Minced tissue preparation, section 2 | |
| Monocryl (absorbable monofilament) | Ethicon | Surgery, Section 1 | |
| NaCl | Sigma-Aldrich | S7653 | Immunocytochemistry; Section 6 |
| NaOH 1 N | Merck Millipore | 106462 | Plastic compression section 4 and cell culture |
| Nylon mesh, 110 μM thick pore size 0.04 sqmm | Plastic compression; Section 4 | ||
| Oculentum simplex APL: ointment for eye protection | APL | Vnr 336164 | Surgery, Section 1 |
| PBS | Gibco | 14190-094 | Plastic compression section 4 |
| Penicillin-Streptomycin | Gibco | 15140-122 | Plastic compression section 4 |
| Phenobarbiturate 15 mg/kg | Pentobarbital, APL, Sweden | Preparing the animal for surgery, Section 1 | |
| PCL Knitted fabric | Plastic compression; Section 4 | ||
| Rat-tail collagen | First LINK, Ltd, UK | 60-30-810 | Plastic compression section 4, keep on ice |
| Scalpel blade - 15 | Preparing the animal for surgery, Section 1 | ||
| Shaving shears | Preparing the animal for surgery, Section 1 | ||
| Stainless stell mesh, 400 μM thick pore size | Plastic compression; Section 4 | ||
| Steril gloves | Preparing the animal for surgery, Section 1 | ||
| Sterile gowns | Preparing the animal for surgery, Section 1 | ||
| Sterile drapes | |||
| Sterilium | Bode Chemie HAMBURG | Preparing the animal for surgery, Section 1 | |
| Suture Thread Ethilon | Preparing the animal for surgery, Section 1 | ||
| TE-solution (antigen unmasking solution) consist of 10 mM Tris and 1 mM EDTA, pH 9.0 | 10 mM Tris/1 mM EDTA, adjust pH to 9.0 | ||
| Tiletamine hypochloride 2.5 mg/kg | Preparing the animal for surgery, Section 1 | ||
| Transferrin | Sigma-Aldrich | T8158 | In vitro culture; Section 5 |
| Trizma Base, H2NC | Sigma-Aldrich | T6066 | Immunocytochemistry; Section 6 |
| Vector VIP kit: Enzyme peroxidase substrate kit | Vector | SK4600 | Immunocytochemistry; Section 6 |
| Vicryl (absorbable braded) | Ethicon | Surgery, Section 1 | |
| Tris buffer pH 7.6 (washing buffer) | TE solution: Make 10x (0.5 M Tris, 1.5 M NaCl) by mixing: 60.6 g Tris (Trizma Base, H2NC(CH2OH)3, M=121.14 g/mol), add 800 ml distilled water adjust the pH till 7.6, add 87.7 g NaCl and fill to 1,000 ml with distilled water. Dilute to 1x with distilled water. | ||
| X-tra solv (solvent) | DALAB | 41-5213-810 | Immunocytochemistry; Section 6. Use under fume hood |
| Zolazepam hypochloride | Zoletil, Virbac, France | Preparing the animal for surgery, Section 1 | |
| Depilatory wax strips | Veet | Preparing the animal for surgery, Section 1 | |
| Pentobarbital sodium | Lundbeck | Termination, Section 3 |
参考文献
- Rheinwald, J. G., Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 6, 331-343 (1975).
- Fossum, M., Nordenskjold, A., Kratz, G. Engineering of multilayered urinary tissue in vitro. Tissue Engineering. 10, 175-180 (2004).
- Salmikangas, P., et al. Manufacturing, characterization and control of cell-based medicinal products: challenging paradigms toward commercial use. Regen Med. 10, 65-78 (2015).
- Fossum, M., et al. Minced skin for tissue engineering of epithelialized subcutaneous tunnels. Tissue Engineering. Part A. 15, 2085-2092 (2009).
- Fossum, M., et al. Minced urothelium to create epithelialized subcutaneous conduits. The Journal of Urology. 184, 757-761 (2010).
- Reinfeldt Engberg, G., Lundberg, J., Chamorro, C. L., Nordenskjold, A., Fossum, M. Transplantation of autologous minced bladder mucosa for a one-step reconstruction of a tissue engineered bladder conduit. BioMed Research International. 2013, 212734 (2013).
- Meek, C. P. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 96, 557-558 (1958).
- Tanner, J. C., Vandeput, J., Olley, J. F. The Mesh skin graft. Plastic and Reconstructive Surgery. 34, 287-292 (1964).
- Svensjo, T., et al. Autologous skin transplantation: comparison of minced skin to other techniques. The Journal of Surgical Research. 103, 19-29 (2002).
- Ajalloueian, F., Zeiai, S., Rojas, R., Fossum, M., Hilborn, J. One-stage tissue engineering of bladder wall patches for an easy-to-use approach at the surgical table. Tissue Engineering. Part C, Methods. 19, 688-696 (2013).
- Engelhardt, E. M., et al. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 32, 3969-3976 (2011).
- Brown, R. A., Wiseman, M., Chuo, C. B., Cheema, U., Nazhat, S. N. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 15, 1762-1770 (2005).
- Fumagalli Romario, U., Puccetti, F., Elmore, U., Massaron, S., Rosati, R. Self-gripping mesh versus staple fixation in laparoscopic inguinal hernia repair: a prospective comparison. Surg Endosc. 27, 1798-1802 (2013).
- Muangman, P., et al. Complex Wound Management Utilizing an Artificial Dermal Matrix. Annals of Plastic Surgery. 57, 199-202 (2006).
- Ajalloueian, F., Zeiai, S., Fossum, M., Hilborn, J. G. Constructs of electrospun PLGA, compressed collagen and minced urothelium for minimally manipulated autologous bladder tissue expansion. Biomaterials. 35, 5741-5748 (2014).
- Orabi, H., AbouShwareb, T., Zhang, Y., Yoo, J. J., Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol. 63, 531-538 (2013).
- Blais, M., Parenteau-Bareil, R., Cadau, S., Berthod, F. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2, 545-551 (2013).
- Serpooshan, V., Muja, N., Marelli, B., Nazhat, S. N. Fibroblast contractility and growth in plastic compressed collagen gel scaffolds with microstructures correlated with hydraulic permeability. J Biomed Mater Res A. 96, 609-620 (2011).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。