면역 형광 현미경 검사법: 파라핀이 내장 된 조직 절편의 면역 형광 염색
Overview
출처: 토마스 채피1,토마스 에스 그리피스2,3,4,캐스린 엘 슈베르트페거1,3,4
1 미네소타 대학교 미니애폴리스 대학교, MN 55455
2 미네소타 대학교 비뇨기과, 미니애폴리스, MN 55455
3 Masonic 암 센터, 미네소타 대학, 미니애폴리스, MN 55455
4 면역학 센터, 미네소타 대학, 미니애폴리스, MN 55455
조직 단면의 병리학적 분석은 정상적인 조직 구조에 대한 더 나은 이해를 얻고 질병의 메커니즘에 대한 우리의 이해에 기여하기 위해 사용될 수 있습니다. 조직 생검은 환자또는 생체 내 실험에서 종종 포름알데히드 또는 파라핀 왁스에 포함시킴으로써 보존됩니다. 이렇게 하면 장기 저장 및 조직을 단면화할 수 있습니다. 조직은 마이크로토메를 사용하여 얇은 (5 μm) 섹션으로 절단되고 단면도는 유리 슬라이드에 부착됩니다. 조직 섹션은 조직 섹션 내의 특정 단백질의 검출을 허용하는 항체로 얼룩질 수 있습니다. 형광에 결합 된 항체로 염색 (또한 형광으로 알려진) - 레이저에 의해 흥분 할 때 특정 파장에서 빛을 방출 하는 화합물 -면역 형광으로 알려져 있다. 단면 내에서 단백질을 검출하는 기능은 조직 내의 세포 유형 이질성, 특정 신호 경로의 활성화 및 바이오마커의 발현과 같은 정보를 제공할 수 있다. 사용된 형광과 분석에 사용할 수 있는 현미경의 유형에 따라 여러 색상을 사용할 수 있으며, 이를 통해 표적의 멀티플렉스 분석을 가능하게 합니다.
다음 프로토콜은 파라핀 임베디드 조직 섹션의 면역 형광 염색에 관여하는 기본적인 단계를 간략하게 설명합니다. 이 프로토콜은 조직의 고정, 파라핀 포함 과정 또는 조직의 단면에 대한 세부 사항을 포함하지 않는다는 점에 유의하는 것이 중요합니다. 조직이 단면화되고 유리 슬라이드에 놓이면 일련의 등급에 대한 에탄올 (EtOH) 배큐어를 통해 수화됩니다. 단면도는 조직 단면에 항체의 비특이적 결합을 감소시키기 위하여 차단 시약으로 배양됩니다. 그 때 단면도는 불소로 직접 표지될 지도 모르거나 아닐 지도 모르다 1 차적인 항체로 배양됩니다. 1차 항체가 직접 표지되지 않으면, 단면도는 불소로 표지된 이차 항체로 인큐베이션됩니다. 상이한 항체는 다른 염색 조건을 요구할 수 있고, 따라서 항체의 최적화를 위한 제안이 포함되어 있습니다. 모든 언바운드 항체를 제거하기 위해 세척한 후, 슬라이드는 DAPI를 함유한 미디어와 함께 장착되어 핵을 형광으로 표시합니다. 마운팅 미디어가 건조되면 슬라이드는 다른 형광을 감지 할 수있는 레이저현미경을 사용하여 이미지를 이미지화 할 수 있습니다.
Procedure
1. 셋업
- 일반적인 염색 프로토콜에는 다음 단계가 포함됩니다.
- 일련의 등급에 따라 슬라이드의 조직 섹션을 다시 수화합니다.
- 조직에 항체의 비 특이적 결합을 차단하고 배경 형광을 줄이는 데 도움이 되는 차단 버퍼로 조직 섹션을 배양합니다.
- 차단 버퍼를 제거하고 1 차 항체에서 섹션을 배양, 이 시간에 항체는 펩티드 표적을 결합합니다.
- 1 차적인 항체를 제거하고 세척 완충제에서 광범위하게 섹션을 세척합니다.
- 1차 항체에 결합을 허용하기 위해 이차 항체로 섹션을 배양하고, 1차 항체가 플루오로포어로 직접 표지되지 않고 이차 항체가 요구된다.
- 슬라이드에서 이차 항체를 세척합니다.
- 슬라이드를 장착 미디어에 장착하고 형광 현미경에 시각화하기 전에 건조할 수 있습니다.
- 슬라이드 홀더(유리 또는 플라스틱), 항아리, 파이펫, PAP 펜, 습한 챔버, 커버 슬립 및 DAPI장착 미디어 등 다음과 같은 항목이 필요합니다.
- 자일렌은 슬라이드의 재수화에 사용됩니다. 자일렌은 위험하며 장갑과 실험실 코트를 포함한 적절한 PPE와 함께 연기 후드에 사용해야합니다.
- 버퍼, 솔루션 및 시약용 레시피
-
등급에 따라 에탄올
에탄올 - 160 mL 200 증거 EtOH 및 40mL ddH2O
에탄올 - 140mL 200 증거 EtOH 및 60mL ddH2O -
항원 회수 용액
10mM 나트륨 구연산염, pH 6.0 -
차단 버퍼
이차 항체가 만들어진 호스트 동물로부터 100 μL 세럼
900 μL 1X PBS
참고: 이것은 10 개의 섹션에 대한 볼륨입니다. 필요에 따라 섹션당 약 100μL 버퍼의 체적을 조정합니다. - 워시 버퍼(1X PBS)
-
등급에 따라 에탄올
2. 프로토콜
- 자일렌과 에탄올로 재수화
- 슬라이드 홀더에 슬라이드를 배치한 다음 슬라이드를 100% 자일렌 이소머 솔루션에 잠수하여 슬라이드가 완전히 솔루션으로 덮여 있는지 확인합니다.
- 100% 자일렌 이소마에서 3분 동안 인큐베이션 슬라이드. 총 3개의 개별 인큐베이션을 두 번 반복합니다. 오염을 최소화하기 위해 새로운 솔루션으로 전송하기 전에 종이 타월로 슬라이드 랙을 닦아야 합니다.
참고: 이러한 인큐베이션은 세 개의 별도 용기에서 수행되는 것이 좋습니다. - 인큐베이트 슬라이드는 100% EtOH에서 2분 동안 슬라이드합니다. 총 3개의 개별 인큐베이션을 두 번 반복합니다.
참고: 이러한 인큐베이션은 세 개의 별도 용기에서 수행되는 것이 좋습니다. - 인큐베이트 슬라이드는 95% EtOH에서 2분 동안 슬라이드합니다.
- 인큐베이트 슬라이드는 80% EtOH에서 2분 동안 슬라이드를 합니다.
- 인큐베이트 슬라이드는 70% EtOH에서 2분 동안 슬라이드를 합니다.
- 1X PBS에서 5분 동안 인큐베이션 슬라이드를 사용할 수 있습니다.
- (선택 사항) 1차 항체에 의해 인식되는 에피토프를 마스크를 풀기 위한 항원 회수
참고 1: 이 절차는 사용되는 항체에 크게 의존하며 항원 회수에 대한 요구 사항을 결정하기 위해 초기 최적화 절차를 수행하는 것이 좋습니다.
참고 2: 이 절차는 아래에 표시된 F4/80 염색으로 수행되지 않았습니다. 항원 회수에 대한 요구 사항은 각각의 새로운 항체에 최적화되어야 합니다.- 내열 플라스틱 또는 유리 슬라이드 홀더에 슬라이드를 배치하고 랙이 슬라이드로 채워져 열 분포를 보장합니다. 랙의 슬롯보다 샘플수가 적은 경우 빈 슬라이드를 사용할 수 있습니다.
- 2L 비커 - 10mL 항원 마스킹 스톡에서 990mL 물에 1000mL의 항원 미마스킹 용액1000mL에 랙을 놓습니다.
- 총 20분 동안 높은 전자레인지에 전자레인지를 돌리면 슬라이드가 물로 덮여 있는지 확인합니다.
- 비커에서 20 분 동안 슬라이드를 식힙니다.
- ddH2O가 들어 있는 슬라이드 홀더에서 슬라이드 랙을 5분 씩 세척합니다. 매번 신선한 ddH2O를 사용하여 총 3개의 개별 배큐어에 대해 두 번 반복합니다. 슬라이드는 세척 할 때마다 동일한 슬라이드 홀더로 세척 할 수 있습니다.
- 1X PBS에서 5분 동안 인큐베이션 슬라이드를 사용할 수 있습니다.
- 파프 펜으로 원을 그리는 섹션입니다. 이렇게 하면 조직 섹션을 커버하는 데 필요한 최소한의 버퍼를 사용할 수 있습니다. 조직 섹션이 건조하지 않도록 하십시오.
- 각 섹션에 차단 버퍼를 추가합니다. 단면을 커버하는 데 필요한 버퍼의 양은 섹션의 크기에 따라 다르지만 25-500 μL범위일 수 있습니다.
참고: 차단 버퍼의 선택은 사용되는 항체에 따라 달라질 수 있다. 예를 들어, 이차 항체가 제기된 숙주 동물로부터의 10% 혈청은 이차 항체의 비특이적 결합을 감소시키는 데 사용될 수 있다(예를 들어, 정상적인 염소 혈청은 염소에서 이차 항체가 제기된 경우 사용될 수 있다). 차단 버퍼의 최적화는 각 1차 항체에 대해 수행되어야 한다. F4/80을 가진 유방 종양 단면을 염색하기 위하여, PBS에서 10% 정상 염소 혈청의 0.1 ml를 이용하였다. - 실온에서 1시간 또는 최대 24시간 동안 가습 챔버에서 버퍼를 차단하는 섹션을 배양합니다. 가습 챔버는 단면이 건조하지 않도록 합니다.
- 슬라이드에서 차단 버퍼를 제거합니다. 또는 파이펫 끝으로 단면을 만지지 않도록 주의하지만 파이펫을 사용하여 차단 버퍼를 제거할 수 있습니다.
- 버퍼차단에서 1차 항체를 희석시다.
참고: 올바른 희석은 각 항체 및 샘플 유형에 대해 결정되어야 합니다. 최적화에는 최적의 염색을 식별하기 위해 일련의 희석을 수행하는 것이 포함됩니다. F4/80을 가진 유방 종양 단면도를 염색하기 위하여는, 조직 단면도는 PBS에 있는 1% 일반적인 염소 혈청에서 0.1 mL 쥐 항F4/80 항체 희석1:100에서 4°C에서 하룻밤 에 배양되었습니다. - 각 섹션에 1 차 항체를 추가하고 4 °C에서 최대 16 시간 (하룻밤) 가습 챔버에서 배양하십시오. 1 차 적인 항체 없이 버퍼를 차단에 적어도 하나의 섹션을 두고 대조군 역할을, 이차 항체에 의해 비 특이적 결합을 식별하는 데 도움이 됩니다.
- 1차 항체 또는 블로킹 버퍼를 단면도에서 빼내고 슬라이드랙에 슬라이드를 배치하고 1배 PBS로 슬라이드 홀더에 배치하여 PBS로 섹션을 세척합니다. 매번 10분 동안 3회 세척합니다.
- 이차 항체를 희석 1:200 에 차단 버퍼. 희석은 이차 항체에 따라 최적화될 수 있다.
- 이차 항체를 대조군을 포함한 모든 섹션에 추가하고 빛으로부터 보호되는 가습 챔버에서 1시간 동안 배양한다.
- 이차 항체를 단면도에서 배출하고, PBS에서 매번 10분 동안 3회 세척한다.
- 슬라이드에 DAPI가 포함된 장착 용지 2-3 방울을 추가하고 샘플에 커버슬립을 배치합니다. 장착 매체가 신속한 건조형 미디어가 아닌 경우, 누출을 방지하고 시료를 장기적으로 유지하기 위해 용융 파라핀 왁스 또는 손톱 광택으로 슬라이드의 가장자리를 밀봉해야 할 수도 있다.
- 슬라이드가 어둠 속에서 밤새 건조되도록 하고 형광 현미경을 사용하여 섹션을 이미지화하십시오.
3. 데이터 분석 및 결과
- 염색된 단면도는 형광 현미경을 사용하여 분석됩니다. 이미지 캡처 및 분석의 구체적인 세부 사항은 현미경의 사양과 분석을 수행하는 데 사용되는 소프트웨어에 따라 달라집니다. 일반적으로 이미지를 단일 색상 이미지로 촬영하고 겹쳐서 여러 색 이미지를 생성할 수 있습니다. 백분율 양성 세포는 긍정적으로 얼룩진 세포의 수를 계산하고 단면 내의 DAPI 염색 세포의 총 수로 나누어 정량화될 수 있다. 유방 종양 섹션의 F4/80 염색의 경우, 소프트웨어 라이카 응용 프로그램 제품군을 사용하여 버전 3.8, 획득 탭 및 이미지 오버레이 획득 모드에서 DAPI 와 RFP가 모두 활성화되었습니다. 노출, 게인 및 감마(DAPI와 RFP의 경우 각각 944.2ms, 1.0x 및 1.08)에 대해 각각 20.0ms, 1.0배, 1.53을 조정했습니다.이 실험마다 다릅니다. 오버레이 획득 버튼은 DAPI 및 RFP 노출의 오버레이 이미지를 만드는 데 사용되었습니다.
- 아래이미지(그림 1)는대식세포 및 기타 골수성 세포에 대한 항원을 검출하는 F4/80으로 염색된 종양 섹션의 예를 보여 주며. 단면도는 DAPI 함유 마운팅 미디어를 사용하여 장착되었고 핵은 파란색으로 나타났다.
Results
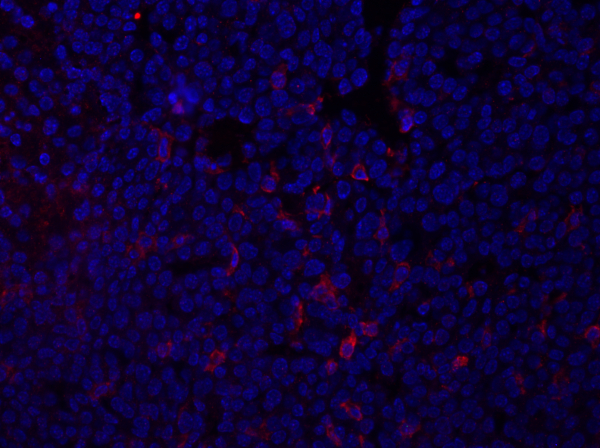
그림 1: 유방 종양 섹션의 F4/80 염색. 고정 후 마우스 유방 종양은 항 F4/80으로 단면화되고 염색되고 DAPI 함유 마운팅 미디어를 사용하여 장착되었습니다. 염색은 세포 표면 F4/80에 의해 빨간색으로 염색에 의해 표시됩니다. 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
이미징에서 얻은 데이터는 조직 섹션 내에서 관심 있는 단백질의 발현의 강도 및 국소화에 관한 정보를 제공할 것이다. 검사되는 단백질에 따라, 이 데이터는 또한 조직 단면도 내의 특정 세포 집단의 주파수에 관하여 정보를 제공할 수 있었습니다. 이것은 긍정적으로 얼룩진 세포의 수를 계산하고 총 세포 집단과 비교하여 정량화될 수 있습니다.
Application and Summary
면역 형광은 조직 섹션의 맥락 내에서 단백질 발현 및 국소화의 조사를 허용합니다. 이 기술은 정상 및 병든 조직에서 단백질 국소화 또는 세포 수를 검토해서 조직의 맥락에서 조직이 어떻게 변화하는지 이해하는 데 사용할 수 있습니다. 지역화 또는 식 패턴의 변경 내용을 결정하 고 샘플의 특정 특성에 연결 될 수 있습니다.
References
- Im K, Mareninov S., Diaz MFP, Yong WH. An Introduction to Performing Immunofluorescence Staining. Yong W. (eds) Biobanking. Methods in Molecular Biology. 1897, Humana Press, New York, NY (2019)
- Ramos-Vara JA. Principles and Methods of Immunohistochemistry. Gautier JC. (eds) Drug Safety Evaluation. Methods in Molecular Biology. 1641, Humana Press, New York, NY (2017)
- Donaldson JG. Immunofluorescence Staining. Current protocols in Cell Biology. 69 (1):1 4.3.1-4.3.7. (2015)
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
면역 형광 현미경 검사법: 파라핀이 내장 된 조직 절편의 면역 형광 염색
Immunology
53.8K Views

유세포 분석 및 형광 활성화 세포 분류 (FACS): 비장 B 림프구의 분리
Immunology
92.9K Views

자기 활성화 세포 분류 (MACS): 흉선 T 림프구 분리
Immunology
22.9K Views

ELISA 분석: 간접, 샌드위치 및 길항
Immunology
238.1K Views

ELISPOT 분석: IFN-γ 분비 비장세포 검출
Immunology
28.4K Views

면역 조직 화학 및 면역 세포 화학: 광학 현미경을 통한 조직 이미징
Immunology
78.8K Views

항체 생성: 융합 세포를 사용한 단일 클론 항체 생성
Immunology
43.5K Views

공 초점 형광 현미경: 쥐 섬유 아세포에서 단백질의 국소화를 결정하는 기술
Immunology
43.1K Views

면역침전반응 기반 기술: 아가로스 비즈를 사용한 내인성 단백질의 정제
Immunology
87.6K Views

세포주기 분석: 세포주기 CFSE 염색 및 유세포 분석을 사용한 자극 후 CD4 및 CD8 T 세포 증식 평가
Immunology
24.2K Views

입양 세포 전송: 기증자 쥐 비장 세포를 숙주 쥐에 도입 후 FACS를 통한 성공 평가
Immunology
22.3K Views

세포 사멸 분석: 세포 독성 능력의 크롬 방출 분석
Immunology
151.4K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유