Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
The Neuromuscular Junction: Measuring Synapse Size, Fragmentation and Changes in Synaptic Protein Density Using Confocal Fluorescence Microscopy
W tym Artykule
Podsumowanie
The neuromuscular junction (NMJ) is altered in a variety of conditions that can sometimes culminate in synaptic failure. This report describes fluorescence microscope-based methods to quantify such structural changes.
Streszczenie
The neuromuscular junction (NMJ) is the large, cholinergic relay synapse through which mammalian motor neurons control voluntary muscle contraction. Structural changes at the NMJ can result in neurotransmission failure, resulting in weakness, atrophy and even death of the muscle fiber. Many studies have investigated how genetic modifications or disease can alter the structure of the mouse NMJ. Unfortunately, it can be difficult to directly compare findings from these studies because they often employed different parameters and analytical methods. Three protocols are described here. The first uses maximum intensity projection confocal images to measure the area of acetylcholine receptor (AChR)-rich postsynaptic membrane domains at the endplate and the area of synaptic vesicle staining in the overlying presynaptic nerve terminal. The second protocol compares the relative intensities of immunostaining for synaptic proteins in the postsynaptic membrane. The third protocol uses Fluorescence Resonance Energy Transfer (FRET) to detect changes in the packing of postsynaptic AChRs at the endplate. The protocols have been developed and refined over a series of studies. Factors that influence the quality and consistency of results are discussed and normative data are provided for NMJs in healthy young adult mice.
Wprowadzenie
The neuromuscular junction (NMJ) is the critical relay synapse that mediates communication between the nervous system and skeletal muscle. It is required for all voluntary movement. Fluorescence microscopy has long been used to study the effects of transgenes on the mouse NMJ 1-3 or to compare the effects of age, diet, exercise and disease upon rodent NMJs 4-11. Such studies have taught us much about the physiology and pathophysiology of the NMJ, but the diverse parameters reported (e.g., AChR area, endplate area, perimeter length, fragmentation indices) often make it difficult to compare the findings of these studies. There is an increasing expectation for pre-clinical researchers to be able to demonstrate reproducibility, particularly in studies with rodent models of disease 12. The protocols described here were refined through a series of studies that investigated developmental, physiological and pathophysiological changes to the NMJ. Such studies require measurement of the area of synaptic specializations at the mouse motor endplate and the relative density of packing of synaptic proteins within postsynaptic specializations13-15.
The utility of these methods is illustrated by recent studies in a mouse model of anti-MuSK myasthenia gravis. Daily injections of IgG from anti-MuSK-positive myasthenia gravis patients into adult mice caused them to become weak within 2 weeks 16. Confocal maximum-projection images of muscle sections that were double-labeled for synaptophysin (in nerve-terminals) and postsynaptic AChRs revealed a progressive decline in the area of AChR staining as the primary change. Importantly the rate of decline was sufficient to explain comparable declines in the amplitude of synaptic potentials, failure of synaptic transmission and muscle weakness 17,18. Qualitatively similar findings were reported by other research groups 10,19. The same NMJ measurement methods have since been used to assess the impact of three drugs for treating anti-MuSK myasthenia gravis in this mouse model 20,21.
Sedentary aging can lead to loss of neuromuscular connections. The protocols described here have revealed an age-associated decline in the area of nerve terminal synaptophysin at motor endplates as mice progress into old age. The same methods revealed that voluntary exercise could largely prevent the reduction in presynaptic nerve terminal area 22, consistent with previous work by other groups 4. Loss of neuromuscular connections also occurs in the SOD1G93A mouse model of amyotrophic lateral sclerosis 9,23.
The studies mentioned above demonstrate that a variety of health conditions may lead to reductions in the area of either pre- or post-synaptic specializations at the NMJ. This may result in impaired synaptic function or may herald complete loss of the neuromuscular connection. Three protocols are described that allow quantitation of the area and density of synaptic specializations. The purpose of the first protocol is to provide a practical and reproducible measure of the areas of pre- and post-synaptic specializations and their alignment at mammalian NMJs, using fluorescence microscopy. Two-dimensional maximum projection confocal images and image analysis with NIH ImageJ is used to detect changes in the area of synaptophysin staining (synaptic vesicles), postsynaptic AChRs and synaptic overlap area. Confocal imaging parameters (gain and offset level) are optimized for each NMJ so as to maximize the visual information used to discern the area of synaptic specialization. Neuromuscular failure can also result from changes in the density of postsynaptic AChR and/or other synaptic proteins. The second protocol can be applied to detect changes in the relative density of postsynaptic proteins such as MuSK, rapsyn, dystroglycan, phosphorylated Src kinase and phosphorylated AChR 18,21.
In myasthenia gravis, a reduced density of AChR within the postsynaptic membrane is the immediate cause of synaptic failure and muscle weakness. The third protocol describes a Fluorescence Resonance Energy Transfer (FRET) method to assess changes in the proximity of adjacent AChRs within postsynaptic membranes 14,15. This method detects energy transfer between neighboring AChRs labeled with fluorescent-α-bungarotoxin (BGT). FRET occurs only when the fluorescent donor and acceptor probes are less than 10 nm apart. This can reveal (submicroscopic) changes in the tightness of AChR packing that may directly relate to the amplitude of synaptic potentials.
These three protocols, refined over the past decade, provide complementary measures of NMJ integrity in a consistent and reproducible way. Use of standardized protocols and parameters should facilitate comparison of the effects of genes and environmental interventions upon the mammalian NMJ.
Protokół
NOTE: Design, conduct and reporting of animal experiments should take account of current guidelines 24. Such work must be approved in advance by the local animal welfare authority (in our case the Animal Ethics Committee of the University of Sydney).
1. Euthanasia of the Animal and Muscle Dissection
- Transfer the mouse from the holding room to a separate room where it is euthanized with an intraperitoneal injection of pentobarbitone solution (30 mg/kg) using the mouse handling method detailed by Shimizu 25. Place the mouse back into its cage.
- Once the breathing of the mouse has stopped for more than 1 min, test the foot-withdrawal reflex by gently pinching the foot, and the corneal reflex by lightly brushing the cornea. Only when reflex responses are absent can the mouse be prepared for dissection.
- Consult an atlas of rodent anatomy such as Chiasson 26 and/or seek the help of an experienced anatomist before attempting dissection of the muscle of interest. In each case remove hair from the overlying skin using a small electric shaver before opening the skin to expose the muscle.
NOTE: The dissection will differ for each anatomically-distinct muscle. - Using blunt forceps free the muscle from overlying membranes and surrounding tissues. Grasp and cut the distal tendon to separate the muscle from its insertion.
- Gently tease and snip the muscle free from surrounding tissue right back to its origin. Briefly place the newly dissected muscle into 0.1 M phosphate buffered saline (PBS) solution or Ringer’s Solution prior to further processing.
2. Preparing the Muscle for Cryosectioning
NOTE: Optimal structural preservation can be achieved by whole animal perfusion as previously detailed 27, or immersion fixation (for small muscles) as described in optional step 2.1. However, 4% paraformaldehyde fixation can impair subsequent staining with many antibody probes and with fluorescent-BGT. Glutaraldehyde particularly should be avoided. If muscles are not to be fixed they must be immediately snap frozen (proceed to 2.3).
- Optional immersion fixation: Pin the muscle to wax in a Petri dish at resting length. Cover the muscle with 2% w/v paraformaldehyde (freshly dissolved in PBS) for 2 hr at RT. Wash it with 3 changes of PBS over 30 min (3 x 10 min) then replace the PBS with 30% w/v sucrose in PBS and incubate O/N at 4 °C.
- Make molds ('boats') in advance by folding 2 cm x 1.5 cm pieces of aluminum foil as shown in Figure 1. Place a piece of nitrocellulose membrane in the bottom of the boat. Gently pour cryostat embedding matrix (Materials table) into the boat to a depth of 2 mm, taking care to avoid air bubbles. Place the muscle into the boat, aligning it with the ball-point pen lines on the nitrocellulose. Add more embedding matrix so as to completely cover the muscle (Figure 1).
- Pre-label polypropylene tubes with an indelible marker. Place a drop of water in each tube and chill the tube in liquid nitrogen.
NOTE: The frozen water drop maintains the vapor pressure and prevents desiccation during prolonged -80 °C storage - Using a face shield, thick protective gloves and a large pair of blunt forceps, partially lower a small metal beaker (3 cm diameter, 8 cm deep) containing 2 cm depth of isopentane into a container of liquid nitrogen for 30 sec. Remove the beaker and place it on the bench top. Using a smaller pair of blunt forceps place the mold containing the muscle and embedding matrix into the chilled isopentane. Take care to avoid liquid nitrogen mixing with the isopentane.
- Allow 2 min for the block to freeze completely before using blunt forceps to lift the frozen block out and seal it in the correct pre-labeled and pre-chilled tube (step 2.3).
- Store the tubes temporarily in the liquid nitrogen prior to transfer to -80 °C. Log all samples in a spreadsheet of freezer contents.
3. Cryosectioning and Fluorescence Staining for En Face Images of NMJs
- Peel away the aluminum mold. Within the -20 °C cryostat chamber attach the frozen block to the cryostat chuck so as to cut 20 μm cryosections parallel to the long axis of the muscle fibers (Figure 1). Pick up the sections on poly-L-lysine or gelatin coated microscope slides.
- NOTE: Omit this step if the tissue is fixed prior to freezing. After allowing 30 min for sections to dry onto the slides, fix them by placing a drop of 2% paraformaldehyde in PBS over each section for 15 min at RT.
- Wash slides 3 x 10 min in PBS in a Coplin jar, and then immerse the slides in PBS containing 0.1 M glycine for 30 min to block residual aldehyde groups.
- Wash slides for 10 min in PBS, then immerse in methanol (chilled to -20 ºC) for 7 min. This permeabilization step is a routine part of double labeling with fluorescent-BGT and anti-synaptophysin but it may adversely affect immunostaining for some other proteins.
- Wash slides 2 x 10 min in PBS then place each slide in a stable and leveled humidified chamber. Immediately cover each section with 20 µl of blocking solution (0.2% Triton X-100, 2% bovine serum albumin (BSA) in PBS) for 1 hr at RT. Sections must not be allowed to dry out at any stage of the immunostaining process.
- Carry out the primary incubation: Taking one slide at a time carefully remove the excess blocking solution from over each section and replace it with 20 µl of rabbit anti-synaptophysin (diluted 1:200 in the blocking solution).
- Include a negative-control slide that will be incubated with blocking solution only. This 'no-primary antibody control' is essential in every immunostaining run.
- Taking care that the primary antibody remains in place over each section, close the humidified chamber and incubate for 1-2 days at 4 ºC.
- Inspect each section to confirm that the primary antibody remains in place. Use a Pasteur pipette to gently rinse each slide with PBS and place it in a Coplin jar. Wash all the slides 3 x 10 min in PBS.
- Carry out secondary incubation. Taking one slide at a time, carefully remove excess PBS, lay it in the humidified chamber and cover each section with 20 µl of a mixture containing FITC-conjugated donkey anti-rabbit IgG and BGT conjugated to tetramethyl rhodamine or another red fluorophor (TRITC-/redBGT; 5 g/ml) diluted in blocking solution. Incubate at RT for 2 hr.
- Wash slides 3 x 10 min in PBS in Coplin jars.
- Taking one slide at a time, carefully remove excess PBS and mount with a coverslip using a minimal volume of, glycerol-based, fade-resisting mounting medium. Seal the edges of the coverslips with clear nail varnish. Allow it to dry hard.
- Store the slides in the dark at 4 ºC for up to one week, or at -20 ºC for longer storage periods (up to several months).
4. Unbiased Sampling and En Face Imaging of Motor Endplates
- Blind the slides by labeling each slide with a random code number that remains known only to a second researcher (not involved in the analysis). As a result the operator remains blind to treatment groups until quantitation of NMJ parameters is complete for all the samples.
- Place the slide on the microscope stage and view it under wide field illumination with the TRITC filter set (63X oil 1.3 N.A. objective). Move progressively (field by field) left to right and back until an endplate appears in the field (Figure 2A).
NOTE: Sampling criterion: Every AChR-stained structure that is relatively flat and faces the objective (i.e., extends <15 m in the z-dimension) is considered an endplate and is imaged for analysis (crescents of AChR staining represent cross-sections through endplates and are therefore excluded). - With the confocal pinhole set to 1.0 Airy unit and low laser power optimize the gain and offset levels for TRITC/red-BGT (532 nm laser) at the endplate that is to be imaged. Next optimize FITC/synaptophysin fluorescence using the 488 nm laser. Collect a z-stack of the endplate with a 0.7 μm interval between each optical slice. Save the images with a file name that includes the date of the imaging session, the code name of the slide and the number of the endplate.
NOTE: The scans using the 488 nm and 532 nm lasers (FITC and TRITC) should be collected sequentially (not simultaneously) to avoid contamination of the FITC channel by fluorescence from the red fluorophore and vice versa (bleed-through). - Repeat the sampling and imaging of steps 4.2-4.3 until 20 endplates are collected from the slide/sample.
- Change to the next coded slide and repeat 4.2-4.4. Repeat this for each of the coded slides.
- Collect a few images of endplates from the control slide (no-primary antibody control) using confocal settings that were found optimal for the experimental slides (the FITC fluorescence channel should appear dark).
- At the end of the confocal session transfer the image files to another computer and back up the original files on an external drive or server.
5. Measuring the Area of Synaptic Specializations in En Face Images
- Use NIH ImageJ freeware (http://imagej.nih.gov/ij/) to prepare maximum projection (MIP) images from each z-stack. Save them as tiff files (Figure 2A & B). Filenames should include the image session date, sample code, endplate number and fluorescent channel (e.g., 060414_5723_7_FITC.tiff ).
- Open the z-projection image in ImageJ. Select the acetylcholine receptor image channel (Figure 3A) and select: Image > Type > 8-bit to convert the 24-bit RGB colored image into three 8-bit grayscale images on the screen.
- Using the ImageJ polygon tool draw a rough outline around the endplate of interest in the redBGT stained (ACHR) channel so as to include all apparent stained regions of the particular individual endplate, whilst excluding any staining that does not originate from the endplate of interest (Figure 3C).
- Apply a minimum intensity threshold to the image by selecting: Image > Adjust > Threshold (Figure 3E and associated ImageJ screenshots).
- Adjust the threshold level so as to isolate the AChR-stained portions while excluding surrounding background signal as sub-threshold (Figure 3E). Open a second window with the original (continuous-tone) image immediately beside the window for comparison, to facilitate the decision about the threshold value. Record the threshold value for later use in colocalization analyses.
- Retaining the polygon outline around the endplate select: Analyze > Analyze Particles. In the pop-up menu specify the range of sizes as: 50 to infinity pixels (this eliminates tiny artifacts arising from electrical noise in the photomultiplier).
- Analyze Particles command generates a window with a list of discrete supra-threshold areas and their fluorescence intensity values numbered as they appear in the binary image (Figure 3G and associated ImageJ screenshot). Copy this data into a labeled spreadsheet.
- Measure the Total endplate area (area within the polygon) by selecting: Analyze > Measure. This yields the total endplate area. Copy and paste the data for AChR areas and intensities into a spreadsheet making sure to label columns appropriately, rows will be used for individual endplates for specific slides.
- Switch to the anti-synaptophysin fluorescence channel and repeat steps 5.1 - 5.5, but for the FITC channel (Figure 3B, D and F). The aim is to adjust the threshold so that it creates a binary image that, as closely as possible, matches the boundaries of staining as perceived by eye. Record the threshold value.
- Measure the area of overlap by applying the following steps: Open the original file containing the two channel images and split it into two separate images by selecting: Image > Stacks > Stack to Images.
- Using the Colocalization plugin (downloaded and installed from the ImageJ webpage) Select: Pluggin > Colocalization and input the threshold values previously recorded for the AChR and nerve channels into the respective channel query box. This will yield an overlap image in white pixels (Figure 3H and associated ImageJ screenshots).
- Convert the newly created overlap image into a grayscale format and apply a threshold to the maximum value. The maximum threshold will only select the white pixels, corresponding to the overlap area of the two previous channels. Record in the spreadsheet the resulting area value of 'colocalization', which represents the area of overlap in pixels.
- Prepare a spreadsheet of data sample means, calculate and plot standard deviations and standard errors as histograms or scatterplots 20,22. Note that the value of n generally represents the number of mice per sample group for statistical purposes.
- Plot endplate AChR areas as scatterplots or frequency histograms to determine whether the data is normally distributed before statistical testing (Figure 6).
6. Relative Staining Intensities Compared Using Transverse Optical Sections
NOTE: For this protocol process all muscle samples together and image in a single confocal session. In planning an experiment allow up to 30 min imaging time per muscle sample.
- Cut 15 µm cryosections transverse to the long axis of the muscle fibers and collect onto slides as described at step 3.1.
- Carry out fluorescence staining as described in steps 3.2-3.13.
- Code the stained slides so that imaging and analyses are undertaken with the operator blind to treatment group, as described in step 4.1.
- Using a 40X fluorescence objective (N.A. 0.75) briefly survey a section from each slide to determine a single gain and offset level setting for AChR that will be suitable for all the endplates across all sample slides. The brightest endplate should then be just below 256 grey on the scale. This optimization should be done separately for the second fluorescence channel (collected successively). Record the fixed gain and offset level settings and do not alter them throughout the imaging session.
- Collect images of a fluorescence standard slide (e.g., non-bleaching fluorescent beads), using the same parameters, at the beginning and end of the confocal session to detect any possible fluctuation in laser intensity.
- Use the AChR channel to scan the slide progressively to locate endplates.
- Focus to find the single optical section plane in each microscope field that contains the most number of AChR-stained endplates.
- Scan this single optical section twice and save the averaged image (Figure 4G).
- Keeping the same focal plane switch to the second fluorescence channel (protein of interest) and collect the image as at step 6.8. Save the image file, including in the file name: date of imaging session, sample code, image number and a symbol to indicate the fluorescent channel. Figure 4A-F shows examples of the endplate distribution of AChR compared to rapsyn, MuSK or -dystroglycan (-DG).
- Move the stage to the next field that contains one or more endplates and repeat step 6.8-6.9. Repeat this until a total of 60 endplates are imaged.
- At the end of the imaging session transfer all files to another computer and back them up.
- Open each original image file and while viewing the AChR channel, select: Image > Stacks > Stack to Images, to split channels.
- Select: Image > Type > 8bit to convert to 8-bit grayscale format on the screen. Do this for both fluorescence channels.
- Select: Image > Stacks > Images to Stack. Open a new stack from two previously separated 8-bit images. One can then switch conveniently between the two fluorescence channels within the single window.
- Use the polygon tool to draw a line tightly around the boundary of the AChR staining (Figure 4I).
- Select: Analyze > Measure to measure the average pixel intensity for AChR within the enclosed area (note the importance of drawing the line tightly). Copy this value into a labeled spreadsheet.
- Retaining the same polygon outline (to define the area to be measured), switch to the second fluorescent channel (e.g., Figure 4B, D, F) and select: Analyze > measure. This will yield the average staining intensity for the protein of interest within the synaptic area defined by AChR staining.
- Choose an area away from visible endplate staining then select: Analyze > Measure to measure the average background fluorescence intensity. Repeat this for the other fluorescence channel/s and copy the background values into the spreadsheet of fluorescence values.
- Subtract the average background values from endplate values to obtain the corrected intensities for AChR and the protein of interest at each endplate.
- Divide the corrected endplate intensity values for the protein of interest by the corrected BGT fluorescence intensity to yield the fluorescence intensity ratios 14,21
7. Comparing the Postsynaptic Membrane AChR Density Using FRET
NOTE: This protocol assesses the extent to which AChRs are closely packed (<10 nm spacing) in the postsynaptic membrane. The precise donor and acceptor fluorophore combination is critical to this FRET assay. Names and details of the fluorophores are given in the Materials table. Their spectral properties, in relation to FRET, are discussed in our previous papers 14,15.
- Prepare fixed transverse cryosections as described in section 6.1. All sample groups must be processed together and imaged in the same confocal session.
- Thoroughly mix 2.5 g/ml red-BGT (FRET donor) with 10 g/ml far red-BGT (FRET acceptor) with blocking solution in a small plastic tube by pipetting up and down 12 times. This 1:4 molar mixture maximizes the efficiency of FRET 14.
- Place each slide in a humidified chamber, carefully cover each section with a drop (12 µl) of the above mixture and incubate for 1.5 hr at RT.
- Control sections: cover small numbers of sections with 2.5 g/ml red-BGT (donor only; labeled C1 controls), and also some sections with 10 g/ml far-red-BGT (acceptor only; labeled C2 controls). Incubate these controls as at step 7.3.
- Wash slides 3 x 10 min in PBS and mount in glycerol-based, fade-resisting mounting medium (see step 3.12).
- Perform sampling of endplates as in step 6.7. Fluorescence from the donor and acceptor should be perfectly co-localized at endplates due to the random binding of the fluorescent-BGT molecules.
- Control images: Using the 40X objective and low laser power optimize redBGT gain and offset level settings for endplates from a control slide C1. Optimize far-redBGT gain and offset levels for endplates from control slide C2. Confirm the absence of any fluorescence bleed-through.
- Without changing the laser power, gain or offset-level settings move to the experimental slides and collect images (pre-photobleach) for both fluorescence channels.
- Selectively photobleach the far-red-BGT over a portion of a single endplate by zooming in the scan area then scanning 10 times with the 633 nm laser at 100% power. The fluorescence in the scanned area should become dim.
- Reset the laser power and zoom and collect post-bleach images on both fluorescent channels using the confocal settings established at 7.7.
- Calculate the FRET efficiency (E) from the percentage increase in donor (red-BGT) fluorescence following photobleach of the acceptor (far-red-BGT) according to the following formula*:
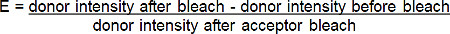
*For all situations where the fluorescence of the donor increases after photobleaching the acceptor.
Wyniki
Measurement of Synaptic Area at the NMJ
Any estimate of area relies upon the drawing of a boundary to define the extent of synaptic specializations. In healthy young adult muscles NMJ images should display well-defined boundaries for both AChR and synaptophysin staining (Figure 2A and B). Fluorescence intensity for both AChR and synaptophysin rises sharply at the boundary between the peri-synaptic and synaptic portion of the motor endplate (
Dyskusje
The protocols described here have enabled us to reliably measure and quantify changes in the properties of the NMJ across a range of conditions, including normal aging and disease states. The methods described for en face NMJ images will allow researchers to compare the area of pre- and postsynaptic specializations and the area of synaptic overlap/alignment. To compare the relative intensity of pre- and postsynaptic proteins the second protocol, which uses transverse optical sections, is preferred. The third protocol spe...
Ujawnienia
The authors declare they have no competing financial interests.
Podziękowania
This work was supported by the National Health and Medical Research Council [570930]. Imaging was carried out at the Bosch Institute Advanced Microscopy Facility. Former members of the lab, whose work is cited, are thanked for their contributions to developing these methods.
Materiały
| Name | Company | Catalog Number | Comments |
| Scanning confocal microscope | Leica | DM 2000 with TCS SP2 system | Most scanning confocal microscopes should be suitable. |
| Zeiss | LSM 510 Meta | ||
| Leica | SPE-II | ||
| Alexa555-a-bungarotoxin (red-BGT) | Life technologies | B35451 | Used for labelling AChRs |
| Alexa647-α-bungarotoxin (far-red-BGT) | Life technologies | B35450 | Far red fluorescence: barely visible through the eyepiece |
| rabbit anti-synaptophysin | Life technologies | 18-0130 | Different batches of primary antibody differ in effective working dilution |
| FITC-anti-rapsyn mab1234 | Milipore | FCMAB134F | Monoclonal antibody conjugated to FITC |
| FITC-donkey anti-rabbit IgG | Jackson | 711-095-152 | Polyclonal secondary antibodies can vary in quality according to source and batch |
| Optimal Cutting Temperature compound (O.T.C.) | ProSciTech | IA018 | Cryostat embedding matrix for freezing muscles |
| DABCO | Sigma | 10981 | Mounting medium that slows photobleaching of fluorophores |
Odniesienia
- Schmidt, N., et al. Neuregulin/ErbB regulate neuromuscular junction development by phosphorylation of α-dystrobrevin. J Cell Biol. 195, 1171-1184 (2011).
- Amenta, A. R., et al. Biglycan is an extracellular MuSK binding protein important for synapse stability. J Neurosci. 32, 2324-2334 (2012).
- Samuel, M. A., Valdez, G., Tapia, J. C., Lichtman, J. W., Sanes, J. R. Agrin and Synaptic Laminin Are Required to Maintain Adult Neuromuscular Junctions. PLOS ONE. 7, e46663 (2012).
- Valdez, G., et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci (USA). 107, 14863-14868 (2010).
- Yampolsky, P., Pacifici, P. G., Witzemann, V. Differential muscle-driven synaptic remodeling in the neuromuscular junction after denervation). Eur J Neurosci. 31, 646-658 (2010).
- Li, Y., Lee, Y., Thompson, W. J. Changes in Aging Mouse Neuromuscular Junctions Are Explained by Degeneration and Regeneration of Muscle Fiber Segments at the Synapse. J Neurosci. 31, 14910-14919 (2011).
- Zhu, H., Bhattacharyya, B. J., Lin, H., Gomez, C. M. Skeletal muscle IP3R1 receptors amplify physiological and pathological synaptic calcium signals. J Neurosci. 31, 15269-15283 (2011).
- Valdez, G., Tapia, J. C., Lichtman, J. W., Fox, M. A., Sanes, J. R. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS ONE. 7, e34640 (2012).
- Perez-Garcia, M. J., Burden, S. J. Increasing MuSK Activity Delays Denervation and Improves Motor Function in ALS Mice. Cell reports. 2, 1-6 (2012).
- Klooster, R., et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 135, 1081-1101 (2012).
- Pratt, S. J., Shah, S. B., Ward, C. W., Inacio, M. P., Stains, J. P., Lovering, R. M. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 591, 559-570 (2013).
- Landis, S. C., et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 490, 187-191 (2012).
- Gervásio, O. L., Phillips, W. D. Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J Physiol. 562, 673-685 (2005).
- Gervásio, O. L., Armson, P. F., Phillips, W. D. Developmental increase in the amount of rapsyn per acetylcholine receptor promotes postsynaptic receptor packing and stability. Dev Biol. 305, 262-275 (2007).
- Brockhausen, J., Cole, R. N., Gervásio, O. L., Ngo, S. T., Noakes, P. G., Phillips, W. D. Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse. Dev Neurobiol. 68, 1153-1169 (2008).
- Cole, R. N., Reddel, S. W., Gervásio, O. L., Phillips, W. D. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol. 63, 782-789 (2008).
- Morsch, M., Reddel, S. W., Ghazanfari, N., Toyka, K. V., Phillips, W. D. Muscle Specific Kinase autoantibodies cause synaptic failure through progressive wastage of postsynaptic acetylcholine receptors. Exp Neurol. 237, 237-286 (2012).
- Cole, R. N., Ghazanfari, N., Ngo, S. T., Gervasio, O. L., Reddel, S. W., Phillips, W. D. Patient autoantibodies deplete postsynaptic Muscle Specific Kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. J Physiol. 588, 3217-3229 (2010).
- Viegas, S., et al. Passive and active immunization models of MuSK-Ab positive myasthenia: Electrophysiological evidence for pre and postsynaptic defects. Exp Neurol. 234, 506-512 (2012).
- Morsch, M., Reddel, S. W., Ghazanfari, N., Toyka, K. V., Phillips, W. D. Pyridostigmine but not 3,4-diaminopyridine exacerbates ACh receptor loss and myasthenia induced in mice by Muscle Specific Kinase autoantibody. J Physiol. 591, 2747-2762 (2013).
- Ghazanfari, N., Morsch, M., Reddel, S. W., Liang, S. X., Phillips, W. D. Muscle Specific Kinase autoantibodies suppress the MuSK pathway and ACh receptor retention at the mouse neuromuscular junction. J Physiol. 592, 2881-2897 (2014).
- Cheng, A., Morsch, M., Murata, Y., Ghazanfari, N., Reddel, S. W., Phillips, W. D. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One. 8, e67970 (2013).
- Schaefer, A. M., Sanes, J. R., Lichtman, J. W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 490, 209-219 (2005).
- Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLos Biol. 8, e1000412 (2010).
- Shimizu, S., Hedrich, H. J., Bullock, G. Routes of Administration. The Laboratory Mouse. , (2004).
- Chiasson, R. B. . Laboratory anatomy of the white rat. , (1988).
- Gage, G. J., Kipke, D. R., Shain, W. Whole Animal Perfusion Fixation for Rodents. J. Vis. Exp. (65), e3564 (2012).
- Mitra, A. K., Stroud McCarthy, M. P., M, R. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22A by low dose electron microscopy and x-ray diffraction to 12.5A. J Cell Biol. 109, 755-774 (1989).
- Paas, Y., et al. Electron microscopic evidence for nucleation and growth of 3D acetylcholine receptor microcrystals in structured lipid-detergent matrices. Proc. Natl Acad. Sci. (USA). 100, 11309-11314 (2003).
- Samson, A. O., Scherf, T., Eisenstein, M., Chill, J. H., Anglister, J. The mechanism for acetylhcoline receptor inhibition by α-neurotoxins and species-specific resistance to α-bungarotoxin revealed by NMR). Neuron. 35, 319-332 (2002).
- Ghazanfari, N., et al. Muscle Specific Kinase: Organiser of synaptic membrane domains. Int J Biochem Cell Biol. 43, 295-298 (2011).
- Ghazanfari, N., Morsch, M., Tse, N., Reddel, S. W., Phillips, W. D. Effects of the β2-adrenoceptor agonist, albuterol, in a mouse model of anti-MuSK myasthenia gravis. PLoS ONE. 9, e87840 (2014).
- Prakash, Y. S., Miller, S. M., Huang, M., Sieck, G. C. Morphology of diaphragm neuromuscular junctions on different fibre types. J Neurocytol. 25, 88-100 (1996).
- Salpeter, M. M., Harris, R. Distribution and turnover rate of acetylcholine receptors throughout the junction folds at a vertebrate neuromuscular junction. J Cell Biol. 96, 1781-1785 (1983).
- Soper, S. A., Nutter, H. L., Keller, R. A., Davis, L. M., Shera, E. B. The photophysical constants of several fluorescent dyes pertaining to ultrasensitive fluorescence spectroscopy. Photochem Photobiol. 57, 972-977 (1993).
- Panchuk-Voloshina, N., et al. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 47, 1179-1188 (1999).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone