Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Monitoring Cell-autonomous Circadian Clock Rhythms of Gene Expression Using Luciferase Bioluminescence Reporters
W tym Artykule
Podsumowanie
Circadian clocks function within individual cells, i.e., they are cell-autonomous. Here, we describe methods for generating cell-autonomous clock models using non-invasive, luciferase-based real-time bioluminescence technology. Reporter cells provide tractable, functional model systems for studying circadian biology.
Streszczenie
In mammals, many aspects of behavior and physiology such as sleep-wake cycles and liver metabolism are regulated by endogenous circadian clocks (reviewed1,2). The circadian time-keeping system is a hierarchical multi-oscillator network, with the central clock located in the suprachiasmatic nucleus (SCN) synchronizing and coordinating extra-SCN and peripheral clocks elsewhere1,2. Individual cells are the functional units for generation and maintenance of circadian rhythms3,4, and these oscillators of different tissue types in the organism share a remarkably similar biochemical negative feedback mechanism. However, due to interactions at the neuronal network level in the SCN and through rhythmic, systemic cues at the organismal level, circadian rhythms at the organismal level are not necessarily cell-autonomous5-7. Compared to traditional studies of locomotor activity in vivo and SCN explants ex vivo, cell-based in vitro assays allow for discovery of cell-autonomous circadian defects5,8. Strategically, cell-based models are more experimentally tractable for phenotypic characterization and rapid discovery of basic clock mechanisms5,8-13.
Because circadian rhythms are dynamic, longitudinal measurements with high temporal resolution are needed to assess clock function. In recent years, real-time bioluminescence recording using firefly luciferase as a reporter has become a common technique for studying circadian rhythms in mammals14,15, as it allows for examination of the persistence and dynamics of molecular rhythms. To monitor cell-autonomous circadian rhythms of gene expression, luciferase reporters can be introduced into cells via transient transfection13,16,17 or stable transduction5,10,18,19. Here we describe a stable transduction protocol using lentivirus-mediated gene delivery. The lentiviral vector system is superior to traditional methods such as transient transfection and germline transmission because of its efficiency and versatility: it permits efficient delivery and stable integration into the host genome of both dividing and non-dividing cells20. Once a reporter cell line is established, the dynamics of clock function can be examined through bioluminescence recording. We first describe the generation of P(Per2)-dLuc reporter lines, and then present data from this and other circadian reporters. In these assays, 3T3 mouse fibroblasts and U2OS human osteosarcoma cells are used as cellular models. We also discuss various ways of using these clock models in circadian studies. Methods described here can be applied to a great variety of cell types to study the cellular and molecular basis of circadian clocks, and may prove useful in tackling problems in other biological systems.
Protokół
1. Construction of Lentiviral Luciferase Reporters
A mammalian circadian reporter construct usually contains an expression cassette in which a circadian promoter is fused with the luciferase gene. Both ligation- and recombination-based strategies are commonly used for DNA cloning. As an example, here we describe a recombination-based Gateway cloning method for generating a P(Per2)-dLuc lentiviral reporter, in which the destabilized luciferase (dLuc) is under control of the mouse Per2 promoter.
- Cloning of Per2 promoter. Use PCR to amplify the Per2 promoter DNA fragment of 526 bp, upstream of the transcription start site from a mouse Per2 BAC clone9-13, using a forward primer (5'-CTCGAGCGGATTACCGAGGCTGGTCACG TC-3') and a reverse primer (5'-CTCGAGTCCCTTGCTCGGCCCGTCAC TTGG-3'), and clone into pENTR5'-TOPO vector (Invitrogen) to generate pENTR5'-P(Per2).
- Cloning of dLuc. The dLuc contains the firefly luciferase gene and a C-terminal PEST sequence for rapid protein degradation as previously described21. Use PCR to amplify the dLuc DNA fragment, and clone into pENTR/D-TOPO vector (Invitrogen) to generate pENTR/D-dLuc.
- Construction of reporter vector. Mix the two pENTR plasmids, pENTR5'-P(Per2) and pENTR/D-dLuc, with the lentiviral destination vector pLV7-Bsd (Bsd, blasticidin resistance gene), and perform the recombination reaction using Clonase to generate a pLV7-Bsd-P(Per2)-dLuc reporter (Figure 1). pLV7-Bsd is a modified version (made in our lab) of pLenti6/R4R2/V5-DEST (Invitrogen) in which the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) sequences22 were inserted immediately downstream of the expression cassette to enhance gene expression.
2. Production of Lentiviral Particles
1. Seed 293T cells (day 1)
- Grow human embryonic kidney (HEK) 293T cells to 90-100% confluence in regular DMEM supplemented with 10% FBS and 1x Penicillin-Streptomycin-Glutamine (PSG) on 10 cm culture dishes. (Rapidly growing cells with low passage number are critical for efficient transfection.)
- Prior to seeding the cells for transfection, coat 6-well culture plates by adding 1 ml of 0.001% poly-L-lysine in PBS to each well and incubate at room temperature for 20 min. Aspirate the solution and rinse once with 1x PBS before use.
- Dissociate 293T cells with trypsin and seed 0.75 x 106 cells onto each well of the pre-coated plates with 2 ml regular DMEM. Swirl the plates thoroughly to obtain an even distribution of cells in each well. Grow the cells in the incubator at 37 °C overnight.
2. Transient transfection via CaPO4/DNA precipitation (day 2)
- Observe the seeded cells from day 1. Cell should reach confluence of 80-90%.
- Prepare plasmid transfection mix in a 1.5 ml microcentrifuge tube by adding 2 μg of a lentiviral reporter plasmid DNA (e.g., pLV7-P(Per2)-dLuc; Liu lab) and the 3 packaging vectors (1.3 μg Gag/Pol, 0.5 μg Rev, and 0.7 μg VSVG; Invitrogen). As a control for both transfection and subsequent infection, we usually include an additional well in transfection for a lentiviral GFP expression vector, pLV156-CMV-EGFP (Figure 1A), harboring enhanced green fluorescent protein (EGFP) under the control of the CMV promoter as described previously20.
- Add 100 μl of 0.25 M CaCl2 (diluted with DNase/RNase-free ddH2O from 2.5 M stock) to the plasmid mix in step 2 and mix thoroughly. Then add 100 μl of 2x BBS solution (50 mM BES, 280 mM NaCl, 1.5 mM Na2HPO4, pH 6.95) and mix gently but thoroughly. Incubate the DNA mix at room temperature for 15 min.
- While waiting, aspirate medium from 293T cells and change to 2 ml fresh medium. Return the plate to the incubator for at least 10 min to equilibrate medium pH before transfection.
- Add transfection mix from step 3 to 293T cells drop by drop. Swirl the plate gently and observe particle formation under a microscope. Incubate at 5% CO2, 37 °C overnight. (Fine particle formation of CaPO4/DNA precipitate is critical for efficient transfection.)
3. Harvest viral particles (days 3-4)
- About 16 hours post-transfection (day 3) by which time cells should reach 100% confluence, aspirate medium from cells and replace with 2 ml fresh regular DMEM. Incubate at 37 °C overnight.
- On day 4, assess transfection efficiency by observing EGFP expression in transfection control cells (Transfection efficiency of 90-100% with high EGFP expression is a reliable predictor of a good viral prep.)
- Collect the medium containing secreted, infectious viral particles. Centrifuge at >2,000 x g for 5 min to remove residual 293T cells and collect the virus-containing supernatant. Alternatively, the medium may be cleared with a 0.45 μm membrane filter. The viral particles are ready for use in infection.
3. Infection of 3T3 Cells
1. Seed 3T3 cells (day 3)
Split and seed appropriate number (~12,000) of 3T3 cells on a 12-well plate to obtain 20-30% confluence by next day. Incubate at 37 °C overnight.
2. Infect 3T3 cells (day 4)
- Observe the seeded cells. Confluence of 20-30% (less than 50%) is desired for infection.
- Add polybrene to a final concentration of 5 μg/ml to the collected medium containing viral particles. Mix well by pipetting.
- Aspirate medium from 3T3 cells, and add 1 ml of the above viral mixture per well. Incubate at 37 °C overnight. (Polybrene is used to enhance infection efficiency, but is not absolutely required. As it may be toxic to some cells, prior testing is recommended.)
3. Select infected cells (day 5 and onward)
- Twenty-four hours post-infection, aspirate medium containing virus and polybrene from infected cells, wash once with 1x PBS, and change to fresh medium. Incubate at 37 °C overnight for recovery and growth.
- When confluent (usually 1-2 days later), split the cells and incubate at 37 °C overnight.
- The following day, aspirate medium from cells (<50% confluence is desired) and replace with fresh medium containing 10 μg/ml Blasticidin to select for stably transduced cells. (Blasticidin kill-curve needs to be empirically determined for a particular cell line.)
- Change to fresh medium containing Blasticidin every 2-3 days for continuous selection of antibiotic-resistant cells expressing clock reporters (generally 4-6 days total).
4. Bioluminescence Recording of Reporter Cells
1. Seed reporter cells
Propagate Blasticidin-resistant reporter cells and split onto 35-mm culture dishes. Incubate at 37 °C until confluent. We usually prepare ≥3 dishes for each reporter cell line under each condition for circadian phenotyping.
2. Synchronization and change to recording medium
- Aspirate medium from confluent reporter cells, wash once with PBS, and replace with DMEM containing 10 μM forskolin (or 200 nM dexamethasone). Incubate at 37 °C for 1 hour to synchronize the cells. (Alternatively, cells can be synchronized by temperature cycles23 or serum shock24.)
- While waiting, prepare recording medium for 3T3 cells as follows: 1x DMEM (HyClone) containing 10% FBS, 1x Pen/Strep/Gln, 1 μM forskolin, 1 mM luciferin, 25 mM HEPES, pH 7.4. Serum and forskolin concentration may be determined empirically. For very dim cells, phenol red-free medium may be used.
- At the end of forskolin treatment, aspirate medium and replace with freshly made recording medium.
3. Bioluminescence recording of reporter cells
- Following medium change, cover culture dishes with 40 mm sterile coverslips and seal in place with vacuum grease to prevent evaporation.
- Load the dishes onto the LumiCycle luminometer, which is kept inside an incubator set at 36 °C without H2O or CO2.
- Start real-time bioluminescence recording. We usually record rhythms for 1 week, followed by medium change and continuous recording for a second week (see Savelyev et al. for details)25. (For recording of 96-well plates, Synergy SL2 was used as the recording device; see Discussion 1.1 for detail.)
5. Data Analysis and Presentation
Reporter cells facilitate high-resolution quantitative luminescence recording, critical for determining phenotypic effects on circadian clock function. To obtain circadian parameters including phase, period length, rhythm amplitude, and damping rate, we use the LumiCycle Analysis program (Actimetrics) to analyze bioluminescence data5,14. Briefly, raw data are baseline fitted first, and baseline-subtracted data are fitted to a sine wave, from which the parameters are determined. For samples that show persistent rhythms, goodness-of-fit of >90% is usually achieved. Due to high transient bioluminescence upon medium change, we usually exclude the first cycle of data from analysis.
For data presentation, we usually plot raw data (bioluminescence, counts/sec) against time (days). When necessary, baseline-subtracted data can be plotted to compare amplitude and phase.
6. Representative Results
1. Phase-specific circadian reporters
The circadian clock is based on a biochemical negative feedback mechanism1. The core feedback loop consists of transcriptional activators BMAL1 and CLOCK, and repressors PERs and CRYs, which act on the circadian E/E'-box enhancer elements to produce rhythmic gene expression (with morning phase, e.g., Rev-erbα). The core loop regulates and integrates at least two other circadian cis-elements, the DBP/E4BP4 binding element (D-box; for day phase, e.g., Per3) and the ROR/REV-ERB binding element (RRE; for night phase, e.g., Bmal1)17. Combinatorial regulation by multiple circadian elements can generate novel intermediate phases. For example, Cry1 transcription is mediated by all three circadian elements (i.e., E/E'-box and D-box elements in the promoter and RREs in the first intron of the Cry1 gene), giving rise to the distinct Cry1 evening-time phase13.
Based on these mechanisms of gene regulation, we generated four different reporter constructs: P(Per2)-dLuc and P(Cry1)-dLuc reporters containing both E/E'-box and D-box elements in the regulatory region17,26,27; P(Cry1)-Intron-dLuc representing combinatorial regulation by all three elements (i.e., E/E'-box, D-box, and RRE)13,17; and P(Bmal1)-dLuc regulated exclusively by RRE9,17,19,21. We introduced these reporters into 3T3 cells to produce the anticipated distinct phases of reporter expression (Figure 2).
2. Gene knockdown via RNAi and pharmacologically active compounds
When transfection efficiency is high, synthetic siRNA can be transiently transfected into cells to knock down gene expression. When transfection is technically difficult, an shRNA expression vector can be stably transduced into cells via lentiviral infection, so that shRNA produced by the cell is processed to siRNA for gene knockdown (KD). Here we present KD effects of Cry1 and Cry2 genes using siRNA in U2OS cells (Figure 3A) and shRNA in 3T3 cells using a pLL3.7 Gateway expression vector9 (Figure 3B). In addition to 3T3 cells, the U2OS model has become another preeminent cellular clock model largely because it meets the key requirements for high-throughput screening of commercially available human siRNA libraries (e.g., human origin, capable of generating robust circadian rhythms, validated function of all known clock genes, and amenable to highly efficient transfection and quantitative luminescence recording). In both cell types, RNAi-mediated KD resulted in clock phenotypes consistent with previous mouse knockout (KO) and cellular KD studies5,10,11,28. For example, Cry1 KD shortens period length and reduces rhythm persistence, whereas Cry2 KD lengthens period. In addition, selected small molecules can be used to pharmacologically target and perturb protein function (Figure 3C).
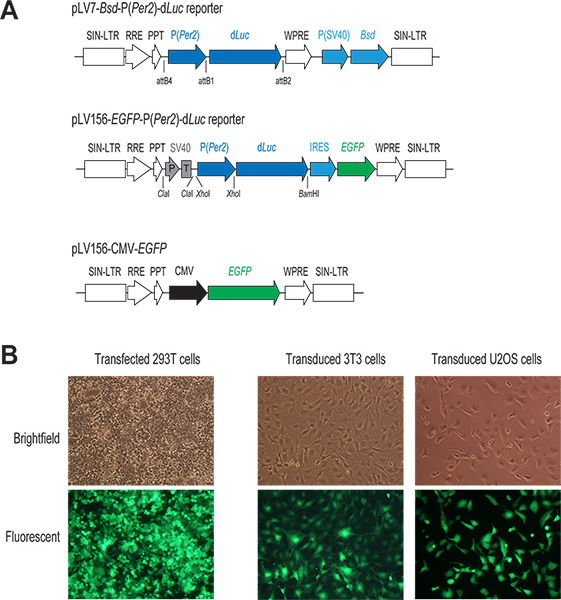
Figure 1. The lentivirus-mediated gene delivery system. (A) Schematic diagram of two lentiviral P(Per2)-dLuc reporter vectors and a CMV-EGFP construct. Only the region for integration into the host cell genome is shown. In both reporter constructs, the transcription of dLuc is under direct control of the Per2 promoter. In the pLV7-Bsd-P(Per2)-dLuc vector (recombination-based cloning), a coexpressed Blasticidin resistance gene (Bsd) facilitates selection of infected cells. In the pLV156-P(Per2)-dLuc vector (ligation-based cloning), EGFP translation is mediated by an internal ribosome entry site (IRES) downstream of dLuc, allowing for visual observation and FACS sorting of infected cells. In addition, an SV40 promoter/terminator (P/T) is used as an insulator (see discussion 1.3). In the CMV-EGFP control vector, EGFP expression is under control of a strong CMV promoter. (B) Fluorescent images of transfected and infected GFP-expressing cells. Typically, we achieve high efficiency both in transient transfection of 293T cells and in lentiviral infection of cell lines of our interest, as indicated by GFP expression in these cells. Click here to view larger figure.
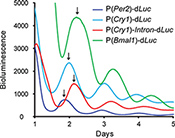
Figure 2. Phase-specific expression of bioluminescence reporters in 3T3 cells. The lentiviral reporter vectors used in this experiment are pLV7-Bsd-P(Per2)-dLuc, P(Cry1)-dLuc, P(Cry1)-Intron-dLuc, and P(Bmal1)-dLuc. Each reporter exhibits a distinct phase of oscillation, as indicated by the arrows. While the Per2 and Cry1 promoters drive peak bioluminescence at morning-day phases and the Bmal1 promoter at night phase, combinatorial regulation by the P(Cry1)-Intron harboring E-box, D-box, and RRE elements confers evening phase of peak bioluminescence. Click here to view larger figure.
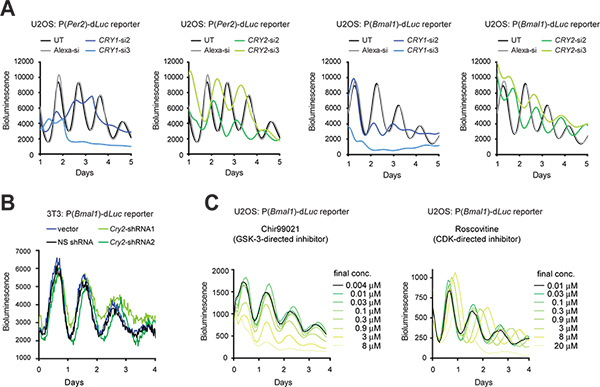
Figure 3. Genetic and pharmacological perturbation of circadian bioluminescence rhythms in reporter cells. (A) Effects of Cry1 and Cry2 knockdown by siRNAs on cellular rhythms of U2OS reporter cells. The LumiCycle luminometer was used for bioluminescence recording of cells in 35 mm dishes. Figure is adapted from Reference #10, with permission from Elsevier (2009). (B) Effects of Cry2 knockdown by shRNAs on cellular rhythms of 3T3 reporter cells. A pLL3.7 Gateway vector containing a U6-shRNA cassette was used for Cry2 gene knockdown. shRNA2 has a better knockdown efficiency than shRNA1 as determined by Western blot analysis (data not shown). A Synergy luminometer was used for bioluminescence recording of cells in a 96-well plate. The settings for recording are as follows: incubator temperature, 33 °C; integration time, 15 sec; interval time, 30 min. (C) Effects of small molecule inhibitors on cellular rhythms of U2OS reporter cells. Chir99021 and Roscovitine are inhibitors directed against GSK-3 and CDK, respectively. The ViewLux system (Chir99021 assay) and a Tecan luminometer (Roscovitine assay) were used for bioluminescence recordings of cells in 384-well plates. Figure is adapted from Reference #19 (Copyright 2008 National Academy of Sciences, U.S.A.). Click here to view larger figure.
Dyskusje
1. Modifications to Current Protocol
1.1 Recording devices and throughput considerations
Because of its commercial availability, the LumiCycle (Actimetrics) has become the most commonly used automated luminometer device for real-time recording4,5,9,19,29-31. The LumiCycle employs photomultiplier tubes (PMTs) as light detectors, which provide extremely high sensitivity and low noise14, and therefore is particularly suitable for data acquisition of ex...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported in part by the National Science Foundation (IOS-0920417) (ACL).
Materiały
| Name | Company | Catalog Number | Comments |
| DMEM | HyClone | SH30243FS | For regular cell growth |
| DMEM | Invitrogen | 12100-046 | For luminometry |
| FBS | HyClone | SH3091003 | |
| Pen/Strep/Gln(100x) | HyClone | SV3008201 | |
| B-27 | Invitrogen | 17504-044 | |
| D-Luciferin | Biosynth | L-8220 | |
| Poly-L-lysine | Sigma | P4707 | |
| Polybrene | Millipore | TR-1003-G | |
| Forskolin | Sigma | F6886 | |
| All other chemicals | Sigma | ||
| Equipment | |||
| Tissue culture incubator | 5% CO2 at 37 °C | ||
| Tissue culture hood | BSL-2 certified | ||
| Light & fluorescent microscope | Phase contrast optional | ||
| LumiCycle | Actimetrics | ||
Odniesienia
- Reppert, S. M., Weaver, D. R. Coordination of circadian timing in mammals. Nature. 418, 935-941 (2002).
- Hastings, M. H., Reddy, A. B., Maywood, E. S. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649-661 (2003).
- Nagoshi, E. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 119, 693-705 (2004).
- Welsh, D. K. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 14, 2289-2295 (2004).
- Liu, A. C. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 129, 605-616 (2007).
- Kornmann, B. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 5, e34 (2007).
- Hogenesch, J. B., Herzog, E. D. Intracellular and intercellular processes determine robustness of the circadian clock. FEBS Lett. 585, 1427-1434 (2011).
- DeBruyne, J. P., Weaver, D. R., Reppert, S. M. Peripheral circadian oscillators require CLOCK. Curr. Biol. 17, 538-539 (2007).
- Liu, A. C. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 4, e1000023 (2008).
- Zhang, E. E. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 139, 199-210 (2009).
- Baggs, J. E. Network features of the mammalian circadian clock. PLoS Biol. 7, e52 (2009).
- Hirota, T. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 8, e1000559 (2010).
- Ukai-Tadenuma, M. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 144, 268-281 (2011).
- Yamazaki, S., Takahashi, J. S. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 393, 288-301 (2005).
- Welsh, D. K., Imaizumi, T., Kay, S. A. Real-time reporting of circadian-regulated gene expression by luciferase imaging in plants and mammalian cells. Methods Enzymol. 393, 269-288 (2005).
- Sato, T. K. Feedback repression is required for mammalian circadian clock function. Nat. Genet. 38, 312-319 (2006).
- Ueda, H. R. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 37, 187-192 (2005).
- Brown, S. A. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 3, e338 (2005).
- Hirota, T. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc. Natl. Acad. Sci. U.S.A. 105, 20746-20751 (2008).
- Tiscornia, G., Singer, O., Verma, I. M. Production and purification of lentiviral vectors. Nat. Protoc. 1, 241-245 (2006).
- Ueda, H. R. A transcription factor response element for gene expression during circadian night. Nature. 418, 534-539 (2002).
- Zufferey, R., Donello, J. E., Trono, D., Hope, T. J. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 73, 2886-2892 (1999).
- Buhr, E. D., Yoo, S. H., Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 330, 379-385 (2010).
- Balsalobre, A., Damiola, F., Schibler, . U.A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 93, 929-937 (1998).
- Savelyev, S. A., Larsson, K. C., Johansson, A., Lundkvist, G. B. S. Slice Preparation, Organotypic Tissue Culturing and Luciferase Recording of Clock Gene Activity in the Suprachiasmatic Nucleus. J. Vis. Exp. (48), e2439 (2011).
- Akashi, M., Ichise, T., Mamine, T., Takumi, T. Molecular mechanism of cell-autonomous circadian gene expression of Period2, a crucial regulator of the mammalian circadian clock. Mol. Biol. Cell. 17, 555-565 (2006).
- Ohno, T., Onishi, Y., Ishida, N. A novel E4BP4 element drives circadian expression of mPeriod2. Nucleic Acids Res. 35, 648-655 (2007).
- Maier, B. A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev. 23, 708-718 (2009).
- Yoo, S. H. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339-5346 (2004).
- Liu, A. C., Lewis, W. G., Kay, S. A. Mammalian circadian signaling networks and therapeutic targets. Nat. Chem. Biol. 3, 630-639 (2007).
- Ko, C. H. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 8, e1000513 (2010).
- Izumo, M., Johnson, C. H., Yamazaki, S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc. Natl. Acad. Sci. U.S.A. 100, 16089-16094 (2003).
- Izumo, M., Sato, T. R., Straume, M., Johnson, C. H. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput. Biol. 2, e136 (2006).
- Chen, Z. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl. Acad. Sci. U.S.A. 109, 101-106 (2011).
- Yamaguchi, S. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 302, 1408-1412 (2003).
- Akashi, M., Hayasaka, N., Yamazaki, S., Node, K. Mitogen-activated protein kinase is a functional component of the autonomous circadian system in the suprachiasmatic nucleus. J. Neurosci. 28, 4619-4623 (2008).
- Hoshino, H., Nakajima, Y., Ohmiya, Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat. Methods. 4, 637-639 (2007).
- Asai, M. Visualization of mPer1 transcription in vitro: NMDA induces a rapid phase shift of mPer1 gene in cultured SCN. Curr. Biol. 11, 1524-1527 (2001).
- Wilsbacher, L. D. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice in vivo. Proc. Natl. Acad. Sci. U.S.A. 99, 489-494 (2002).
- Yamazaki, S. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 288, 682-685 (2000).
- Welsh, D. K., Noguchi, T., Yuste, R. Cellular bioluminescence imaging. Imaging: A Laboratory Manual. , 369-385 (2011).
- Nakajima, Y. Enhanced beetle luciferase for high-resolution bioluminescence imaging. PLoS One. 5, e10011 (2010).
- Guilding, C. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol. Brain. 2, 28 (2009).
- O'Neill, J. S. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 320, 949-953 (2008).
- Fuller, P. M., Lu, J., Saper, C. B. Differential rescue of light- and food-entrainable circadian rhythms. Science. 320, 1074-1077 (2008).
- Mukherjee, S. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol. Psychiatry. 68, 503-511 (2010).
- Saijo, K. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 137, 47-59 (2009).
- Elias, G. M. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 52, 307-320 (2006).
- Isojima, Y. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc. Natl. Acad. Sci. U.S.A. 106, 15744-15749 (2009).
- Bucan, M., Abel, T. The mouse: genetics meets behaviour. Nat. Rev. Genet. 3, 114-123 (2002).
- Hughes, M. E. Harmonics of circadian gene transcription in mammals. PLoS Genet. 5, e1000442 (2009).
- Atwood, A. Cell-autonomous circadian clock of hepatocytes drives rhythms in transcription and polyamine synthesis. Proc. Natl. Acad. Sci. U.S.A. 108, 18560-18565 (2011).
- Panda, S. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 109, 307-320 (2002).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone