Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Minced Tissue in Compressed Collagen: A Cell-containing Biotransplant for Single-staged Reconstructive Repair
W tym Artykule
Podsumowanie
Tissue engineering often includes in vitro expansion in order to create autografts for tissue regeneration. In this study a method for tissue expansion, regeneration, and reconstruction in vivo was developed in order to minimize the processing of cells and biological materials outside the body.
Streszczenie
Conventional techniques for cell expansion and transplantation of autologous cells for tissue engineering purposes can take place in specially equipped human cell culture facilities. These methods include isolation of cells in single cell suspension and several laborious and time-consuming events before transplantation back to the patient. Previous studies suggest that the body itself could be used as a bioreactor for cell expansion and regeneration of tissue in order to minimize ex vivo manipulations of tissues and cells before transplanting to the patient. The aim of this study was to demonstrate a method for tissue harvesting, isolation of continuous epithelium, mincing of the epithelium into small pieces and incorporating them into a three-layered biomaterial. The three-layered biomaterial then served as a delivery vehicle, to allow surgical handling, exchange of nutrition across the transplant, and a controlled degradation. The biomaterial consisted of two outer layers of collagen and a core of a mechanically stable and slowly degradable polymer. The minced epithelium was incorporated into one of the collagen layers before transplantation. By mincing the epithelial tissue into small pieces, the pieces could be spread and thereby the propagation of cells was stimulated. After the initial take of the transplants, cell expansion and reorganization would take place and extracellular matrix mature to allow ingrowth of capillaries and nerves and further maturation of the extracellular matrix. The technique minimizes ex vivo manipulations and allow cell harvesting, preparation of autograft, and transplantation to the patient as a simple one-stage intervention. In the future, tissue expansion could be initiated around a 3D mold inside the body itself, according to the specific needs of the patient. Additionally, the technique could be performed in an ordinary surgical setting without the need for sophisticated cell culturing facilities.
Wprowadzenie
Most tissue engineering studies on transplantation to the skin and urogenital tract include autologous cell harvests from healthy tissue and cell expansion in specially equipped cell-culturing facilities1,2.
After cell expansion, cells are usually stored for later use when the patient is prepared to receive the autograft. Nitrogen freezers allow long-term storage at low temperatures of -150 °C or lower. The process of freezing must be careful and controlled in order not to lose the cells. One risk of cell death is crystallization of intracellular water during the thawing process, which can lead to rupture of the cell membranes. Cell freezing is usually performed by slow and controlled cooling (-1 °C per min), using a high concentration of cells, fetal bovine serum, and dimethyl sulfoxide. After thawing, the cells need to be processed again by removing the freezing medium and culturing on cell culture plastic or a biomaterial before transplantation back to the patient.
All the above-mentioned steps are time-consuming, laborious, and costly3. In addition, all in vitro processing of cells intended for patient transplantation are highly regulated and requires well-trained and accredited personnel and laboratories4. All in all, to procure a safe and reliable manufacturing process, the technique could only be established in a very small number of technically advanced centers and a wider use in common surgical disorders is doubtful.
In order to overcome the limitations of cell culturing in the laboratory environment, the concept of transplanting minced tissue for cell expansion in vivo is introduced by using the body itself as a bioreactor. For these purposes, the autografts would preferentially be transplanted on a 3D mold according to the shape that is needed for the final reconstruction of the organ of interest5-7.
Originally, the idea of transplanting minced epithelium was presented by Meek in 1958 when he described how epithelium grows from the edges of a wound. He demonstrated that a small piece of skin would increase its margins and thereby its potential for cell expansion by 100% by cutting the piece twice in perpendicular directions (Figure 1)8. The theory has been supported by the use of meshed partial thickness skin grafts for skin transplantation9 and in skin wound healing models10.
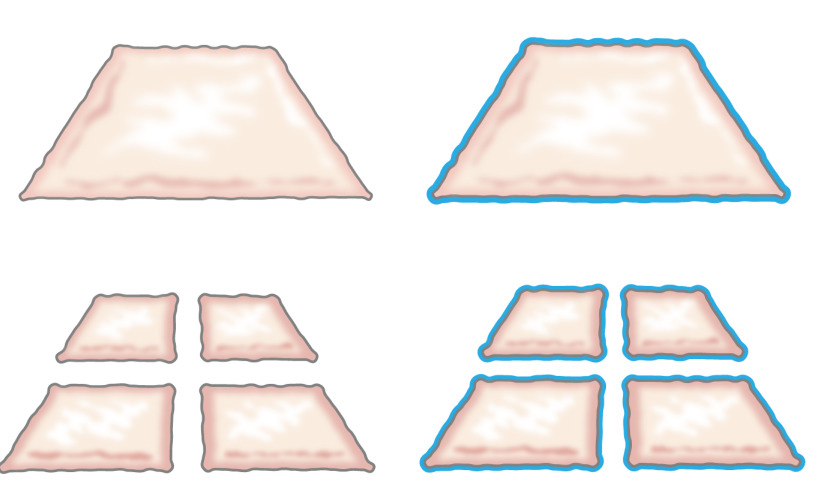
Figure 1: Meek theory. According to Meek’s theory, epithelium grows from the edges of a wound. By increasing the area exposed by the mincing technology, minced tissue epithelializes wounds from many spots.
The present study is based on the hypothesis that the same principle could be applied to the subcutaneous tissue by placing minced epithelium around a mold. The epithelial cells would mobilize from minced transplants (reorganize), cover the wound areas (migrate) and divide (expand) in order to form a continuous neoepithelium that covers the wound area and separates the foreign body (the mold) from the inner body (Figure 2).
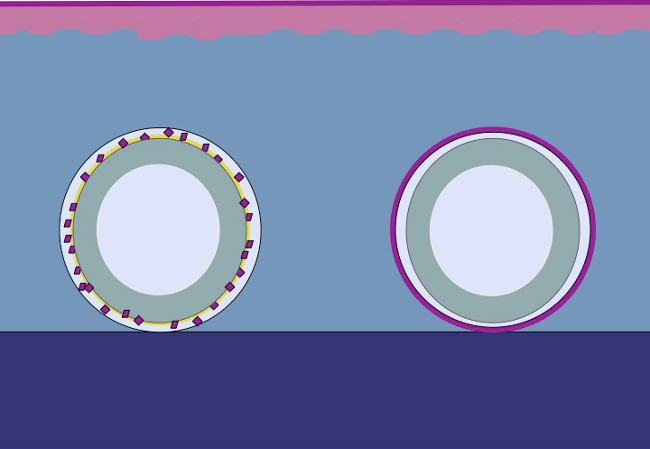
Figure 2: Cartoon of a 3D mold with minced epithelium for in vivo intracorporal tissue expansion according to the theory of Meek. By using minced tissue placed on a mold and then transplanted to the subcutaneous tissue, the hypothesis is that the epithelial cells migrate from the edges of the minced tissue, reorganize, and expand so as to form a continuous neoepithelium that covers the wound area and separates the foreign body (the mold) from the inner body.
Although previous in vivo studies show promising results, further improvements could be achieved by reinforcing the autografts so that the regenerated epithelium could withstand mechanical trauma better7. For these purposes, important prerequisites for a successful biomaterial were identified, such as: easy diffusion of nutrients and waste products, possibility to mold in a 3D manner and easiness of surgical handling. Conclusions were made that these needs could be met by adding a composite biomaterial to the minced tissue.
The current study aimed at developing a scaffold composed of minced tissue in plastic-compressed collagen containing a reinforcing core of a biodegradable fabric. By these means, viable cells could migrate from the minced tissue particles and proliferate with morphological features characteristic of the original epithelium (skin or urothelium). Using plastic compression, the scaffold was reduced in size from 1 cm to about 420 µm as the minced particles were encased in the upper layer collagen. The core fabric could be any polymer but needs to be modified with a hydrophilic surface in order to interlink with the covering collagen layers11.
The method provided an enhanced scaffold integrity by incorporating a knitted mesh consisting of poly(ε-caprolactone) (PCL) within two plastic compressed collagen gels using it as a scaffold for culturing minced bladder mucosa or minced skin from pigs. The construct was maintained in cell culture conditions for up to 6 weeks in vitro, demonstrating successful formation of a stratified, multilayered urothelium or squamous skin epithelium on the top of a well-consolidated hybrid construct. The construct was easy to handle and could be sutured in place for bladder augmentation purposes or covering of skin defects. All parts of the tissue scaffold are FDA-approved and the technique could be used for single-stage procedures by tissue harvesting, mincing, plastic compression, and transplanting back to the patient as a single-staged intervention. The procedure could be performed for tissue expansion and reconstruction under sterile conditions in any general surgery unit.
Protokół
All animal protocols were pre-approved by the Stockholm County Committee on Animals and all procedures conformed to the regulations for animal use, as well as relevant federal statutes.
1. Animal Procedures
- Preparing the Animal for Surgery
- Prepare the surgical table with all the materials and instruments needed for the operation under sterile conditions. Perform the surgery exclusively under sterile conditions to reduce the risk of infection and optimize the conditions in survival surgery.
- Fast the animal for 12 hr prior to surgery and measure its weight. Administer an intramuscular injection of azaperone (2 mg/kg) for premedication. Anesthetize the animal with intramuscular injections of tiletamine hypochloride (2.5 mg/kg), zolazepam hypochloride (2.5 mg/kg), medetomidine (25 µg/kg) and atropine (25 µg/kg).
- Inject phenobarbiturate (15 mg/kg) prior to endotracheal intubation and continue general anesthesia with 0.8%–2% isoflurane. Insert a peripheral vein catheter in one ear and infuse glucose (25 mg/ml) intravenously during the procedure to maintain the well-being of the animal.
- Place monitoring devices on the ear or tail to check temperature, blood pressure, saturation, and peripheral pulse. Control anesthesia by pain stimulation of the ears or hooves. Apply ointment to eyes to prevent dryness while under anesthesia.
- Excision of Bladder Biopsy Specimen
- Catheterize the bladder using a 10-French silicone catheter by introducing a speculum to view the urethra and insert the catheter through the urethra and into the urinary bladder to empty the urine under semi-sterile conditions. Fill the bladder with sterile saline solution to a pressure of 20–25 cm H2O, about 100–300 ml, and then empty to 20 cm H2O (approx. 8 ml/kg of body weight).
- With the pig carefully turned to a side position, perform a basic preoperative sterilization of the skin with application of chlorhexidine gluconate. Apply a diathermy plate to the shoulder after removing the pelage with shaving shears.
- Turn the pig carefully into a supine position and remove the abdominal pelage using shaving shears and do a basic preoperative sterilization of the abdominal skin with application of chlorhexidine gluconate.
- Embed the extremities with soft clothing to minimize the risk of hyperextension damage to the joints in the extremities. Sterilize the abdominal skin of the animal with successive applications of chlorhexidine gluconate and place sterile draping around the surgical field.
- Prior to skin incision, apply an intravenous analgesic consisting of buprenorphine (45 µg/kg), carprofen (3 mg/kg) and local injection of lidocaine in the midline below the umbilicus. Check for pain reaction by grasping the skin with forceps. Make a lower midline incision through the fascia and peritoneum using diathermy for control of bleeding. Localize the bladder that has a fully intraperitoneal position in the pig and can be freely exposed by mobilizing it through the surgical wound.
- Take hold of the bladder with the forceps and measure it with a sterilized measuring tape and mark an elliptic-shaped biopsy specimen approximately 2 cm longitudinally and 1 cm transversally, or smaller, using a sterilized pen (the pig tolerates a quarter reduction of bladder size very well). Excise the marked area, using a scalpel, and place the bladder biopsy specimen in DMEM under sterile conditions.
- Perform autotransplantation with the biotransplant or close the bladder by suturing it with 5-0 Vicryl in two layers. Close the abdominal fascia carefully with 2-0 or 3-0 running Vicryl. Close the subcutis with 3-0 Vicryl and the skin with 3-0 Ethilon. Place a dressing on the wound and carefully attend to the animal until it has recovered sufficiently from the anesthesia and does not express pain.
- Move the animal to the animal care facility to secure conditions for postoperative care. Put the animal in a single cage with a heating lamp and attend to the animal until full recovery from the anesthesia and then let the animals be stalled in pairs.
- Provide an uneventful recovery regarding pain and well-being and administer buprenorphine (45 µg/kg) intramuscularly for postoperative analgesia and trimetoprim (4 mg/kg) and sulfonamide (20 mg/kg) twice daily for three days and once daily for five days to reduce the risk of postoperative infections.
- Excision of Skin Biopsy Specimen
- Prepare the surgical table with all the necessary materials. Anesthetize the animal as previously described in 1.1. Remove the pelage using wax, wash and sterilize the incision area with betadine and 70% alcohol and then place sterile draping around the incision area.
- Use a dermatome to harvest a 0.3 mm partial thickness skin biopsy specimen. Place the skin specimen in DMEM before mincing, as described in 2.2. Cover the wound area with fatty ointment and a dressing.
2. Minced Tissue Preparation
- Bladder Mucosa
- Wash the bladder biopsy twice in DMEM. Place the bladder biopsy specimen onto a sterilized dissecting plate with the mucosa facing upwards and fix one of the sides to the plate using dissection pins.
- Separate the mucosal tissue from the detrusor muscle using fine scissors and forceps (Figure 3) and keep the mucosa moist by dripping saline or DMEM over it.
- Use the mincing device by placing it on the mucosa and then pass the device from one end to the other vertically and horizontally, applying manual pressure to obtain pieces of minced tissue of 0.8 mm x 0.8 mm (0.8 mm is the distance between the rotating cutting blades).
- Skin
- If the skin biopsis are thick: place the skin onto a sterile dissecting plate and use surgical scissors to separate the epidermis from subcutaneous fat and dermis. The epidermis is thin and translucent (approx. 0.3 mm) when it is ready for mincing.
- Use the mincing device by placing it on the epidermis. With pressure, pass the device from one end to the other vertically and horizontally to obtain pieces of minced tissue of 0.8 mm x 0.8 mm.
3. Preparation of Plastic Compressed PCL/Collagen Autografts
- Place all ingredients on ice to keep cold. Utensils needed: Falcon tube, 10x DMEM, 1x DMEM, 1 N NaOH and rat tail collagen type 1.
- Mix 2 ml of 10x DMEM (carefully to avoid bubbles) with 12 ml of collagen type 1. Add 1 N NaOH, drop by drop, to bring pH up to 7.4–8 (color in the medium should indicate the pH by changing from intense yellow to pink). In addition, use a pH strip.
- Carefully add 2 ml of 1x DMEM and mix the solution. Plate approximately 2 ml of collagen in each well of the steel rectangular mold (20x30x10 mm) and incubate at 37 °C in 5% CO2 for 10 min.
NOTE: The concentration of collagen should be 2.06 mg/ml in 0.6% acetic acid and the amount of collagen 1 ml/cm2. - Once the collagen sets into the mold, place the biomaterial (PCL) on top of the collagen gel (20 mm x 30 mm) and pour the remaining collagen (about 6 ml) on top of it. Incubate at 37 °C in 5% CO2 for 20 min.
- Place the minced tissue (for 1:6 expansion) on the top of collagen gel. Press the water out of the construct by mechanical force using plastic compression as follows (Figures 3 and 4).
- Place a thick layer of gauze pads on a sterile surface. Place one stainless steel mesh (400 µm thick) on top of the gauze pads and then a sheet of nylon mesh (110 µm thick). Carefully transfer the collagen gel/minced tissue onto the nylon mesh and carefully remove the rectangular steel mold.
- Place a new layer of nylon mesh on top of the collagen gel/minced tissue. Place a second steel mesh on top of the nylon mesh. Place in position the pressure or loading plate weighing a minimum of 120 g (i.e., a glass plate) for 5 min.
- Remove the weight, nylon, and steel meshes. The autografts are now ready to be sutured to the pig bladder in the full-thickness skin wounds or cultured in vitro.
- For in vitro culturing, cut the thin construct into small pieces fitting 12-well plates. Add 1 ml of keratinocyte medium. Place the plates in the incubator at 37 °C, 5% CO2 and culture up to 6 weeks and change the medium 3 times per week.
4. Suture of Autografts
- Suture of autograft with minced bladder mucosa to the pig bladder
- Keep the autograft moist in DMEM during the waiting time. Suture the autograft with fine running monofilament sutures. Use non-absorbable 5-0 Ethilon for research purposes.
- Check if watertight by filling the bladder with saline through the indwelling urinary catheter. If possible, cover the autograft with a layer of greater omentum. Close the abdominal wall, subcutaneous tissue, and skin as described in 1.2.6. Apply a wound dressing.
- Suture of the Autograft with Minced Skin Epidermis to a Full-thickness Wound
- Keep the autograft moist in DMEM during the waiting time.
- Suture the autograft to the bottom of the skin full-thickness wound by interrupted sutures in the corners and in the middle of the autograft to keep the autograft closely attached to the underlying surface.
- Cover the wound with a plastic dressing that keeps the wound moist.
5. Termination
- Sedate the animal with intramuscular injection of zolazepam hypochloride (2.5 mg/kg) and medetomidine (25 µg/kg) prior to termination and apply monitoring devices to the ear or tail to check for pulse and blood pressure.
- Euthanize the animal by administering a lethal dose of pentobarbital sodium (60-140 mg/kg) intravenously. Check pulse and blood pressure until death has occurred.
6. In Vitro Culture
NOTE: To evaluate histologically the progression of the minced tissue in the PCL/collagen constructs in vitro, the collagen/PCL/minced patches are cultured in 12-well plates using keratinocyte medium.
- Preparation of keratinocyte medium:
- Sterilize a 500 ml glass bottle.
- Mix 400 ml of DMEM with 100 ml of Ham’s F12 (4:1 mixture). Supplement with 10% fetal bovine serum, 5 µg/ml insulin, 0.4 µg/ml hydrocortisone, 21 µg/ml adenine, 10-10 mol/L cholera toxin, 2 x 10-9 mol/L triiodothyronine, 5 µg/ml transferrin, 10 ng/ml epidermal growth factor, 50 U/ml penicillin and 50 µg/ml streptomycin.
- Sterilize by filtrating through a 0.2 μm filter and collect the filtrate in the sterile 500 ml bottle.
7. Immunohistochemistry
NOTE: The immunohistochemistry protocol is generally divided into the following steps: (1) fixation and paraffin embedding, (2) micro-sectioning to 5 µm slices, placement on slides, deparaffination, and rehydration, (3) antigen unmasking, staining and mounting. Before starting the last steps in the immunohistochemistry procedure, prepare the washing buffers and the antigen unmasking solution (see separate material details). Prepare the ABC complex solution at least 30 min before use.
- Fixation
NOTE: At the end of the in vitro culture, fix the patches as follows:- Prepare Eppendorf tubes with 1 ml of 4% buffered formaldehyde (PFA) (Caution: formaldehyde is toxic. Please read material safety data sheets before working with this chemical. Wear gloves and safety glasses and prepare the solution inside a fume hood).
- Transfer each of the collagen patches to an Eppendorf tube containing 4% PFA. Fix over night at room temperature.
- Place samples in 70% ethanol for long-term storage at 4 °C. Samples are now ready for dehydration and embedding in paraffin blocks before sectioning.
- Rehydration
- Place the slides in a staining jar with X-tra solv for 15 min. Repeat by using a new staining jar with X-tra solv. Place the slides in a staining jar with absolute ethanol for 10 min. Repeat by using a new staining jar with absolute ethanol. Place the slides in a staining jar with 95% ethanol for 10 min and thereafter into a staining jar with 70% ethanol for 10 min. Finally wash the slides twice for 5 min with distilled water.
- Antigen Unmasking
- Put slides in a Coplin jar with TE-solution and put the jar in a water bath to boil for 20 min. Take the jar out of the water bath carefully. Cool the slides to room temperature for 30 min and wash twice for 5 min in Tris buffer. Place the slides in a staining jar with 3% hydrogen peroxide for 10 min. Wash the slides twice for 5 min in Tris buffer. Draw a circle around the samples using a water repellent marking pen.
- Block nonspecific binding of the antibody using 100–300 µl of blocking solution. Remove the blocking solution and add 100–300 µl of primary antibody dissolved at the recommended concentration in Tris buffer. Incubate overnight. Remove the antibody solution and wash sections in Tris buffer twice for 5 min.
- Incubate with the secondary antibody for 1 hr at room temperature. Wash twice for 5 min in Tris buffer. Incubate 30 min using the ABC Elite Kit (follow the manufacturer’s instructions). Wash twice in Tris buffer.
- Develop antibody reaction by using the Vector VIP Kit, following the manufacturer’s instructions (1–7 min incubation generally produces a clear violet intensity). Put the slides in distilled water. Counterstain with Mayer’s hematoxylin for 30 sec.
- Wash in running water for 5 min. Place the slides in a staining jar with 70% ethanol for 1 min. Repeat by using a new staining jar with 70% ethanol. Place the slides in a staining jar with 95% ethanol for 1 min. Repeat by using a new staining jar with 95% ethanol.
- Place the slides in a staining jar with X-tra solv for 5 min. Remove, one at a time to keep moist. Place a drop of mounting medium on top of each slide and put a cover glass on top (do so carefully to avoid air bubbles). Let the slides dry overnight and view slides under a microscope.
Wyniki
This study presents a method that shows how to produce a biomaterial for transplantation using plastic compression of collagen and minced tissue.
Bladder mucosa and skin can be harvested and then mechanically minced into small particles (Figure 3). By plastic compression, the minced particles are incorporated within the composite scaffold composed of a centrally placed biodegradable polymer that is mechanically strong within outer layers of a collagen gel (Figure 4
Dyskusje
This study presents an easy-to-use approach to produce bladder wall patches with autologous tissue for transplantation at the surgical table. The patches are formed by the combination of a biodegradable polymer knitting in the middle and collagen with and without minced tissue in the outer surfaces in combination with plastic compression. Plastic compression is a method previously described by other authors and can be defined as a rapid expulsion of fluid from collagen gels12,13. Minced tissue of bladder mucos...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors thank the Swedish Society for Medical Research, the Promobilia Foundation, the Crown Princess Lovisa Foundation, the Freemason Foundation for Children’s Welfare, the Swedish Society of Medicine, the Solstickan Foundation, Karolinska Institutet, and the Stockholm City Council for financial support.
Materiały
| Name | Company | Catalog Number | Comments |
| Silicone catheter 10-French | Preparing the animal for surgery, Section 1 | ||
| DMEM 10x | Gibco | 31885-023 | Plastic compression section 4 |
| 24 well plates | Falcon | 08-772-1 | Plastic compression section 4 |
| 3',3',5-Triiodothyronine | Sigma-Aldrich | IRMM469 | In vitro culture; Section 5 |
| 4% PFA | Labmed Solutions | 200-001-8 | Immunocytochemistry; Section 6 |
| 70% ethanol | Histolab | Immunocytochemistry; Section 6 | |
| ABC Elite kit: Biotin-Streptavidin detection kit | Vector | PK6102 | Immunocytochemistry; Section 6 |
| Absolute ethanol | Histolab | 1399.01 | Immunocytochemistry; Section 6 |
| Adenine | Sigma-Aldrich | A8626 | In vitro culture; Section 5 |
| Atropine 25 μg/kg | Temgesic, RB Pharmaceuticals, Great Britain | Preparing the animal for surgery, Section 1 | |
| Azaperone 2 mg/kg | Stresnil, Janssen-Cilag, Pharma, Austria | Preparing the animal for surgery, Section 1 | |
| Biosafety Level 2 hood | Plastic compression; Section 4 | ||
| Blocking solution: Normal serum from the same species as the secondary secondary antibody was generated in. | Vector | The blocking solution depends of the origin of first antibody | Immunocytochemistry; Section 6 |
| Buprenorphine 45 μg/kg | Atropin, Mylan Inc, Canonsburg, PA | Preparing the animal for surgery, Section 1 | |
| Carprofen 3 mg/kg | Rimadyl, Orion Pharma, Sweden | Preparing the animal for surgery, Section 1 | |
| Chlorhexidine gluconate | Hibiscrub 40 mg/mL, Regent Medical, England | Preparing the animal for surgery, Section 1 | |
| Cholera toxin | Sigma-Aldrich | C8052 | In vitro culture; Section 5 |
| Coplin jar: staining jar for boiling | Histolab | 6150 | Immunocytochemistry; Section 6 |
| Stainless mold (33 mm x 22 mm x 10 mm) custom made | Plastic compression; Section 4 | ||
| DMEM | Gibco | 3188-5023 | Plastic compression section 4. Keep on ice when using it in plastic compression |
| Epidermal growth factor | Sigma-Aldrich | E9644 | In vitro culture; Section 5 |
| Ethilon (non-absorbable monofilament for skin sutures) | Ethicon | Surgery, Section 1 | |
| Fetal bovine serum (FBS) | Gibco | 10437-036 | Plastic compression section 4 |
| Forceps (Adison with tooth) | Preparing the animal for surgery, Section 1 | ||
| Gauze (Gazin Mullkompresse) | Preparing the animal for surgery, Section 1 | ||
| Ham's F12 | Gibco | 31765-027 | Plastic compression section 4 |
| Hematoxylin | Histolab | 1820 | Immunocytochemistry; Section 6 |
| Humidity chamber | DALAB | Immunocytochemistry; Section 6 | |
| Hydrocortisone | Sigma-Aldrich | H0888 | In vitro culture; Section 5 |
| Hydrogen peroxide Solution 30% | Sigma-Aldrich | H1009 | Immunocytochemistry; Section 6 |
| Insulin | Sigma-Aldrich | I3536 | In vitro culture; Section 5 |
| Isoflurane | Isoflurane, Baxter, Deerfield, IL | Preparing the animal for surgery, Section 1 | |
| Lidocaine 5 mg/ml | Xylocaine, AstraZeneca, Sweden | Preparing the animal for surgery, Section 1 | |
| Lucose 25 mg/ml | Baxter, Deerfield, IL | Preparing the animal for surgery, Section 1 | |
| Marker pen pap pen | Sigma-Aldrich | Z377821-1EA | Immunocytochemistry; Section 6 |
| Medetomidine 25 μg/kg | Domitor, Orion Pharma, Sweden | Preparing the animal for surgery, Section 1 | |
| Mincing device | Applied Tissue Technologies LLC | Minced tissue preparation, section 2 | |
| Monocryl (absorbable monofilament) | Ethicon | Surgery, Section 1 | |
| NaCl | Sigma-Aldrich | S7653 | Immunocytochemistry; Section 6 |
| NaOH 1 N | Merck Millipore | 106462 | Plastic compression section 4 and cell culture |
| Nylon mesh, 110 μM thick pore size 0.04 sqmm | Plastic compression; Section 4 | ||
| Oculentum simplex APL: ointment for eye protection | APL | Vnr 336164 | Surgery, Section 1 |
| PBS | Gibco | 14190-094 | Plastic compression section 4 |
| Penicillin-Streptomycin | Gibco | 15140-122 | Plastic compression section 4 |
| Phenobarbiturate 15 mg/kg | Pentobarbital, APL, Sweden | Preparing the animal for surgery, Section 1 | |
| PCL Knitted fabric | Plastic compression; Section 4 | ||
| Rat-tail collagen | First LINK, Ltd, UK | 60-30-810 | Plastic compression section 4, keep on ice |
| Scalpel blade - 15 | Preparing the animal for surgery, Section 1 | ||
| Shaving shears | Preparing the animal for surgery, Section 1 | ||
| Stainless stell mesh, 400 μM thick pore size | Plastic compression; Section 4 | ||
| Steril gloves | Preparing the animal for surgery, Section 1 | ||
| Sterile gowns | Preparing the animal for surgery, Section 1 | ||
| Sterile drapes | |||
| Sterilium | Bode Chemie HAMBURG | Preparing the animal for surgery, Section 1 | |
| Suture Thread Ethilon | Preparing the animal for surgery, Section 1 | ||
| TE-solution (antigen unmasking solution) consist of 10 mM Tris and 1 mM EDTA, pH 9.0 | 10 mM Tris/1 mM EDTA, adjust pH to 9.0 | ||
| Tiletamine hypochloride 2.5 mg/kg | Preparing the animal for surgery, Section 1 | ||
| Transferrin | Sigma-Aldrich | T8158 | In vitro culture; Section 5 |
| Trizma Base, H2NC | Sigma-Aldrich | T6066 | Immunocytochemistry; Section 6 |
| Vector VIP kit: Enzyme peroxidase substrate kit | Vector | SK4600 | Immunocytochemistry; Section 6 |
| Vicryl (absorbable braded) | Ethicon | Surgery, Section 1 | |
| Tris buffer pH 7.6 (washing buffer) | TE solution: Make 10x (0.5 M Tris, 1.5 M NaCl) by mixing: 60.6 g Tris (Trizma Base, H2NC(CH2OH)3, M=121.14 g/mol), add 800 ml distilled water adjust the pH till 7.6, add 87.7 g NaCl and fill to 1,000 ml with distilled water. Dilute to 1x with distilled water. | ||
| X-tra solv (solvent) | DALAB | 41-5213-810 | Immunocytochemistry; Section 6. Use under fume hood |
| Zolazepam hypochloride | Zoletil, Virbac, France | Preparing the animal for surgery, Section 1 | |
| Depilatory wax strips | Veet | Preparing the animal for surgery, Section 1 | |
| Pentobarbital sodium | Lundbeck | Termination, Section 3 |
Odniesienia
- Rheinwald, J. G., Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 6, 331-343 (1975).
- Fossum, M., Nordenskjold, A., Kratz, G. Engineering of multilayered urinary tissue in vitro. Tissue Engineering. 10, 175-180 (2004).
- Salmikangas, P., et al. Manufacturing, characterization and control of cell-based medicinal products: challenging paradigms toward commercial use. Regen Med. 10, 65-78 (2015).
- Fossum, M., et al. Minced skin for tissue engineering of epithelialized subcutaneous tunnels. Tissue Engineering. Part A. 15, 2085-2092 (2009).
- Fossum, M., et al. Minced urothelium to create epithelialized subcutaneous conduits. The Journal of Urology. 184, 757-761 (2010).
- Reinfeldt Engberg, G., Lundberg, J., Chamorro, C. L., Nordenskjold, A., Fossum, M. Transplantation of autologous minced bladder mucosa for a one-step reconstruction of a tissue engineered bladder conduit. BioMed Research International. 2013, 212734 (2013).
- Meek, C. P. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 96, 557-558 (1958).
- Tanner, J. C., Vandeput, J., Olley, J. F. The Mesh skin graft. Plastic and Reconstructive Surgery. 34, 287-292 (1964).
- Svensjo, T., et al. Autologous skin transplantation: comparison of minced skin to other techniques. The Journal of Surgical Research. 103, 19-29 (2002).
- Ajalloueian, F., Zeiai, S., Rojas, R., Fossum, M., Hilborn, J. One-stage tissue engineering of bladder wall patches for an easy-to-use approach at the surgical table. Tissue Engineering. Part C, Methods. 19, 688-696 (2013).
- Engelhardt, E. M., et al. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 32, 3969-3976 (2011).
- Brown, R. A., Wiseman, M., Chuo, C. B., Cheema, U., Nazhat, S. N. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 15, 1762-1770 (2005).
- Fumagalli Romario, U., Puccetti, F., Elmore, U., Massaron, S., Rosati, R. Self-gripping mesh versus staple fixation in laparoscopic inguinal hernia repair: a prospective comparison. Surg Endosc. 27, 1798-1802 (2013).
- Muangman, P., et al. Complex Wound Management Utilizing an Artificial Dermal Matrix. Annals of Plastic Surgery. 57, 199-202 (2006).
- Ajalloueian, F., Zeiai, S., Fossum, M., Hilborn, J. G. Constructs of electrospun PLGA, compressed collagen and minced urothelium for minimally manipulated autologous bladder tissue expansion. Biomaterials. 35, 5741-5748 (2014).
- Orabi, H., AbouShwareb, T., Zhang, Y., Yoo, J. J., Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol. 63, 531-538 (2013).
- Blais, M., Parenteau-Bareil, R., Cadau, S., Berthod, F. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med. 2, 545-551 (2013).
- Serpooshan, V., Muja, N., Marelli, B., Nazhat, S. N. Fibroblast contractility and growth in plastic compressed collagen gel scaffolds with microstructures correlated with hydraulic permeability. J Biomed Mater Res A. 96, 609-620 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone