Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Method for Measuring Metabolism in Sorted Subpopulations of Complex Cell Communities Using Stable Isotope Tracing
W tym Artykule
Podsumowanie
This article describes a method for studying cellular metabolism in complex communities of multiple cell types, using a combination of stable isotope tracing, cell sorting to isolate specific cell types, and mass spectrometry.
Streszczenie
Mammalian cell types exhibit specialized metabolism, and there is ample evidence that various co-existing cell types engage in metabolic cooperation. Moreover, even cultures of a single cell type may contain cells in distinct metabolic states, such as resting or cycling cells. Methods for measuring metabolic activities of such subpopulations are valuable tools for understanding cellular metabolism. Complex cell populations are most commonly separated using a cell sorter, and subpopulations isolated by this method can be analyzed by metabolomics methods. However, a problem with this approach is that the cell sorting procedure subjects cells to stresses that may distort their metabolism.
To overcome these issues, we reasoned that the mass isotopomer distributions (MIDs) of metabolites from cells cultured with stable isotope-labeled nutrients are likely to be more stable than absolute metabolite concentrations, because MIDs are formed over longer time scales and should be less affected by short-term exposure to cell sorting conditions. Here, we describe a method based on this principle, combining cell sorting with liquid chromatography-high resolution mass spectrometry (LC-HRMS). The procedure involves analyzing three types of samples: (1) metabolite extracts obtained directly from the complex population; (2) extracts of "mock sorted" cells passed through the cell sorter instrument without gating any specific population; and (3) extracts of the actual sorted populations. The mock sorted cells are compared against direct extraction to verify that MIDs are indeed not altered by the cell sorting procedure itself, prior to analyzing the actual sorted populations. We show example results from HeLa cells sorted according to cell cycle phase, revealing changes in nucleotide metabolism.
Wprowadzenie
Higher organisms contain complex communities of distinct cell types that collaborate to bring about more complex functions. For example, tumors contain not only cancerous cells, but also fibroblasts, cells that constitute blood vessels, and often immune cell infiltrates1; blood contains a complex mixture of dozens of immune cell subtypes2; and even cultured cell lines may consist of multiple subpopulations, such as the luminal and basal subtypes of breast cancer cells3. Moreover, distinct cell types that coexist can exhibit metabolic "collaboration". For example, in the brain, astrocytes are thought to convert glucose to lactate, which is then "fed" to neurons that oxidize this substrate4; T lymphocytes are in some contexts dependent on adjacent dendritic cells as a source of cysteine5; and cancer cells may collaborate with associated fibroblasts in tumors6. To understand the metabolic behavior of such systems, it is essential to separate and measure the metabolic activities of the various cell types present.
By far the most widely used method for separating cell types is fluorescence-activated cell sorting. This method is broadly applicable, provided that the cell type or state of interest can be "labeled" using fluorescent antibodies, expression of engineered fluorescent proteins, or other dyes. One option is to initially separate cells types through a cell sorter, re-culture the individual cell types obtained, and then perform metabolism studies of these cultures7. However, this is only feasible if the cell type or phenotype is stable in culture conditions, and cannot capture transient behavior such as cell cycle states, nor the metabolic cooperation in co-cultures. For such cases, metabolism must be measured directly on sorted cells. This is challenging since the cell sorting procedure subjects cells to stresses that may distort their metabolism8, and we are aware of only a few studies taking this approach9,10. In particular, we have found that major metabolites such as amino acids may leak from cells kept in cell sorting buffer, so that measurements of absolute metabolite abundance are no longer reliable11 (although relative comparison between sorted fractions may still be valuable).
To circumvent these issues, we label cells with stable isotopes prior to sorting, and focus on the MIDs in cellular metabolites, rather than metabolite abundances. Since MIDs are formed over longer time scales, they should be less affected by short-term exposure to sorting conditions. We quantify MIDs using full-scan high-resolution mass spectrometry, which is sensitive enough to provide data on hundreds of metabolites starting from around 500,000 sorted cells, requiring about 30-60 min of cell sorting time. A comparison between a "mock sorted" control (cells passed through the cell sorter instrument without gating any specific population) and metabolite extraction directly from the culture dish is made to ensure that the observed MIDs are representative of those in the original culture. Depending on the choice of stable isotope tracers, various metabolic pathways can be studied with this method.
Protokół
1. Metabolite Extraction
- Extraction from dish
- Culture cells in a 6-well plate in triplicates in stable isotope labeled culture media + dialyzed supplements (serum or other growth supplements) until cells become 75% confluent.
NOTE: Here culture HeLa cells for 48 h in RPMI containing 40% U-13C-Glucose and 70% U-13C, 15N2-Glutamine and 5% dialyzed FBS (Fetal Bovine Serum). Dialyzed FBS is used to get rid of the small molecular weight metabolites which might contaminate the labeled media. Culturing cells in dialyzed supplement prior to the real experiment is recommended to ensure cells are growing normally in the medium. Supplements are dialyzed in 0.15 M NaCl solution overnight using snake skin dialysis tubing. - At the day of extraction discard culture media, rinse wells twice with 500 µL cold HBSS and then discard it.
NOTE: Here use HBSS containing 40% U-13C-Glucose since it is also in the culture media. - Add 600 µL 100% methanol pre-cooled on dry ice.
- Transfer the dish to dry ice and remove cell material with a cell scraper.
- Carefully pipette the cell extracts to a microcentrifuge tube and store at -80 °C until mass spectrometry analysis.
- Culture cells in a 6-well plate in triplicates in stable isotope labeled culture media + dialyzed supplements (serum or other growth supplements) until cells become 75% confluent.
- Extraction of mock sorted cells
- Culture cells in a 100 mm dish in triplicates in stable isotope labeled culture media + dialyzed supplements.
NOTE: Here culture HeLa cells for 48 h in 100 mm dish because a high number of cells (~4 x 106) are needed to obtain 500,000 sorted cells. This number of HeLa cells extract was required to obtain good measurement of metabolites. The culture media used was RPMI containing 40% U-13C-Glucose and 70% U-13C, 15N2-Glutamine and 5% dialyzed FBS. - At the day of extraction, discard culture media, rinse wells with 1.5 mL warm HBSS and then discard it.
- Detach cells by adding 1.5 mL trypsin/EDTA for 4 min at 37 °C. Perform the following steps in 4 °C or in ice.
- Deactivate trypsin by adding 3 mL ice cold HBSS (Hank's Balanced Salt Solution) + dialyzed supplement.
- Collect cells in a 15 mL tube and centrifuge at 750 x g for 3 min.
- Resuspend the pellet in HBSS + dialyzed supplement + 1 mM EDTA at a concentration 1-2 x 106 cells/mL, pass through 40 µm cell strainers to obtain single cells, and transfer to a 5 mL tube.
- Sort cells through cell sorter gating only for singlets, and centrifuge sorted cells at 750 x g for 3 min at 4 °C.
NOTE: Here sort HeLa cells at a rate 1,000 events/s, with instrument pressure 27 psi, and using a 100 µm nozzle. HeLa cells are big so they should be sorted at a slow event rate and large nozzle to get as intact pellet as possible. Keep the cells in cold blocks throughout sorting to decrease the metabolism. A thorough description of sorting procedure is described previously12. - Discard the supernatant and resuspend the pellet in 50 µL ice cold dH2O to obtain a homogenous pellet before adding methanol. Addition of cold methanol directly to pellet forms a solid pellet which is hard to resuspend.
- Extract metabolites by adding 540 µL methanol kept in dry ice and keep extracts at -80 °C until liquid chromatography – high resolution mass spectrometry (LC-HRMS) analysis.
- Culture cells in a 100 mm dish in triplicates in stable isotope labeled culture media + dialyzed supplements.
- Extraction of cell cycle sorted cells
- Culture cells in a 100 mm dish in triplicates in culture media + dialyzed supplements.
NOTE: Here culture HeLa cells containing Geminin Fucci Green (mAG1-hGem) probe13 which allows for sorting of G1 (negative) and SG2M (positive) cells. Cells can be cultured in stable isotope tracing media for as long as required. Here we have cultured them for 46 h in unlabeled media, then we have switched to RPMI containing 40% U-13C-Glucose and 70% U-13C, 15N2-Glutamine 2 h before starting sorting. This was done in order to be able to study the cell cycle phases which requires a short pulse labeling. - At the day of extraction, discard culture media, rinse wells with 1.5 mL warm HBSS and then discard it.
- Detach cells by adding 1.5 mL trypsin/EDTA for 4 min at 37 °C. Perform the following steps in 4 °C or in ice.
- Deactivate trypsin by adding 3 mL ice cold HBSS + dialyzed supplement.
- Collect cells in a 15 mL tube and centrifuge at 750 x g for 3 min.
- Resuspend the pellet in HBSS + dialyzed supplement + 1 mM EDTA at a concentration 1-2 x 106 cells/mL, pass through 40 µm cell strainers to obtain single cells, and transfer to a 5 mL tube.
- Sort cells through the cell sorter gating out debris and doublets, then gating for the cell marker of interest, and centrifuge sorted cells at 750 x g for 3 min at 4 °C.
NOTE: Here sort HeLa cells at a rate 1,000 events/s, with instrument pressure 27 psi, and using a 100 µm nozzle. Keep the cells in cold blocks throughout sorting to decrease the metabolism rate. - Discard the supernatant and resuspend the pellet in 50 µL ice cold dH2O to obtain a homogenous pellet before adding methanol. Addition of cold methanol directly to pellet forms a solid pellet which is hard to resuspend.
- Extract the metabolites by adding 540 µL methanol kept in dry ice and keep extracts at -80 °C until LC-HRMS analysis.
- Culture cells in a 100 mm dish in triplicates in culture media + dialyzed supplements.
2. Mass Spectrometry Analysis
Note: Here we describe the protocol for analyzing cell extracts on a LC-HRMS system. Any metabolomics methods for analysis of cell extracts can be used. Full scan analysis might be useful for detecting a wide range of metabolites.
- Calibrate the instrument using a mass spectrometry reference calibration mix.
- Thaw cell extracts on ice for 30 min and vortex for 15 s.
- Transfer 100 µL of the cell extract to a spin filter and centrifuge for 10 min at 13,000 x g at 4 °C.
- Inject 12.5 µL of the filtrate onto the LC-HRMS system.
- Separate metabolites using a Zwitterionic Hydrophilic Interaction Liquid Chromatography (ZIC-HILIC) column (150 mm x 4.6 mm, 5 µm particle size) fitted with a ZIC-HILIC guard column (20 mm × 2.1 mm) using a gradient elution of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). Start the gradient elution at 20% of solvent A and increase up to 80% in 17 min. Maintain this percentage during 4 min with a flow of 400 µL min-1 and column temperature and sample tray at 23 °C and 4 °C, respectively.
- Use an instrument coupled to the chromatographic separation for metabolites detection, a heated electrospray (H-ESI II) in both positive and negative modes as ionization source, and a full scan acquisition mode at a mass resolving power of 70,000 Full Width Half Maximum (FWHM) (m/z 200).
- Use nitrogen (purity >99.995%) for the sheath gas and auxiliary gas at a flow rate of 45 and 10 a.u. (arbitrary units) and set vaporizer temperature at 350 °C and the electrospray voltage at 4 kV in positive mode and -3.5 kV in negative mode.
3. Data Analysis
- Select a number of metabolites for which standards are available and which show good quality peaks in the samples. Good quality peaks have high signal to noise ratio. It is important to verify peak quality and make sure not to include false isotopes. Isotopic peaks which differ in shape and/or retention time are likely false.
- For isotope-labeled samples calculate mass isotopomer (MI) fractions by dividing the peak area of each MI with total peak areas of all MIs.
- Calculate the labeled carbon/nitrogen fractions, enrichment of 13C and 15N, respectively, as
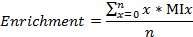
where n is the total number of carbons (or nitrogens, respectively) in the metabolite, and MIx is the MI fraction of x.
NOTE: All calculations can be performed using programming languages.
Wyniki
As an example, here we describe an experiment investigating the metabolism of HeLa cells sorted according to cell cycle phase. To label a wide range of central metabolites on both carbons and nitrogens, we cultured cells for 48 h using U-13C-glucose and U-13C, 15N-glutamine as tracers. To obtain rich MIDs for the validation experiment, we here chose a mixture of 40% U-13C-Glucose and 70% U-13C,15N2-Glutamine, as intermediat...
Dyskusje
Our method is based on the principle that MIDs in cellular metabolites reflect the "history" of metabolic activities of a cell. This makes it possible to investigate metabolic activities in subpopulation of cells, as they occurred in the complex community of cells, prior to the cell sorting procedure. In contrast, peak areas of metabolites differ markedly between extracts of sorted cells and direct extraction from the culture dish11. In part this is because the different chemical compositi...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to thank Dr. Anas Kamleh for valuable help with optimizing mass spectrometry methods, and Annika von Vollenhoven for assistance with cell sorting. This research was supported by the Swedish Foundation for Strategic Research (grant no. FFL12-0220) and the Strategic Programme in Cancer Research (IR, RN); the Swedish Heart-Lung Foundation (CEW, HG); and Mary Kay Foundation (JW, MJ).
Materiały
| Name | Company | Catalog Number | Comments |
| HBSS | Sigma | H6648 | |
| INFLUX (inFlux v7 Sorter) | BD Biosciences | ||
| U-13C-Glucose | Cambridge isotopes | 40762-22-9 / GLC-018 | |
| U-13C,15N2-Glutamine | Cambridge isotopes | CNLM-1275-H-0.1 | |
| Methanol (JT Baker), HPLC grade | VWR | BAKR8402.2500 | |
| Ultrafree - MC - VV centrifugal Filters. Durapore PVDF 0.1 µm | Millipore | UFC30VV00 | |
| Ultimate 3,000 UHPLC | Thermo Fisher scientific | ||
| Q-Exactive Orbitrap Mass spectrometer | Thermo Fisher scientific | ||
| Merk-Sequant ZIC HILIC column (150 mm x 4.6 mm, 5 µm) | Merck KGaA | 1.50444.0001 | |
| Merk-Sequant ZIC HILIC guard column (20 mm x 2.1 mm) | Merck KGaA | ||
| Acetonitrile Optima LC-MS, amber glass | Fisher Scientific | A955-212 | |
| Milli-Q water | Millipore | Produced with a Milli-Q Gradient system | |
| Myrsyra 99.5% Optima (Formic acid) | Fisher Scientific | 11423423 | |
| X100 Screw Vial 1.5 ml, 8-425 32x11.6 mm, amber, 100 units | Thermo Fisher scientific | 10560053 | |
| X100 Lock Skruv Vitt PTFE Packing 8-425 (Screw caps) | Thermo Fisher scientific | 12458636 | |
| ProteoMass LTQ/FT-Hybrid ESI Pos. Mode Cal Mix | Sigma-Aldrich | MSCAL5 | Calibration kit |
| SNAKESKIN 10K MWCO | Thermo Fisher scientific | 88245 | |
| Mathematica v.10 | Wolfram Research |
Odniesienia
- Gregersen, P. K. Cell type-specific eQTLs in the human immune system. Nat. Genet. 44 (5), 478-480 (2012).
- Heppner, G. H. Tumor heterogeneity. Cancer Res. 44 (6), 2259-2265 (1984).
- Prat, A., et al. Characterization of cell lines derived from breast cancers and normal mammary tissues for the study of the intrinsic molecular subtypes. Breast Cancer Res. Treat. 142 (2), 237-255 (2013).
- Magistretti, P. J., Allaman, I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron. 86 (4), 883-901 (2015).
- Angelini, G., et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. U. S. A. 99, 1491-1496 (2002).
- Koukourakis, M. I., Giatromanolaki, A., Harris, A. L., Sivridis, E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: A metabolic survival role for tumor-associated stroma. Cancer Res. 66 (2), 632-637 (2006).
- Hollenbaugh, J. A., Munger, J., Kim, B. Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC-MS/MS analysis. Virology. 415 (2), 153-159 (2011).
- Richardson, G. M., Lannigan, J., Macara, I. G. Does FACS perturb gene expression. Cytom. Part A. 87 (2), 166-175 (2015).
- Moussaieff, A., et al. High-resolution metabolic mapping of cell types in plant roots. Proc. Natl. Acad. Sci. U. S. A. 110 (13), E1232-E1241 (2013).
- Johnson, C., et al. A metabolic signature of colon cancer initiating cells. Cancer Metab. 2, 32 (2014).
- Roci, I., et al. Metabolite Profiling and Stable Isotope Tracing in Sorted Subpopulations of Mammalian Cells. Anal. Chem. 88 (5), 2707-2713 (2016).
- Shapiro, H. . Practical flow cytometry. , (2003).
- Sakaue-Sawano, A., et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 132 (3), 487-498 (2008).
- Noack, S., Wiechert, W. Quantitative metabolomics: A phantom. Trends Biotechnol. 32 (5), 238-244 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone