このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
ペプチド:主要組織適合遺伝子複合体四量体を用いたマウス肺由来の希少抗原特異的T細胞の同定
要約
私たちは、磁気ビーズベースのT細胞濃縮およびペプチド:主要組織適合遺伝子複合体(MHC)四量体を通じて、マウス肺の希少抗原特異的T細胞集団を単離および同定するための詳細なプロトコールを提供します。
要約
健康および疾患における抗原特異的T細胞の同定と特性評価は、免疫病態生理学の理解を深めるための鍵であり続けています。内因性T細胞レパートリー内の抗原特異的T細胞集団を追跡するという技術的課題は、ペプチド:MHCテトラマー試薬の開発によって大幅に進歩しました。抗原性ペプチドエピトープに複合体を形成したMHCクラスIまたはクラスII分子のこれらの蛍光標識可溶性マルチマーは、対応するT細胞受容体(TCR)特異性を持つT細胞に直接結合するため、 ex vivo によって誘導される機能的応答を必要とせずに、天然状態の抗原特異的T細胞集団を同定できます刺激。非常にまれな集団の場合、四量体に結合したT細胞を磁気的に濃縮して、検出の感度と信頼性を高めることができます。
組織に常在するT細胞免疫の研究が深まるにつれ、非リンパ組織に輸送され、非リンパ組織に存在する抗原特異的T細胞の同定が急務となっています。このプロトコルでは、マウスの肺内に存在する抗原特異的T細胞の単離と特性評価のための詳細な指示を提示します。これには、消化された肺組織からのT細胞の単離と、フローサイトメトリー分析とソーティングのための一般的なT細胞磁気濃縮ステップとテトラマー染色が含まれます。このプロトコルで強調されているステップは、一般的な技術と容易に利用可能な試薬を利用しているため、マウスT細胞免疫学に従事するほぼすべての研究者がアクセスでき、肺内に存在している低頻度抗原特異的T細胞集団のさまざまなダウンストリーム分析に非常に適応性があります。
概要
適応免疫系の中心には、T細胞が特定の抗原を認識して応答する能力があります。T細胞がいつ、どこでその同族抗原に応答するかによって、感染と自己免疫、恒常性と癌、健康と病気のバランスが決まります1。したがって、免疫の特定の状況におけるT細胞の研究は、関心のある関連抗原に対する特異性を持つ細胞に焦点を当てるべきです。抗原特異的T細胞集団を特徴付ける能力を大幅に向上させた技術的進歩の中には、「ペプチド:MHC四量体」としてよく知られている抗原性ペプチドエピトープに複合体化された主要組織適合遺伝子複合体(MHC)クラスIまたはクラスII分子の蛍光標識可溶性多量体(通常は四量体)があります2,3,4,5 .ペプチド:MHCクラスIおよびクラスII四量体は、T細胞抗原受容体(TCR)の天然リガンドを発現することにより、免疫系のT細胞の内因性レパートリー内の抗原特異的CD8+およびCD4+ T細胞をそれぞれ直接同定する手段を提供します。四量体は、抗原特異的T細胞の研究において、TCRトランスジェニックT細胞養子導入モデル6よりもエレガントなアプローチであり、実験マウスモデルとヒト疾患4,5の両方で、外来および自己抗原特異的T細胞集団を同定するためにますます使用されています。
四量体は、抗原刺激に応答して増殖したT細胞の高頻度集団を容易に同定できますが、ナイーブT細胞、自己抗原特異的T細胞、またはメモリーT細胞への使用は、これらの集団の頻度が非常に低いために制限されます7。私たちのグループと他のグループは、マウスリンパ組織8,9,10,11におけるこれらの細胞集団の研究を可能にするために、検出の感度を高める四量体ベースの磁気濃縮戦略を開発し、普及させました。
この分野での組織常在性T細胞の出現により、非リンパ性領域のT細胞を調査する新しい方法の開発にますます重点が置かれています。他の多くの粘膜表面と同様に、肺のT細胞は、宿主上皮、共生微生物および感染性微生物、およびアレルゲンを含む環境実体に由来するさまざまな自己抗原および外来抗原に遭遇します。非リンパ組織(NLT)から採取されたT細胞の転写解析は、しばしば人身売買と組織恒常性に向けられた、独特の組織特異的な運命と機能を持つ記憶のような表現型を示しています12。さらに、組織に常在するメモリーT細胞(Trms)は、循環中のT細胞よりもクローン性が制限される傾向がある13。NLTにおいて、抗原がどのように、そしてなぜT細胞の居住を促進するのかを明らかにすることは、免疫系がどのように感染から保護し、組織の恒常性を維持し、時には自己免疫に進化するかを理解するために重要です。しかし、肺からの組織常在性T細胞の消耗は、他のNLTと比較して大きいようです14。したがって、特定の抗原特異性を持つ肺の内因性T細胞を同定し、特徴付ける能力は、その固有の希少性によって制限されます。
磁気ビーズを用いた細胞濃縮技術とペプチド:MHC四量体染色を組み合わせることで、マウス肺において増殖しているがまれな自己抗原特異的T細胞を検出することに成功しました15,16。ここでは、マウスの肺に存在する希少な抗原特異的T細胞集団を確実に単離し、特性評価するために最適化したプロトコールについて詳しく説明します(図1)。このプロトコールは、組織常在T細胞と血管T細胞を区別するためのin vivo抗体染色ステップ17と、それに続くリソースの利用可能性に対応するための肺組織処理のための2つの異なる方法を組み込んでいます。その後、一般的なT細胞磁気濃縮ステップ、四量体染色、フローサイトメトリーによる解析が行われます。このプロトコールでは、誘導性一酸化窒素合成酵素(iNOS)を介したT細胞活性化誘導性アポトーシスを阻害するアミノグアニジン18とTCRのダウンレギュレーションを制限するダサチニブ19を添加することにより、細胞生存率と四量体染色がさらに強化されます。このプロトコールで強調されているステップは、一般的な技術とすぐに利用できる試薬を利用しているため、マウスT細胞免疫学に従事するほぼすべての研究者がアクセスでき、さまざまなダウンストリーム分析に非常に適応性があります。ナイーブT細胞が肺に見つかる可能性は低いですが、このプロトコルは、肺の自己抗原特異的T細胞とTrmsの研究に特に役立つと考えています。
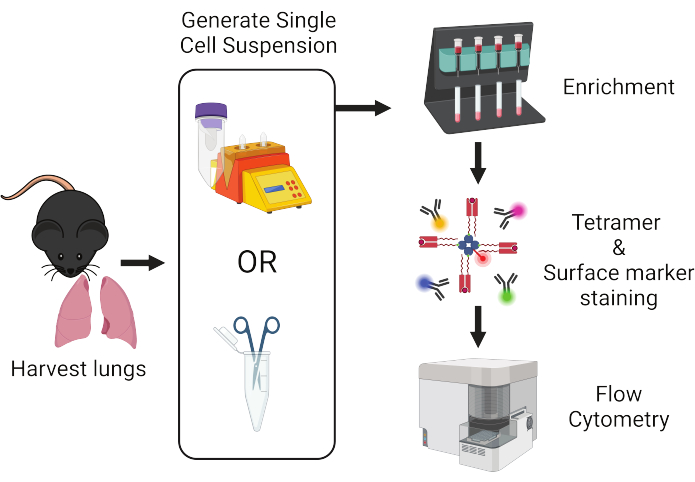
図1:プロトコルワークフローの概要。 肺はマウスから採取され、単一細胞に解離されます。その後、サンプルをT細胞に濃縮してから、ペプチド:MHC四量体および蛍光標識抗体で染色し、フローサイトメトリー分析を行います。 この図の拡大版を表示するには、ここをクリックしてください。
プロトコル
このプロトコルに記載されている手順は、米国実験動物ケア認定協会(AAALAC)認定の動物管理プログラムであるマサチューセッツ総合病院の動物管理委員会(IACUC)が定めたガイドラインに従って承認および開発されています。実験は、MGH動物施設で特定の病原体のない条件下で飼育および維持されたC57BL / 6遺伝的背景で、8〜12週齢の雄および雌マウスで行われました。
1. 原液の調製
- 10 mM HEPES、5 mM KCl、1.8 mM CaCl2、150 mM NaCl、および1 mM MgCl2を溶解してHEPES緩衝液を調製し、pH 7.4に調整します。
- 700 μg/mL のリベラーゼサーモリシン培地(TM)( 材料表を参照)を Ca++ および Mg++ を含む Hank's Balanced Salt Solution ( 材料表を参照) に溶解し、10x リベラーゼストック溶液を調製します。
- 100 mMアミノグアニジン半硫酸塩( 材料表を参照)をCa++ およびMg++ ( 材料表を参照)を含むハンク平衡塩溶液に溶解することにより、10xアミノグアニジンストック溶液を調製します。
- EHAA培地( 材料表を参照)と10%ウシ胎児血清(FBS)、100 U/mLペニシリン/ストレプトマイシン、50 μg/mLゲンタマイシン、2 mM L-グルタミン、および55 μM 2-メルカプトエタノールを混合して、完全なイーグルハムアミノ酸(EHAA)培地を調製します。
注:RPMIやDMEMなどの他の一般的なT細胞培地も使用できます。 - 1x PBSを2% FBSおよび0.05%アジ化ナトリウムと混合して、ソーターバッファーを調製します。
- 精製した抗マウスCD16/32抗体( 材料表を参照)をソーターバッファー中で1:100 v/vで希釈することにより、Fcブロックを調製します。
- L-グルタミンを含まないRPMI 1640培地に100 μg/mL Liberase TMと50 μg/mL DNase I( 材料表を参照)を加えて、Liberase TM/DNase消化溶液を調製します( 材料表を参照)。
- 生理食塩水(0.9%NaCl)に10 mg / mLケタミン( 材料の表を参照)と1 mg / mLのキシラジン( 材料の表を参照)を加えて、ケタミン/キシラジン混合物を準備します( 材料の表を参照)。
2. 自動組織解離器による肺組織からの単一細胞懸濁液の生成
- 各マウスについて、4 mLの氷冷HEPESバッファーを特殊な組織解離チューブ( 材料の表を参照)に調製し、氷上に保ちます。
- 150-200 μL のケタミン/キシラジン混合物の腹腔内注射によりマウスを麻酔します。100 μLの生理食塩水で希釈した1〜3 μgの蛍光色素標識抗マウスCD45抗体( 材料表を参照)を麻酔した各マウスに静脈内注射します。抗体投与の3分後に、ケタミン/キシラジン混合物500〜600μLの腹腔内注射によりマウスを安楽死させます。
- 肺を切除し、氷の上で冷やしたそれぞれの組織解離チューブに入れます。チューブを自動組織解離器( 材料の表を参照)に置き、肺組織の最初のプログラム(プリセットプログラム)を実行します。
- 解離した肺組織を含む各チューブに、500 μLのリベラーゼストック溶液と500 μLのアミノグアニジンストック溶液を加えます。ニューティングミキサーで37°Cで30分間インキュベートします。
- チューブを解離器に戻し、肺組織用の2番目のプログラム(プリセットプログラム)を実行します。
- 組織解離チューブからの単一細胞懸濁液を100 μmのセルストレーナー( 材料の表を参照)を通し、50 mLのコニカルチューブに注ぎます。組織解離チューブを5 mLの完全EHAA(または同等のT細胞培地)ですすぎ、ストレーナーを通過して50 mLチューブに入ります。
- ストレーナーを取り外し、細胞懸濁液の容量を完全なEHAAで50mLに増やします。細胞懸濁液を400 x g で4°Cで5分間遠心分離します。 上清を慎重に吸引し、約100 μLの上清と細胞ペレットを残します。
- 約100μLのFcブロック溶液を加えて総容量を200μLまで上げ、激しく渦巻いてペレットを再懸濁します。
3.代替プロトコル:手動解離により肺組織から単一細胞懸濁液を生成する
- 各マウスについて、1.5 mLのマイクロチューブに1 mLの完全EHAAを調製し、氷上に保ちます。
- 150-200 μL のケタミン/キシラジン混合物の腹腔内注射によりマウスを麻酔します。100 μLの生理食塩水で希釈した1〜3 μgの蛍光色素標識抗マウスCD45抗体を、麻酔をかけた各マウスに静脈内注射します。抗体投与の3分後に、ケタミン/キシラジン混合物500〜600μLの腹腔内注射によりマウスを安楽死させます。
- 肺を切除し、氷上で冷やしたそれぞれの1.5mLマイクロチューブに入れます。
- すべての肺を採取したら、細胞培地を含まない新しいマイクロチューブに各肺を移します。
- マイクロチューブ内のハサミを使用して、肺を小さな断片(~2〜3 mmの塊)に切ります。
- 各チューブに1 mLのLiberase TM/DNase I消化カクテルを加えます。
- 水浴中で37°Cで30分間インキュベートします。その後、サンプルを氷に戻して、過剰消化を防ぎます。
- 50 mLのコニカルチューブの上に置いた100 μmのセルストレーナーにサンプルを注ぎます。滅菌済みの1mLシリンジからプランジャーのゴム端でメッシュに対して組織をつぶします。
- ストレーナーをコールドソーターバッファーですすぎ、チューブ内に最終容量7mLを収集します。細胞懸濁液を400 x g で4°Cで5分間遠心分離します。 上清を慎重に吸引し、約100 μLの上清と細胞ペレットを残します。
- 100 μLのFcブロック溶液を加えて総容量を200 μLまで上げ、激しく渦巻いてペレットを再懸濁します。
4. 磁気ビーズ濃縮によるT細胞の肺サンプルの濃縮
- 50 μL の抗マウス CD90.2 マイクロビーズを添加します ( 材料の表を参照)。ボルテックスし、4°Cで10分間インキュベートします。
注:CD90.2は、C57BL/6マウスのT細胞によって発現される対立遺伝子に対応します。マウスの他の系統が使用されている場合は、対立遺伝子の遺伝子型を確認してください。 - それまでの間、常磁性細胞分離カラム( 材料の表を参照)を1ポジションまたは4ポジションの細胞分離磁石( 材料の表を参照)の上に置きます。開いた15 mLコニカルチューブを各カラムの下に配置して、流れを捕捉します。3 mLのコールドソーターバッファーでカラムをプライムし、重力によってカラムを下の円錐形チューブに排出します。
- 細胞をマイクロビーズと10分間インキュベートしたら、コールドソーターバッファーを1 mLの容量に加え、カラムの上に置いた100 μmの細胞ストレーナーに移します。カラムの流れを、カラムの下に配置された新しい15 mLコニカルチューブに集めます。
- 細胞懸濁液が重力によってカラムを完全に排出したら、元のチューブを追加の3 mLのコールドソーターバッファーですすぎ、メッシュを介してカラムに適用してカラムを洗浄し、ストレーナをすすぎます。ストレーナを取り外します。
- サンプルが再びカラムに完全に排出され、カラムからそれ以上のフロースルーが出なくなったら、カラムを磁石から取り外し、新しい15 mLコニカルチューブの上に置きます。
- 5 mLのコールドソーターバッファーをカラムに加えます。
- カラムプランジャーを1回の連続動作で上部に押し込み、ソーターバッファーをカラムの底部から新しいチューブに押し込むことにより、カラムに結合した細胞をすぐに溶出します。
- 溶出したサンプルを400 x g で5分間、4°Cで遠心分離します。 上清を慎重に吸引し、約100 μLの上清と細胞ペレットを残します。
- 100 μLのコールドソーターバッファーを加えて総容量を200 μLまで上げ、激しくボルテックスしてペレットを再懸濁します。
- 必要に応じて、非結合細胞画分を含むフロースルーサンプルを分析します。
5. 抗原特異的T細胞のペプチド:MHC四量体による染色
- 10 μM ダサチニブ 1 μL ( 材料表を参照) を各サンプルに加え、ボルテックスでボルテックスし、室温で 5 分間インキュベートします。
- PEまたはAPC標識ペプチド:MHC四量体を最終濃度10 nM(または特定の四量体に対して経験的に最適化された濃度)まで添加します。
- ボルテックスし、室温(または経験的に最適化された時間と温度)で1時間暗闇でインキュベートします。
6. フローサイトメトリーによる四量体標識T細胞の解析
- サンプルをペプチド:MHC四量体で染色している間に、抗体のマスターミックスを調製して細胞の表面マーカーを染色します(表1)。
注:静脈内投与されたCD45抗体または四量体と競合する蛍光色素は避けてください。 - テトラマー染色のインキュベーション期間が残り15分になったら、表面抗体マスターミックスを1:100(または経験的に最適化された濃度)で各サンプルに加えます。
- 1時間のインキュベーション期間の残りの間、RTで暗闇でインキュベートを続けます。
- 50,000個のフローサイトメトリーカウントビーズ( 材料表を参照)を添加し、コールドソーターバッファーを最終容量約5 mLまで追加します。染色したサンプルを400 x g で5分間、4°Cで遠心分離します。 上清を慎重に吸引し、約100 μLの上清と細胞/ビーズペレットを残します。
- サンプルを200 μLのソーターバッファーで再懸濁し、サンプルを5 mLの蛍光活性化セルソーティング(FACS)チューブに移します。
- フローサイトメーターでサンプルを分析します。できるだけ多くのセルを、最大で合計2,500,000のイベントまで集めましょう。少なくとも 20,000 個のビーズ イベントが収集されていることを確認します。取得レートを毎秒 5,000 イベント以下に維持します。
- すべてのデータをFCSファイルとして保存します。
| 蛍光色素 | 抗体 |
| BUV395 | CD90.2の |
| パシフィックブルー | CD45(点滴注入により先に添加) |
| パシフィックオレンジ | CD8 |
| ブリリアントバイオレット785 | CD4の |
| フィッツ | CD3 |
| パーCP-Cy5.5 | ダンプ(B220、CD11b、CD11c、F4/80) |
| PEの | pMHC四量体または表現型マーカー |
| PE-Cy7 | 表現型マーカー(例:CD44、PD-1、CD69など) |
| APCの | pMHC四量体または表現型マーカー |
| アレクサフロー 700 | 表現型マーカー(例:CD44、PD-1、CD69など) |
| APC-Cy7の | ライブ/デッドステイン |
表1:サンプル染色マトリックス。 抗原特異的T細胞の同定と特性評価に利用される蛍光色素標識フローサイトメトリー抗体の典型的なパネルです。
7. データの分析
- 適切なフローサイトメトリーソフトウェアを使用してFCSデータファイルを解析します。
- 1)リンパ球様、2)単一、3)ライブ、4)血管外、5)ダンプ陰性、および6)CD4またはCD8陽性のいずれかであるCD90.2陽性イベントを特定するために、連続するインクルージョンゲートのシーケンスを設定します(図2)。
- これらの集団内の四量体陽性T細胞を同定します。
- サンプル分析中に収集されたビーズの数に添加されたビーズの比率(50,000)を計算し、その値に収集されたテトラマー陽性細胞の総数を掛けることにより、絶対的なテトラマー陽性細胞数を決定します。
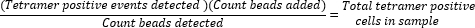
結果
図2 は、ペプチド:MHCクラスII四量体を用いた肺内の希少抗原特異的CD4+ T細胞の同定に使用される代表的なゲーティング戦略を示しています。同じプロセスを、ペプチド:MHCクラスI四量体(データは示さず)を持つ抗原特異的CD8+ T細胞にも適用できます。
肺には非リンパ系細胞が多数存在するため、四量体による希少抗原特異的T細胞の信...
ディスカッション
肺からの抗原特異的T細胞の以前の特性評価は、鼻腔内免疫や感染などの急性プライミングイベント後に増殖する抗原特異的T細胞の強力な数の恩恵を受けてきました20,21,22。しかし、自己抗原特異的T細胞や組織常在メモリーT細胞など、肺内のまれなT細胞集団は、目的のT細胞15,16,23に対する何らかの形のサンプル濃縮なし?...
開示事項
著者には開示すべき対立はありません。
謝辞
組織処理とテトラマー製造に関する技術支援を提供してくださったL.Kuhnに感謝します。この研究は、国立衛生研究所(R01 AI107020およびP01 AI165072 to J.J.M.、T32 AI007512 to D.S.S.)、Massachusetts Consortium on Pathogen Readiness(J.J.M.)、およびMassachusetts General Hospital Executive Committee on Research(J.J.M.)によって資金提供されました。
資料
| Name | Company | Catalog Number | Comments |
| 100 mm cell strainer | Fisher Scientific | 22-363-549 | |
| 10x PBS without Ca++ or Mg++ | Corning | 46-013-CM | |
| 1x PBS without Ca++ or Mg++ | Corning | 21-031-CV | |
| AccuCheck Counting Beads | Invitrogen | PCB100 | |
| Aminoguanidine Hemisulfate Salt | Sigma-Aldrich | A7009 | |
| CD90.2 microbeads, mouse | Miltenyi | 130-121-278 | |
| Cell separation magnet (MidiMACS Separator) | Miltenyi | 130-042-302 | Holds single LS column |
| Cell separation magnet (QuadroMACS Separator) | Miltenyi | 130-090-976 | Holds 4 LS columns |
| Dasatinib | Sigma-Aldrich | CDS023389 | |
| DNase I | Roche | 10104159001 | |
| Eagle’s Ham’s Amino Acids medium | Sigma-Aldrich | C5572 | |
| gentleMACS | Miltenyi | 130-093-235 | Automated tissue dissociator |
| gentleMACS C Tubes | Miltenyi | 130-093-237 | Automated tissue dissociator tubes |
| Hank's Balanced Salt Solution with Ca++ or Mg++ | Corning | 21-020-CM | |
| HEPES | Gibco | 15630080 | |
| Ketamine | Vedco | NDC 50989-996-06 | |
| Liberase TM | Roche | 5401119001 | |
| Pacific Blue anti-mouse CD45 antibody (Clone: 30-F11) | Biolegend | 103126 | |
| Paramagnetic cell separation columns (LS Columns) | Miltenyi | 130-042-401 | Comes with plunger |
| Purified anti mouse CD16/32 antibody (Clone: 93) | Biolegend | 101302 | |
| RPMI 1640 medium without L-glutamine | Corning | 15-040-CM | |
| Sodium Chloride 0.9% (Normal Saline) | Cytiva | Z1376 | |
| Xylazine | Pivetal | NDC 466066-750-02 |
参考文献
- Sun, L., Su, Y., Jiao, A., Wang, X., Zhang, B. T cells in health and disease. Signal Transduct Target Ther. 8 (1), 235 (2023).
- Altman, J. D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274 (5284), 94-96 (1996).
- Crawford, F., Kozono, H., White, J., Marrack, P., Kappler, J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 8 (6), 675-682 (1998).
- Nepom, G. T., et al. HLA class II tetramers: tools for direct analysis of antigen-specific CD4+ T cells. Arthritis Rheum. 46 (1), 5-12 (2002).
- Davis, M. M., Altman, J. D., Newell, E. W. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat Rev Immunol. 11 (8), 551-558 (2011).
- Moon, J. J., et al. Tracking epitope-specific T cells. Nat Protoc. 4 (4), 565-581 (2009).
- Jenkins, M. K., Moon, J. J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 188 (9), 4135-4140 (2012).
- Moon, J. J., et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27 (2), 203-213 (2007).
- Obar, J. J., Khanna, K. M., Lefrancois, L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 28 (6), 859-869 (2008).
- Kotturi, M. F., et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 181 (3), 2124-2133 (2008).
- Legoux, F. P., Moon, J. J. Peptide:MHC tetramer-based enrichment of epitope-specific T cells. J Vis Exp. (68), e4420 (2012).
- Szabo, P. A., Miron, M., Farber, D. L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. 4 (34), 9673 (2019).
- Poon, M. M. L., et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat Immunol. 24 (2), 309-319 (2023).
- Zheng, M. Z. M., Wakim, L. M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15 (3), 379-388 (2022).
- Legoux, F. P., et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T Cells rather than deletion. Immunity. 43 (5), 896-908 (2015).
- Shin, D. S., et al. Lung injury induces a polarized immune response by self-antigen-specific CD4(+) Foxp3(+) regulatory T cells. Cell Rep. 42 (8), 112839 (2023).
- Anderson, K. G., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 9 (1), 209-222 (2014).
- Vig, M., et al. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J Clin Invest. 113 (12), 1734-1742 (2004).
- Lissina, A., et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 340 (1), 11-24 (2009).
- Hogan, R. J., et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 193 (8), 981-986 (2001).
- Hogan, R. J., et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 166 (3), 1813-1822 (2001).
- Zhao, J., et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 44 (6), 1379-1391 (2016).
- Hondowicz, B. D., et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 44 (1), 155-166 (2016).
- Jungblut, M., Oeltze, K., Zehnter, I., Hasselmann, D., Bosio, A. Standardized preparation of single-cell suspensions from mouse lung tissue using the gentleMACS Dissociator. J Vis Exp. (29), e1266 (2009).
- Faustino, L. D., et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol. 21 (11), 1371-1383 (2020).
- Atif, S. M., Gibbings, S. L., Jakubzick, C. V. Isolation and identification of interstitial macrophages from the lungs using different digestion enzymes and staining strategies. Methods Mol Biol. 1784, 69-76 (2018).
- Pape, K. A., Taylor, J. J., Maul, R. W., Gearhart, P. J., Jenkins, M. K. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 331 (6021), 1203-1207 (2011).
- Naeher, D., et al. A constant affinity threshold for T cell tolerance. J Exp Med. 204 (11), 2553-2559 (2007).
- Moon, J. J., et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 108 (35), 14602-14607 (2011).
- Koehli, S., Naeher, D., Galati-Fournier, V., Zehn, D., Palmer, E. Optimal T-cell receptor affinity for inducing autoimmunity. Proc Natl Acad Sci U S A. 111 (48), 17248-17253 (2014).
- Zhang, Z., Legoux, F. P., Vaughan, S. W., Moon, J. J. Opposing peripheral fates of tissue-restricted self antigen-specific conventional and regulatory CD4(+) T cells. Eur J Immunol. 50 (1), 63-72 (2020).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved