Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Identification of Rare Antigen-Specific T Cells from Mouse Lungs with Peptide:Major Histocompatibility Complex Tetramers
W tym Artykule
Podsumowanie
We provide a detailed protocol for isolating and identifying rare antigen-specific T cell populations in mouse lungs through magnetic bead-based T cell enrichment and peptide:major histocompatibility complex (MHC) tetramers.
Streszczenie
The identification and characterization of antigen-specific T cells during health and disease remains a key to improving our understanding of immune pathophysiology. The technical challenges of tracking antigen-specific T cell populations within the endogenous T cell repertoire have been greatly advanced by the development of peptide:MHC tetramer reagents. These fluorescently labeled soluble multimers of MHC class I or class II molecules complexed to antigenic peptide epitopes bind directly to T cells with corresponding T cell receptor (TCR) specificity and can, therefore, identify antigen-specific T cell populations in their native state without a requirement for a functional response induced by ex vivo stimulation. For exceedingly rare populations, tetramer-bound T cells can be magnetically enriched to increase the sensitivity and reliability of detection.
As the investigation of tissue-resident T cell immunity deepens, there is a pressing need to identify antigen-specific T cells that traffic to and reside in nonlymphoid tissues. In this protocol, we present a detailed set of instructions for the isolation and characterization of antigen-specific T cells present within mouse lungs. This involves the isolation of T cells from digested lung tissue followed by a general T cell magnetic enrichment step and tetramer staining for flow cytometry analysis and sorting. The steps highlighted in this protocol utilize common techniques and readily available reagents, making it accessible for nearly any researcher engaged in mouse T cell immunology, and are highly adaptable for a variety of downstream analyses of any low frequency antigen-specific T cell population residing within the lungs.
Wprowadzenie
At the heart of the adaptive immune system lies the ability of a T cell to recognize and respond to a specific antigen. When and where a T cell responds to its cognate antigen determines the balance of infection and autoimmunity, homeostasis and cancer, health and disease1. It follows that the study of T cells in a specific context of immunity should focus on the cells with specificity for a relevant antigen of interest. Among the technological advancements that have greatly enhanced the ability to characterize antigen-specific T cell populations are fluorescently labeled soluble multimers (usually tetramers) of major histocompatibility complex (MHC) class I or class II molecules complexed to antigenic peptide epitopes, better known as "peptide:MHC tetramers"2,3,4,5. By representing the natural ligands of T cell antigen receptors (TCRs), peptide:MHC class I and class II tetramers provide a means to directly identify antigen-specific CD8+ and CD4+ T cells, respectively, within the endogenous repertoire of T cells in the immune system without a requirement for a response to antigen stimulation in an assay. Tetramers represent a more elegant approach to the study of antigen-specific T cells than TCR transgenic T cell adoptive transfer models6, and have been increasingly used to identify foreign and self antigen-specific T cell populations in both experimental mouse models and human disease4,5.
While tetramers can readily identify high-frequency populations of T cells that have expanded in response to antigen stimulation, their use for naive, self antigen-specific, or memory T cells is limited by the very low frequencies of these populations7. Our group and others have developed and popularized tetramer-based magnetic enrichment strategies that increase the sensitivity of detection to enable studies of these cell populations in mouse lymphoid tissues8,9,10,11.
The emergence of tissue-resident T cells in the field has placed a heightened emphasis on developing new ways to investigate T cells in the nonlymphoid space. Like many other mucosal surfaces, T cells in the lungs encounter a range of self and foreign antigens derived from host epithelium, commensal and infectious microbes, and environmental entities, including allergens. Transcriptional analysis of T cells harvested from nonlymphoid tissue (NLT) demonstrates a memory-like phenotype that bears unique tissue-specific fate and function, often directed at trafficking and tissue homeostasis12. Moreover, tissue-resident memory T cells (Trms) tend to be more clonally restricted than those in circulation13. Determining how and why antigens drive T cell residence in NLT is critical to understanding how the immune system protects against infection, maintains tissue homeostasis, and, at times, devolves into autoimmunity. However, there appears to be greater attrition amongst tissue-resident T cells from the lungs compared to other NLT14. Accordingly, the ability to identify and characterize endogenous T cells of the lung with a given antigen-specificity is limited by their inherent rarity.
By combining the use of magnetic bead-based cell enrichment techniques and peptide:MHC tetramer staining, we have succeeded in detecting expanded but rare self antigen-specific T cells in mouse lungs15,16. Here, we present a detailed description of a protocol that we have optimized to reliably isolate and characterize any rare antigen-specific T cell population present in mouse lungs (Figure 1). This protocol incorporates an in vivo antibody staining step to distinguish tissue-resident from vascular T cells17 followed by two different methods for lung tissue processing to accommodate resource availability. This is then followed by a general T cell magnetic enrichment step, tetramer staining, and analysis by flow cytometry. Cell viability and tetramer staining is further enhanced in this protocol by the addition of aminoguanidine, which blocks inducible nitric oxide synthase (iNOS)-mediated T cell activation-induced apoptosis18 and Dasatinib which limits TCR downregulation19. The steps highlighted in this protocol utilize common techniques and readily available reagents, making it accessible for nearly any researcher engaged in mouse T cell immunology and is highly adaptable for a variety of downstream analyses. Although naive T cells are not likely to be found in the lungs, we believe this protocol will be particularly helpful for the study of self antigen-specific T cells and Trms in the lungs.
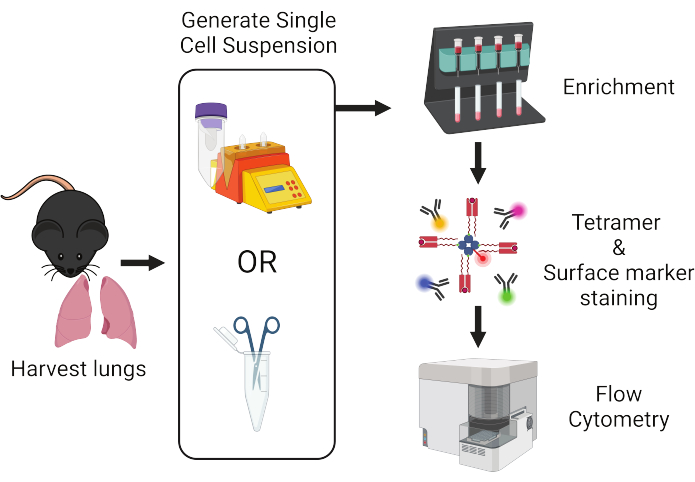
Figure 1: Overview of protocol workflow. Lungs are harvested from mice and dissociated into single cells. Samples are subsequently enriched for T cells prior to staining with peptide:MHC tetramers and fluorescently-labeled antibodies for flow cytometric analysis. Please click here to view a larger version of this figure.
Protokół
The procedures described in this protocol are approved and developed in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital, an American Association for the Accreditation of Laboratory Animal Care (AAALAC)-accredited animal management program. Experiments were performed on 8-12-week-old male and female mice on a C57BL/6 genetic background bred and maintained in the MGH animal facility under specific pathogen-free conditions.
1. Preparing stock solutions
- Prepare HEPES buffer by dissolving 10 mM HEPES, 5 mM KCl, 1.8 mM CaCl2, 150 mM NaCl, and 1 mM MgCl2, and adjust to pH 7.4.
- Prepare 10x Liberase stock solution by dissolving 700 µg/mL of Liberase Thermolysin Medium (TM) (see Table of Materials) in Hank's Balanced Salt Solution with Ca++ and Mg++ (see Table of Materials).
- Prepare 10x aminoguanidine stock solution by dissolving 100 mM Aminoguanidine Hemisulfate Salt (see Table of Materials) in Hank's Balanced Salt Solution with Ca++ and Mg++ (see Table of Materials).
- Prepare complete Eagle's Ham's Amino Acids (EHAA) medium by mixing EHAA medium (see Table of Materials) with 10% fetal bovine serum (FBS), 100 U/mL penicillin/streptomycin, 50 µg/mL gentamycin, 2 mM L-glutamine, and 55 µM 2-mercaptoethanol.
NOTE: Other common T cell media, such as RPMI or DMEM, may also be used. - Prepare sorter buffer by mixing 1x PBS with 2% FBS and 0.05% sodium azide.
- Prepare Fc block by diluting purified anti-mouse CD16/32 antibody (see Table of Materials) at 1:100 v/v in sorter buffer.
- Prepare Liberase TM/DNase digestion solution by adding 100 µg/mL Liberase TM and 50 µg/mL DNase I (see Table of Materials) in RPMI 1640 medium without L-glutamine (see Table of Materials).
- Prepare ketamine/xylazine mixture by adding 10 mg/mL ketamine (see Table of Materials) and 1 mg/mL xylazine (see Table of Materials) in normal saline (0.9% NaCl) (see Table of Materials).
2. Generating single-cell suspension from lung tissue via automated tissue dissociator
- For each mouse, prepare 4 mL of ice-cold HEPES buffer in a specialized tissue dissociator tube (see Table of Materials) and keep it on ice.
- Anesthetize mice by intraperitoneal injection of 150-200 µL of ketamine/xylazine mixture. Inject 1-3 µg of fluorophore-conjugated anti-mouse CD45 antibody (see Table of Materials) diluted in 100 µL normal saline intravenously into each anesthetized mouse. Euthanize the mouse 3 min after antibody administration with an intraperitoneal injection of 500-600 µL of ketamine/xylazine mixture.
- Resect the lungs and place them in their respective tissue dissociator tube chilled on ice. Place the tubes onto the automated tissue dissociator (see Table of Materials) and run the first program (preset program) for lung tissue.
- Add 500 µL of Liberase stock solution and 500 µL of aminoguanidine stock solution to each tube containing the dissociated lung tissue. Incubate on a nutating mixer for 30 min at 37 °C.
- Place the tubes back on the dissociator and run the second program (preset program) for lung tissue.
- Pour the single-cell suspension from the tissue dissociator tube through a 100 µm cell strainer (see Table of Materials) into a 50 mL conical tube. Rinse the tissue dissociator tube with 5 mL of complete EHAA (or any equivalent T cell medium) and pass through the strainer into the 50 mL tube.
- Remove the strainer and raise the volume of the cell suspension to 50 mL with complete EHAA. Centrifuge the cell suspension at 400 x g for 5 min at 4 °C. Carefully aspirate the supernatant, leaving behind approximately 100 µL of supernatant and cell pellet.
- Add approximately 100 µL of Fc Block solution to bring the total volume up to 200 µL and vortex vigorously to resuspend the pellet.
3. Alternative protocol: Generating single-cell suspension from lung tissue via manual dissociation
- For each mouse, prepare 1 mL of complete EHAA in a 1.5 mL microfuge tube and keep on ice.
- Anesthetize mice by intraperitoneal injection of 150-200 µL of ketamine/xylazine mixture. Inject 1-3 µg of fluorophore-conjugated anti-mouse CD45 antibody diluted in 100 µL of normal saline intravenously into each anesthetized mouse. Euthanize the mouse 3 min after antibody administration with an intraperitoneal injection of 500-600 µL of ketamine/xylazine mixture.
- Resect the lungs and place them in their respective 1.5 mL microfuge tube chilled on ice.
- Once all the lungs have been harvested, transfer each lung to a fresh microfuge tube without any cell media.
- Cut the lungs into small fragments (~2-3 mm chunks) using scissors within the microfuge tube.
- Add 1 mL of Liberase TM/DNase I digestion cocktail to each tube.
- Incubate for 30 min at 37 °C in a water bath. Return the samples to ice afterward to prevent over-digestion.
- Pour the sample over a 100 µm cell strainer placed on top of a 50 mL conical tube. Mash the tissue against the mesh with the rubber end of the plunger from a sterile 1 mL syringe.
- Rinse the strainer with cold sorter buffer, collecting a final volume of 7 mL in the tube. Centrifuge the cell suspension at 400 x g for 5 min at 4 °C. Carefully aspirate the supernatant, leaving approximately 100 µL of supernatant and cell pellet.
- Add 100 µL of Fc Block solution to bring the total volume up to 200 µL and vortex vigorously to resuspend the pellet.
4. Enriching the lung sample for T cells via magnetic bead enrichment
- Add 50 µL of anti-mouse CD90.2 microbeads (see Table of Materials). Vortex and incubate for 10 min at 4 °C.
NOTE: CD90.2 corresponds to the allele expressed by T cells in C57BL/6 mice. Check allele genotype if other strains of mice are used. - In the meantime, place a paramagnetic cell separation column (see Table of Materials) onto a one or four position cell separation magnet (see Table of Materials). Position an open 15 mL conical tube under each column to capture the flow through. Prime the column with 3 mL of cold sorter buffer, allowing the column to drain by gravity into the conical tube below.
- Once the cells have incubated with the microbeads for 10 min, add cold sorter buffer to a volume of 1 mL and transfer through a 100 µm cell strainer placed atop the column. Collect the column flow through into a fresh 15 mL conical tube placed below the column.
- Once the cell suspension has completely drained through the column by gravity, wash the column by rinsing the original tube with an additional 3 mL of cold sorter buffer and applying it to the column through the mesh, thereby rinsing the strainer. Remove the strainer.
- When the sample has again completely drained into the column, and no further flowthrough is exiting the column, remove the column from the magnet and place it atop a fresh 15 mL conical tube.
- Add 5 mL of cold sorter buffer to the column.
- Immediately elute the cells bound to the column by pushing the column plunger into the top in one continuous motion and forcing the sorter buffer out of the bottom of the column into the new tube.
- Centrifuge the eluted samples at 400 x g for 5 min at 4 °C. Carefully aspirate the supernatant, leaving behind approximately 100 µL of supernatant and cell pellet.
- Add 100 µL of cold sorter buffer to bring the total volume up to 200 µL and vortex vigorously to resuspend the pellet.
- Analyze the flowthrough sample containing the unbound cell fraction, if desired.
5. Staining the antigen-specific T cells with peptide:MHC tetramers
- Add 1 µL of 10 µM Dasatinib (see Table of Materials) to each sample, vortex, and incubate for 5 min at RT.
- Add PE- or APC-conjugated peptide:MHC tetramer to a final concentration of 10 nM (or empirically optimized concentration for a given tetramer).
- Vortex and incubate in the dark for 1 h at RT (or empirically optimized time and temperature).
6. Analyzing the tetramer-labeled T cells by flow cytometry
- While the sample is being stained with peptide:MHC tetramers, prepare a master mix of antibodies to stain the surface markers of the cells (Table 1).
NOTE: Avoid fluorophores that conflict with the intravenously administered CD45 antibody or the tetramers. - With 15 min left in the incubation period for tetramer-staining, add surface antibody master mix at 1:100 (or empirically optimized concentration) to each sample.
- Continue to incubate in the dark at RT for the remainder of the 1 h incubation period.
- Add 50,000 flow cytometry count beads (see Table of Materials) and add cold sorter buffer to a final volume of approximately 5 mL. Centrifuge the stained sample at 400 x g for 5 min at 4 °C. Carefully aspirate the supernatant, leaving behind approximately 100 µL of supernatant and cell/bead pellet.
- Resuspend the sample with an additional 200 µL of sorter buffer and transfer the sample to a 5 mL fluorescence activated cell sorting (FACS) tube.
- Analyze the sample on a flow cytometer. Collect as many cells as possible, up to a maximum of 2,500,000 total events. Ensure that at least 20,000 count bead events have been collected. Keep the acquisition rate at or below 5,000 events per second.
- Save all data as FCS files.
| Fluorochrome | Antibody |
| BUV395 | CD90.2 |
| Pacific Blue | CD45 (added earlier by i.v. injection) |
| Pacific Orange | CD8 |
| Brilliant Violet 785 | CD4 |
| FITC | CD3 |
| PerCP-Cy5.5 | Dump (B220, CD11b, CD11c, F4/80) |
| PE | pMHC tetramer or phenotypic marker |
| PE-Cy7 | Phenotypic marker (e.g. CD44, PD-1, CD69, etc.) |
| APC | pMHC tetramer or phenotypic marker |
| AlexaFluor 700 | Phenotypic marker (e.g. CD44, PD-1, CD69, etc.) |
| APC-Cy7 | Live/dead stain |
Table 1: Sample staining matrix. A typical panel of fluorophore-conjugated flow cytometry antibodies utilized for identifying and characterizing antigen-specific T cells.
7. Analyzing data
- Analyze the FCS data files using appropriate flow cytometry software.
- Set up a sequence of successive inclusion gates to identify 1) lymphocyte-like, 2) single, 3) live, 4) extravascular, 5) dump-negative, and 6) CD90.2-positive events that are either CD4 or CD8 positive (Figure 2).
- Identify the tetramer-positive T cells within these populations.
- Determine absolute tetramer-positive cell counts by calculating the ratio of beads added (50,000) to the number of beads collected during the sample run and multiplying the value with the total number of tetramer-positive cells collected.
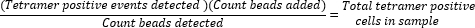
Wyniki
Figure 2 depicts the representative gating strategy used in the identification of rare antigen-specific CD4+ T cells in the lungs with peptide:MHC class II tetramers. The same process can be applied for antigen-specific CD8+ T cells with peptide:MHC class I tetramers (data not shown).
Due to the high number of nonlymphoid cells in the lungs, the reliable detection of rare antigen-specific T cells by tetramer requires some form of prior enrich...
Dyskusje
Prior characterizations of antigen-specific T cells from the lungs have benefitted from the robust numbers of antigen-specific T cells that expand following an acute priming event such as intranasal immunization or infection20,21,22. However, rarer T cell populations in the lungs, such as self antigen-specific T cells or tissue-resident memory T cells, are difficult to detect without some form of sample enrichment for the T cell...
Ujawnienia
The authors have no conflicts to disclose.
Podziękowania
We thank L. Kuhn for technical assistance with tissue processing and tetramer production. This work was funded by the National Institutes of Health (R01 AI107020 and P01 AI165072 to J.J.M., T32 AI007512 to D.S.S.), the Massachusetts Consortium on Pathogen Readiness (J.J.M), and the Massachusetts General Hospital Executive Committee on Research (J.J.M.).
Materiały
| Name | Company | Catalog Number | Comments |
| 100 mm cell strainer | Fisher Scientific | 22-363-549 | |
| 10x PBS without Ca++ or Mg++ | Corning | 46-013-CM | |
| 1x PBS without Ca++ or Mg++ | Corning | 21-031-CV | |
| AccuCheck Counting Beads | Invitrogen | PCB100 | |
| Aminoguanidine Hemisulfate Salt | Sigma-Aldrich | A7009 | |
| CD90.2 microbeads, mouse | Miltenyi | 130-121-278 | |
| Cell separation magnet (MidiMACS Separator) | Miltenyi | 130-042-302 | Holds single LS column |
| Cell separation magnet (QuadroMACS Separator) | Miltenyi | 130-090-976 | Holds 4 LS columns |
| Dasatinib | Sigma-Aldrich | CDS023389 | |
| DNase I | Roche | 10104159001 | |
| Eagle’s Ham’s Amino Acids medium | Sigma-Aldrich | C5572 | |
| gentleMACS | Miltenyi | 130-093-235 | Automated tissue dissociator |
| gentleMACS C Tubes | Miltenyi | 130-093-237 | Automated tissue dissociator tubes |
| Hank's Balanced Salt Solution with Ca++ or Mg++ | Corning | 21-020-CM | |
| HEPES | Gibco | 15630080 | |
| Ketamine | Vedco | NDC 50989-996-06 | |
| Liberase TM | Roche | 5401119001 | |
| Pacific Blue anti-mouse CD45 antibody (Clone: 30-F11) | Biolegend | 103126 | |
| Paramagnetic cell separation columns (LS Columns) | Miltenyi | 130-042-401 | Comes with plunger |
| Purified anti mouse CD16/32 antibody (Clone: 93) | Biolegend | 101302 | |
| RPMI 1640 medium without L-glutamine | Corning | 15-040-CM | |
| Sodium Chloride 0.9% (Normal Saline) | Cytiva | Z1376 | |
| Xylazine | Pivetal | NDC 466066-750-02 |
Odniesienia
- Sun, L., Su, Y., Jiao, A., Wang, X., Zhang, B. T cells in health and disease. Signal Transduct Target Ther. 8 (1), 235 (2023).
- Altman, J. D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274 (5284), 94-96 (1996).
- Crawford, F., Kozono, H., White, J., Marrack, P., Kappler, J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 8 (6), 675-682 (1998).
- Nepom, G. T., et al. HLA class II tetramers: tools for direct analysis of antigen-specific CD4+ T cells. Arthritis Rheum. 46 (1), 5-12 (2002).
- Davis, M. M., Altman, J. D., Newell, E. W. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat Rev Immunol. 11 (8), 551-558 (2011).
- Moon, J. J., et al. Tracking epitope-specific T cells. Nat Protoc. 4 (4), 565-581 (2009).
- Jenkins, M. K., Moon, J. J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 188 (9), 4135-4140 (2012).
- Moon, J. J., et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27 (2), 203-213 (2007).
- Obar, J. J., Khanna, K. M., Lefrancois, L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 28 (6), 859-869 (2008).
- Kotturi, M. F., et al. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol. 181 (3), 2124-2133 (2008).
- Legoux, F. P., Moon, J. J. Peptide:MHC tetramer-based enrichment of epitope-specific T cells. J Vis Exp. (68), e4420 (2012).
- Szabo, P. A., Miron, M., Farber, D. L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol. 4 (34), 9673 (2019).
- Poon, M. M. L., et al. Tissue adaptation and clonal segregation of human memory T cells in barrier sites. Nat Immunol. 24 (2), 309-319 (2023).
- Zheng, M. Z. M., Wakim, L. M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15 (3), 379-388 (2022).
- Legoux, F. P., et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T Cells rather than deletion. Immunity. 43 (5), 896-908 (2015).
- Shin, D. S., et al. Lung injury induces a polarized immune response by self-antigen-specific CD4(+) Foxp3(+) regulatory T cells. Cell Rep. 42 (8), 112839 (2023).
- Anderson, K. G., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 9 (1), 209-222 (2014).
- Vig, M., et al. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J Clin Invest. 113 (12), 1734-1742 (2004).
- Lissina, A., et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 340 (1), 11-24 (2009).
- Hogan, R. J., et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 193 (8), 981-986 (2001).
- Hogan, R. J., et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 166 (3), 1813-1822 (2001).
- Zhao, J., et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 44 (6), 1379-1391 (2016).
- Hondowicz, B. D., et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 44 (1), 155-166 (2016).
- Jungblut, M., Oeltze, K., Zehnter, I., Hasselmann, D., Bosio, A. Standardized preparation of single-cell suspensions from mouse lung tissue using the gentleMACS Dissociator. J Vis Exp. (29), e1266 (2009).
- Faustino, L. D., et al. Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol. 21 (11), 1371-1383 (2020).
- Atif, S. M., Gibbings, S. L., Jakubzick, C. V. Isolation and identification of interstitial macrophages from the lungs using different digestion enzymes and staining strategies. Methods Mol Biol. 1784, 69-76 (2018).
- Pape, K. A., Taylor, J. J., Maul, R. W., Gearhart, P. J., Jenkins, M. K. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 331 (6021), 1203-1207 (2011).
- Naeher, D., et al. A constant affinity threshold for T cell tolerance. J Exp Med. 204 (11), 2553-2559 (2007).
- Moon, J. J., et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 108 (35), 14602-14607 (2011).
- Koehli, S., Naeher, D., Galati-Fournier, V., Zehn, D., Palmer, E. Optimal T-cell receptor affinity for inducing autoimmunity. Proc Natl Acad Sci U S A. 111 (48), 17248-17253 (2014).
- Zhang, Z., Legoux, F. P., Vaughan, S. W., Moon, J. J. Opposing peripheral fates of tissue-restricted self antigen-specific conventional and regulatory CD4(+) T cells. Eur J Immunol. 50 (1), 63-72 (2020).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone