Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Using a Microfluidics Device for Mechanical Stimulation and High Resolution Imaging of C. elegans
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
New tools for mechanobiology research are needed to understand how mechanical stress activates biochemical pathways and elicits biological responses. Here, we showcase a new method for selective mechanical stimulation of immobilized animals with a microfluidic trap allowing high-resolution imaging of cellular responses.
Streszczenie
One central goal of mechanobiology is to understand the reciprocal effect of mechanical stress on proteins and cells. Despite its importance, the influence of mechanical stress on cellular function is still poorly understood. In part, this knowledge gap exists because few tools enable simultaneous deformation of tissue and cells, imaging of cellular activity in live animals, and efficient restriction of motility in otherwise highly mobile model organisms, such as the nematode Caenorhabditis elegans. The small size of C. elegans makes them an excellent match to microfluidics-based research devices, and solutions for immobilization have been presented using microfluidic devices. Although these devices allow for high-resolution imaging, the animal is fully encased in polydimethylsiloxane (PDMS) and glass, limiting physical access for delivery of mechanical force or electrophysiological recordings. Recently, we created a device that integrates pneumatic actuators with a trapping design that is compatible with high-resolution fluorescence microscopy. The actuation channel is separated from the worm-trapping channel by a thin PDMS diaphragm. This diaphragm is deflected into the side of a worm by applying pressure from an external source. The device can target individual mechanosensitive neurons. The activation of these neurons is imaged at high-resolution with genetically-encoded calcium indicators. This article presents the general method using C. elegans strains expressing calcium-sensitive activity indicator (GCaMP6s) in their touch receptor neurons (TRNs). The method, however, is not limited to TRNs nor to calcium sensors as a probe, but can be expanded to other mechanically-sensitive cells or sensors.
Wprowadzenie
The sense of touch provides animals with crucial information about their environment. Depending on the applied force, touch is perceived as innocuous, pleasurable, or painful. The tissue deformation during touch is detected by specialized mechanoreceptor cells embedded in the skin that express receptor proteins, most commonly ion channels. The steps linking force perception to ion channel activation during touch and pain are not fully understood. Even less is known about how the skin tissue filters mechanical deformation and whether mechanoreceptors detect changes in strain or stress1,2,3. This gap in understanding arises, in part, from a lack of suitable tools to apply precise mechanical stimulations to the surface of the skin of a living animal while observing the responses at the cellular level. Whereas atomic force microscopy has been used extensively to apply and measure forces in isolated cells4,5 and also to activate Piezo1 receptors in living cells6, similar experiments using living animals, especially C. elegans, have been notoriously challenging due to the intrinsic mobility of the subject. This challenge is traditionally circumvented by using veterinary- or surgical-grade cyanoacrylate glue to immobilize individual animals on agar pads1,7,8,9. This approach has been productive, but has limitations related to the skill required for immobilization by gluing and the soft agar surface on mechanical compliance. A microfluidics strategy is a complimentary alternative that avoids some of the complications linked to gluing.
The nematode C. elegans is a genetic model organism with a completely mapped nervous system that, due to the animal's size, is a good fit for microfluidics technology. Microfluidics-based devices offer the advantage that the otherwise extremely mobile animals can be restrained while performing high-resolution imaging and delivery of relevant neuro-modulatory stimuli. With the help of microfluidic technologies, living animals can be immobilized without harm10,11, enabling monitoring of behavioral activity over the entire lifetime12,13 and high-resolution imaging of neuronal activity14,15,16,17. Further, many mechanoreceptor neurons needed for the sense of touch and pain can be characterized on their physiological1,8, mechanical4,18,19, and molecular level20,21,22.
C. elegans senses gentle mechanical stimuli to its body wall using six TRNs, three of which innervate the animal's anterior (ALML/R and AVM) and three of which innervate the animal's posterior (PLML/R and PVM). The ion channel molecules needed for transducing an applied force into a biochemical signal have been extensively studied in its TRNs8. This article presents a microfluidic platform23 that enables researchers to apply precise mechanical forces to the skin of an immobilized C. elegans roundworm, while reading out the deformation of its internal tissues by optical imaging. In addition to presenting well-defined mechanical stimuli, calcium transients can be recorded in mechanoreceptor neurons with subcellular resolution and correlated with morphological and anatomical features. The device consists of a central trapping channel that holds a single animal and presents its skin next to six pneumatic actuation channels (Figure 1 and Figure 2). The six channels are positioned along the trapping channel to deliver mechanical stimuli to each of the worm's six TRNs. These channels are separated from the trapping chamber by thin PDMS diaphragms, which can be driven by an external air pressure source (Figure 1). We calibrated the deflection with respect to pressure and provide the measurements in this article. Each actuator can be addressed individually and used to stimulate a mechanoreceptor of choice. The pressure is delivered using a piezo-driven pressure pump but any alternative device can be used. We show that the pressure protocol can be used to activate TRNs in vivo and demonstrate operating devices suitable for delivering mechanical stimuli to adult C. elegans, loading adult animals into devices, performing calcium imaging experiments, and analyzing the results. Device fabrication consists of two main steps: 1) photolithography to make a mold from SU-8; and 2) molding PDMS to make a device. For the sake of brevity and clarity, readers are referred to previously published articles and protocols24,25 for instructions on how to produce the molds and devices.
Protokół
1. Device Fabrication
- Download the attached mask file (Supplemental File 1) and generate a chrome mask using a commercial service or in-house facility. As the smallest dimension on the device is 10 µm (actuator membrane thickness), ensure that the mask has sufficiently high resolution, within ± 0.25 µm, to reliably produce the features.
- Follow standard SU-8 photolithography methods (e.g., references24,25,26) to fabricate the mold for subsequent production of PDMS devices; a summary of the steps is listed below.
NOTE: SU-8 is a photosensitive material, which crosslinks upon exposure to ultraviolet light (maximum absorption ~ 365 nm). Use the manufacturer instructions as a starting point to determine the processing parameters for the soft-bake, exposure (UV), and post-bake.- Deposit SU-8 2002 onto a silicon wafer and spin-coat it to an approximate thickness of 2 µm, for better adhesion of small structures. Soft-bake on a hot plate for 1 min at 95 °C, slightly over-expose (UV) the entire surface (~ 100 mJ/cm2), and post-bake on a hot plate for 2 min at 95 °C.
- Spin-coat a 47-µm thick layer of SU-8 2050 for defining the device features. Use a spin speed of 500 rpm for 15 s (130 rpm/s acceleration) and then 2,000 rpm for 90 s (260 rpm/s acceleration). If necessary, repeat and adjust the spin speed to get the proper thickness of photoresist.
- Soft-bake on a hot plate for 2 min at 65 °C, and then for 7 min at 95 °C. Expose the photoresist according to the manufacturer instructions (~ 160 mJ/cm2) using a chrome mask and long-pass filter to ensure straight side walls.
- Remove the wafer and post-bake on a hot plate for 1 min at 65 °C, and for 7 min at 95 °C.
- Dissolve unexposed SU-8 with developer, and clean with 2-propanol.
- Bake the wafer on a hotplate at 180 °C for 30 min (hard-bake) to stabilize photoresist features.
- Make a PDMS replica from the SU-8 model using standard replica molding methods27.
- Treat the SU-8 mold with trichloromethylsilane (TCMS) vapor to reduce PDMS adhesion (silanization).
CAUTION: TCMS is toxic and water-reactive.- Place the patterned wafer in a wafer rack within a bell-jar vacuum desiccator in a fume hood free of water or water-soluble reagents.
- Under the hood, use a dropper to apply 1 drop of TCMS to a glass dish and place inside the desiccator.
- Close the desiccator lid and allow TCMS vapor to coat the wafer for at least 20 min.
- Vent and then open the desiccator. Open the bell jar lid and remove the wafer using plastic tweezers. Place into a Petri dish or aluminum foil boat for PDMS replica molding. Dispose of TCMS-coated materials in the proper hazardous waste.
- Mix PDMS (ratio of 10:1) using 40 g of the base polymer and 4 g PDMS curing agent.
- Pour the mixture over the SU-8 mold and degas the mixture for at least 30 min in a vacuum chamber.
- Cure for at least 6 h at 70 °C in an oven.
- Treat the SU-8 mold with trichloromethylsilane (TCMS) vapor to reduce PDMS adhesion (silanization).
- Lift PDMS off the wafer by setting the wafer horizontal on a flat surface and carefully peeling the PDMS off. Do not bend the wafer upwards during this step, as it can break the mold.
NOTE: Incomplete adhesion of SU-8 to the wafer or incomplete silanization can result in destruction of the SU-8 structures during this step. - Cut PDMS around the devices into individual chips such that the devices will fit on a 24 mm x 60 mm coverslip.
- Using a 1-mm biopsy punch, make holes in the inlet, two outlets, and six actuators using a 1-mm biopsy punch. Aim for the 2 mm-diameter circles around the edges of the device.
- Bond PDMS chips to glass cover slips.
- Expose both surfaces to 80 W oxygen plasma (30 s).
- Gently place the exposed PDMS surface onto the exposed surface of the cover slip for a conformal seal.
- Anneal the device on a hot plate (100 °C, 10 min).
NOTE: Insufficient bonding can lead to the failure of the device due to leakage.
2. Preparation of the Microscope
NOTE: Transgenic animals: express a calcium indicator such as GCaMP6s28 or other genetically encoded activity probe in the neuron(s) of interest (e.g., TRN); co-express an activity-independent fluorescent protein emitting at a different wavelength to correct for small lateral and out-of-focus movement artifacts that arise due to mechanical stimulation. The automated analysis software tracks and compensates for movement-induced changes in the intensity of the activity probe. The worm strains GN69223 (or AQ323629) express GCaMP6s (or GCaMP6m) and the calcium-independent tagRFP under the control of mec-7 promotor. Additionally, GN692 contains the mutation lite-1(ce314), which prevents activation of TRNs due to the blue light sensor lite-130 during excitation of the GCaMP6s fluorescence23.
- Set up a microscope system for simultaneous excitation of GCaMP and RFP.
- Use either a continuous light source and a dual band excitation filter that transmits only cyan and yellow light, or use a light source that emits only wavelengths in a defined bandwidth such as simultaneous cyan (0.77 mW) and yellow (1.21 mW) LED excitation sources for simultaneous excitation of the calcium-dependent and independent fluorescence, respectively.
- Use a digital camera to enable recording of microscopy images.
NOTE: Most cameras come with a software for image acquisition. Alternatively, there are freely available software that can be used to control the camera and potentially also other parts of the microscopy system. - Adjust excitation intensity based on fluorescence intensity to avoid camera saturation.
- Use a fluorescence cube that depending of the choice of the excitation source needs to be equipped with an excitation filter.
NOTE: A 488-nm GFP and 580-nm mCherry excitation filter was used here.- Add a beam splitter to the cube for reflecting cyan and yellow light and transmitting green and red light.
- Equip the microscope with a 10X objective and a high-magnification objective (e.g., 63X/1.32 NA oil) to focus the excitation light onto the sample.
- Mount a beam splitter in front of the camera for simultaneous recording of the GCaMP and the calcium-independent signal. Ensure that the beam splitter has a dichroic mirror (long pass, cut-off at 570 nm) to separate green and red light using one emission filter for green (passband centered at 525 nm with 50 nm width) and one emission filter for red light (passband centered at 632 nm with 60 nm width).
- Project green and red fluorescence onto the upper half (green) and lower half (red), respectively of the camera chip (see Figure 3). This orientation is a prerequisite for the provided analysis software.
3. Animal Preparation
- Prepare age-synchronized young adult or adult day one C. elegans, as described previously by Porta-de-la-Riva et al.31
- Prepare the microfluidic chip.
- Connect the gravity flow reservoir (~ 60 cm above chip level) containing filtered (0.2-µm polyethersulfone syringe filter) M9 buffer to one outlet of the chip. Connect the other outlet to one outlet of a two-outlet waste container, i.e., a filter flask. Connect the other outlet of the waste container to a peristaltic pump.
NOTE: Use polyethylene (PE) tubing for all connections and use metal tube fittings to connect the PE tubing to the chip. This way all waste solutions will be flushed out of the chip and collected in the waste container. The flow through the outlet channels creates a gentle suction that will keep the worm snug during the later trapping process. - Prepare interconnects consisting of 50-mm long PE tubing (0.9652-mm OD, 0.5842-mm ID) with metal tubing (gauge size 23TW, 0.635-mm OD) on both ends. Press-fit these interconnects into each of the six actuation fingers and into the worm inlet (see Figure 1). Leave these interconnectors in the chip, as repeated removal leads to wear on the PDMS holes.
- Connect the gravity flow reservoir (~ 60 cm above chip level) containing filtered (0.2-µm polyethersulfone syringe filter) M9 buffer to one outlet of the chip. Connect the other outlet to one outlet of a two-outlet waste container, i.e., a filter flask. Connect the other outlet of the waste container to a peristaltic pump.
- Place the chip on the microscope. Pick 2–5 worms into a drop of filtered (0.2-µm syringe filter) M9 buffer and use a 1-mL syringe to draw them up into a PE tubing (0.9652-mm OD, 0.5842-mm ID) connected to a 1-mL syringe by pulling the plunger gently. Keep the animals in the PE tube and not in the syringe.
NOTE: Too many worms or unfiltered solutions in the chip can lead to clogging. - Connect the PE tubing of the syringe to the interconnect at the worm inlet (Figure 1 and Figure 2) of the chip. Activate the gravity flow by opening the valve and start the peristaltic pump. Then gently press the syringe plunger of the syringe to move the animals into the trapping channel while observing the channel under a microscope with a 10X lens in brightfield mode.
NOTE: Occasionally-appearing air bubbles do not pose a problem; they are usually automatically moved into the outlet. Several animals can be 'parked' in the waiting chamber and used sequentially.- After loading the animal into the trapping channel (see also Figure 2), adjust its position by gently pushing or pulling the plunger of the syringe to position the head of the animal in the tapered shape of the front of the channel.
- Make sure the worm (adult day 1) has the right size to be trapped in the chip.
- If the worm does not fill the entire cross-section of the channel from the nose to near the end of the body (not including the tip of the tail) or the worm moves along the axis of the channel without moving the plunger of the syringe, remove the worm by pressing the plunger of the syringe until it disappears from the trapping channel and load a new one (see step 3.8).
- Switch to fluorescence mode of the microscope and a higher magnification lens (40X or higher) and check whether the neurite of the neuron of interest comes to lie across the diaphragm of one of the actuators. If not, adjust the position of the animal by pulling or pushing the plunger. If that does not help, remove the worm and load a new one.
- Focus on the cell body of the neuron of interest and connect the interconnect of the actuator closest to the neuron on the anterior side of the cell body to a programmable pressure pump using PE tubing.
- If the experiment requires measuring the distance between the actuator and the neuron, move the field of view so both are in the field of view and the channelwall is parallel to the upper and lower edge of the image.
- Define a pressure protocol using the programmable pressure pump.
NOTE: This protocol can be adjusted to the desired experiment.- Start with a constant pressure of 0 kPa for a time corresponding to at least 50 images of the image sequence (necessary for normalization). For an imaging rate of 10 Hz this corresponds to 5 s. Add a desired stimulus waveform and pressure and define the length of the stimulus as a second step.
NOTE: For stimulating the TRNs, a sinusoidal waveform of (i.e., 75 kPa, 10 Hz) superimposed with a step (i.e., 275 kPa) is recommended as it generates a large neuronal response. - If the experiment requires additional stimuli, include a period of at least 10 s at a constant pressure of 0 kPa in between stimuli.Before switching the pressure pump on, make sure the instrument pressure is at constant 0 kPa to avoid accidental stimulation of the worm before the actual experiment.
- Start with a constant pressure of 0 kPa for a time corresponding to at least 50 images of the image sequence (necessary for normalization). For an imaging rate of 10 Hz this corresponds to 5 s. Add a desired stimulus waveform and pressure and define the length of the stimulus as a second step.
- Run the imaging and pressure protocol.
- In the image acquisition software of the camera, set up an image sequence at 10 Hz using an exposure time of 100 ms for the duration of the pressure protocol. Adjust the excitation intensity such that the maximal fluorescence increase does not saturate the camera. Save the images as a *.tif file with at least 50 images before the first stimulus for normalization.
- Start the imaging protocol in the image acquisition software and the pressure protocol in the software of the programmable pump. During recording, observe whether the neuron of interest is the brightest spot in an area of 10 x 10 pixels, does not move farther than 10 pixels in sequential images, and stays in the field of view during the recording.
NOTE: If this is not done, the analysis software will fail. Bright spots (i.e., autofluorescence) around the neuron make clean recordings of the neurons difficult. To correct this, remove the worm from the trap and load a new worm into the chip. If the worm moves too much, try a new recording of the same worm and observe the worm motion. If the problem persists the worm might be too small to be trapped. In this case remove this worm and load a slightly bigger worm into the trap. - If desired, record signals from multiple neurons in the field of view simultaneously as long as the neurons are separated by at least 10 pixels during the entire recording.
- To investigate habituation, perform the experiment repetitively on an animal.
- Remove the worm from the trapping channel.
- If it is desired to keep the worm for subsequent studies, disconnect the outlets of the chip towards the gravity flow and the waste container. Press the plunger of the syringe gently until the entire worm is pushed through the trapping channel into the flow channel.
- Continue pressing the syringe plunger until the animal appears in a droplet outside the chip. Disconnect the syringe including the PE tubing from the worm inlet, use it to aspirate the worms in the droplet and transfer it onto an agar plate.
- If it is not desired to keep the worm, remove the worm by pressing the plunger of the syringe until the entire worm is pushed through the trapping channel into the flow channel; the gravity flow and the suction of the peristaltic pump will carry the animal out of the chip and into the waste container.
4. Analysis
- Download and install the newest Fiji version32.
- Download and install the newest java SDK version.
NOTE: If necessary, delete or rename the java folder inside the Fiji folder for Fiji to use the newly installed java compiler. - Open Fiji software.
- Check whether the software is running the newly installed java compiler by opening 'Plugins| Utilities| ImageJ| Properties'.
- Download the pokinganalyzer*.java file (https://github.com/HFehlauer/Poking-Analyzer, see Supplementary File 2).
- Drag and drop the .java file into the software window to open it as a plugin.
- Compile and run the pokinganalyzer*.java file by pressing Ctrl+R keys in Fiji; a graphical user interface (Poking Analyzer) will open (Figure 3).
- Click on the "Help" tab and read about the requirements of the software and how to perform the analysis. To begin, select the "Open video" tab. Specify the video file location for the analyzer: click the "Open a Video" button, navigate to the location of the video, and open it.
NOTE: The software starts with this tab opened. If it is not open when starting a new analysis click on the "Open video" tab. If the video is in a.tif format, the program will internally open the video and display the first image in the lower part of the interface. If the image is not of the video that needs to be analyzed, click on the "Open a video" button to open another video. - To continue, click on the "Define ROIs" tab. In this tab, define the position of the TRN. Note that the first image of the opened video is displayed in the lower part of this tab. Click on the "Define TRN" button, and click on the neuron in the upper part of the displayed image which should display the GCaMP fluorescence.
- Optional: If the actuator is visible in the upper half of the image the program can automatically track the distance between the neuron and the actuator if desired. For this, check the "Define the actuator?"-box, click on the "Define Actuator" button, and click on the actuator in the upper half of the image.
- Click on the "Analysis" tab. Define the image acquisition rate. Either enter a number in the field or click the up or down buttons to increase or decrease the number. If in the previous tab the "Define the actuator?"-box is checked, define the camera cell size, the magnification, and the binning factor, so the program can calculate the distance.
- Click on the "Start Analysis" button.
NOTE: The program will now track the neuron by its fluorescence in the image sequence in the upper half (calcium dependent) and in the lower half (calcium independent) of the image sequence. To account for neuron motion in the plane of recording the program will investigate an area of 10 x 10 pixels around the location of the neuron in the previous image to find the position of the neuron in the following image. It will calculate the fluorescence in both halves by correcting for the background fluorescence (Fbg) and dividing by the fluorescence in the first 50 images (F0):
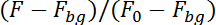
Fluorescence changes in the green, activity-dependent channel (FCa2+) that are caused by neuron motion out of the plane of recoding are then corrected using the fluorescence in the red, activity-independent channel (Fcorr) and the pre-stimulus standard deviation of the calcium-dependent (sdCa2+) and independent fluorescence (sdcorr) by:

- The program will show its progress in the status bar in the lower part of the interface; when the status bar reaches 100%, click on the "Results" tab. If the status bar stops before 100%, the program will give an error message indicating the reason the program could not analyze the video.
- If the signal to noise ratio is too low, the program cannot identify the neuron; adjust the imaging parameters in subsequent recordings.
- If the neuron leaves the field of view during the recording, the program cannot track it anymore and will stop. For subsequent recordings, place the neuron in the middle of the field of view at the beginning of the recording.
- If some regions in the field of view are oversaturated, the automated analysis will produce invalid results. For subsequent recordings, make sure the fluorescence signal does not saturate the camera sensor.
- In the 'results' tab, the result is displayed in the upper part. Save a tab-separated *.txt file of the result table by clicking on "Save the Result Table".
NOTE: This table consists of the time in seconds and the normalized calcium-dependent fluorescence (corrected by the calcium-independent fluorescence). If previously defined, it also contains the total distance between the TRN and the actuator in µm and the distance between the TRN and the actuator perpendicular to the channel wall in µm. Representative results of the fluorescence intensity increase after stimulation versus the distance of the cell body to the closest actuator are plotted in Figure 4. - If there are multiple neurons in one recording (separated by more than 10 pixels), click on the "Define ROIs" tab again and return to step 4.6.2 with the second neuron.
- Click on the "Help" tab and read about the requirements of the software and how to perform the analysis. To begin, select the "Open video" tab. Specify the video file location for the analyzer: click the "Open a Video" button, navigate to the location of the video, and open it.
Wyniki
SU-8 Lithography and Chip Bonding
The lithography protocol and PDMS molding follow standard procedures. Details can be found elsewhere23,24,25,26. The PDMS should peel off the wafer without problems after curing. If the SU-8 features rip off during PDMS peeling, either the SU-8 adhesion layer or the silanization was insufficient. If plasma...
Dyskusje
This protocol demonstrates a method for delivering precise mechanical stimulation to the skin of a roundworm trapped in a microfluidic chip. It is intended to facilitate the integration of physical stimuli for answering biological questions and aims to streamline mechanobiology research in biological labs. This method extends previous assays to assess the function of mechanosensory neurons in C. elegans. Previous quantitative and semi-quantitative techniques measured forces1,
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Sandra N. Manosalvas-Kjono, Purim Ladpli, Farah Memon, Divya Gopisetty, and Veronica Sanchez for support in device design and generation of mutant animals. This research was supported by NIH grants R01EB006745 (to BLP), R01NS092099 (to MBG), K99NS089942 (to MK), F31NS100318 (to ALN) and received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 715243 to MK).
Materiały
| Name | Company | Catalog Number | Comments |
| Chrome mask | Compugraphics (http://www.compugraphics-photomasks.com/) | 5'', designed in AutoCAD (Autodesk, Inc.) | |
| Chrome mask | Mitani-Micronics (http://www.mitani-micro.co.jp/en/) | 5'', designed in AutoCAD (Autodesk, Inc.) | |
| Chrome mask | Kuroda-Electric (http://www.kuroda-electric.eu/ | 5'', designed in AutoCAD (Autodesk, Inc.) | |
| 4'' Silicon wafer (B-test) | Stanford Nanofabrication Facility | ||
| SU-8 2002 | MicroChem | ||
| SU-8 2050 | MicroChem | ||
| Spin-coater | Laurell Technologies | WS-400BZ-6NPP/LITE | |
| Exposure timer | Optical Associates, Inc | OAI 150 | |
| Illumination controller | Optical Associates, Inc | 2105C2 | |
| SU-8 developer | MicroChem | ||
| 2-Propanol | Fisher Scientific | A426F-1GAL | |
| Acetone | Fisher Scientific | A18-4 | |
| Trichloromethylsilane (TCMS) | Sigma-Aldrich | 92361-500ML | Caution: TCMS is toxic and water-reactive |
| Sylgard 184 Elastomer Kit | Dow Corning | PDMS prepolymer | |
| Biopsy punch, 1 mm | VWR | 95039-090 | |
| Oxygen Plasma Asher | Branson/IPC | ||
| Small metal tubing (0.635 mm OD, 0.4318 mm ID, 12.7 mm long); gage size 23TW | New England Small Tube Corporation | NE-1300-01 | |
| Nalgene syringe filter, 0.22 μm | Thermo Scientific | 725-2520 | to filter all solution, small particles would clog the chip |
| Polyethylene tubing; 0.9652 mm OD, 0.5842 mm ID | Solomon Scientific | BPE-T50 | |
| Syringe, 1 ml | BD Scientific | 309628 | for worm trapping and release |
| Syringe, 20 ml | BD Scientific | 309661 | for gravity-based flow |
| Gilson Minipuls 3, Peristaltic pump | Gilson | to suck solutions and worms out of the chip | |
| Microfluidic flow controller, equipped with 0–800 kPa pressure channel | Elveflow | OB1 MK3 | pressure delivery |
| Water-Resistant Clear Poly- urethane Tubing, 4 mm ID and 6 mm OD | McMaster-Carr | 5195 T52 | connection from house air to pressure pump |
| Water-Resistant Clear Polyurethane Tubing, 2.6mm ID and 4mm OD | McMaster-Carr | 5195 T51 | connect pressure pump to small tubng |
| Push-to-Connect Tube Fitting for Air | McMaster-Carr | 5111K468 | metric - imperial converter |
| Straight Connector for 6 mm × 1/4″ Tube OD | McMaster-Carr | 5779 K258 | |
| Leica DMI 4000 B microscopy system | Leica | ||
| 63×/1.32 NA HCX PL APO oil objective | Leica | 506081 | |
| Hamamatsu Orca-Flash 4.0LT digital CMOS camera | Hamamatsu | C11440-42U | |
| Lumencor Spectra X light engine | Lumencor | With cyan and green/yellow light source | |
| Excitation beam splitter | Chroma | 59022bs | in the microscope |
| Hamamatsu W-view Gemini Image splitting optics | Hamamatsu | A12801-01 | to split green and red emission and project them on different areas on the camera chip |
| Emission beam splitter | Chroma | T570lpxr | in the image splitter |
| Emission filters GCamp6s | Chroma | ET525/50m | in the image splitter |
| Emission filters mCherry | Chroma | ET632/60m | in the image splitter |
Odniesienia
- Eastwood, A. L., et al. Tissue mechanics govern the rapidly adapting and symmetrical response to touch. Proc. Natl. Acad. Sci. 15 (50), E6955-E6963 (2015).
- Katta, S., Krieg, M., Goodman, M. B. Feeling Force: Physical and Physiological Principles Enabling Sensory Mechanotransduction. Annu. Rev. Cell Dev. Biol. 31, 347-371 (2015).
- Krieg, M., Dunn, A. R., Goodman, M. B. Mechanical systems biology of C. elegans touch sensation. BioEssays. 37 (3), 335-344 (2015).
- Krieg, M., Dunn, A. R., Goodman, M. B. Mechanical control of the sense of touch by β-spectrin. Nat. Cell Biol. 16 (3), 224-233 (2014).
- Krieg, M., et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 10 (4), 429-436 (2008).
- Gaub, B. M., Müller, D. J. Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 17 (3), 2064-2072 (2017).
- Geffeney, S. L., et al. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a c. Elegans nociceptor. Neuron. 71 (5), 845-857 (2011).
- O'Hagan, R., Chalfie, M., Goodman, M. B. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8 (1), 43-50 (2005).
- Suzuki, H., et al. In Vivo Imaging of C. elegans Mechanosensory Neurons Demonstrates a Specific Role for the MEC-4 Channel in the Process of Gentle Touch Sensation. Neuron. 39 (6), 1005-1017 (2003).
- Kopito, R. B., Levine, E. Durable spatiotemporal surveillance of Caenorhabditis elegans response to environmental cues. Lab Chip. 14 (4), 764-770 (2014).
- Chokshi, T. V., Ben-Yakar, A., Chronis, N. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip. 9 (1), 151 (2009).
- Hulme, S. E., Shevkoplyas, S. S., McGuigan, A. P., Apfeld, J., Fontana, W., Whitesides, G. M. Lifespan-on-a-chip: microfluidic chambers for performing lifelong observation of C. elegans. Lab Chip. 10 (5), 589-597 (2010).
- Li, S., Stone, H. a., Murphy, C. T. A microfluidic device and automatic counting system for the study of C. elegans reproductive aging. Lab Chip. 15 (2), 524-531 (2015).
- Chokshi, T. V., Bazopoulou, D., Chronis, N. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab Chip. 10 (20), 2758-2763 (2010).
- Mishra, B., et al. Using microfluidics chips for live imaging and study of injury responses in Drosophila larvae. J. Vis. Exp. , e50998 (2014).
- Chronis, N., Zimmer, M., Bargmann, C. I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods. 4 (9), 727-731 (2007).
- Krajniak, J., Lu, H. Long-term high-resolution imaging and culture of C. elegans in chip-gel hybrid microfluidic device for developmental studies. Lab Chip. 10 (14), 1862-1868 (2010).
- Vasquez, V., Krieg, M., Lockhead, D., Goodman, M. B. Phospholipids that Contain Polyunsaturated Fatty Acids Enhance Neuronal Cell Mechanics and Touch Sensation. CellReports. 6 (1), 70-80 (2013).
- Krieg, M., et al. Genetic defects in β-spectrin and tau sensitize C. elegans axons to movement-induced damage via torque-tension coupling. Elife. 6 (2010), e20172 (2017).
- Arnadóttir, J., O'Hagan, R., Chen, Y., Goodman, M. B., Chalfie, M. The DEG/ENaC protein MEC-10 regulates the transduction channel complex in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 31 (35), 12695-12704 (2011).
- Lockhead, D., et al. The tubulin repertoire of Caenorhabditis elegans sensory neurons and its context-dependent role in process outgrowth. Mol. Biol. Cell. 27 (23), 3717-3728 (2016).
- Goodman, M. B., Ernstrom, G. G., Chelur, D. S., O'hagan, R., Yao, C. A., Chalfie, M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 415 (6875), 1039-1042 (2002).
- Nekimken, A., Fehlauer, H., Kim, A., Goodman, M., Pruitt, B. L., Krieg, M. Pneumatic stimulation of C. elegans mechanoreceptor neurons in a microfluidic trap. Lab Chip. , (2017).
- Brower, K., White, A. K., Fordyce, P. M. Multi-step Variable Height Photolithography for Valved Multilayer Microfluidic Devices. J. Vis. Exp. (119), e55276 (2017).
- Jenkins, G. Rapid prototyping of PDMS devices using SU-8 lithography. Methods Mol. Biol. 949 (1), 153-168 (2013).
- Faustino, V., Catarino, S. O., Lima, R., Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 49 (11), 2280-2292 (2016).
- Xia, Y., Whitesides, G. M. SOFT LITHOGRAPHY. Annu. Rev. Mater. Sci. 28 (1), 153-184 (1998).
- Chen, T. -. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Cho, Y., Porto, D., Hwang, H., Grundy, L., Schafer, W. R., Lu, H. Automated and controlled mechanical stimulation and functional imaging in vivo in C. elegans. Lab Chip. , (2017).
- Edwards, S. L., et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6 (8), 1715-1729 (2008).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp. (64), e4019 (2012).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9 (7), 676-682 (2012).
- Cho, Y., Porto, D. A., Hwang, H., Grundy, L. J. High-Throughput Controlled Mechanical Stimulation and Functional Imaging In Vivo. BiorXiv. , (2017).
- Petzold, B. C., Park, S. -. J., Mazzochette, E. A., Goodman, M. B., Pruitt, B. L. MEMS-based force-clamp analysis of the role of body stiffness in C. elegans touch sensation. Integr. Biol. (Camb). 5 (6), 853-864 (2013).
- Nekimken, A. L., Mazzochette, E. A., Goodman, M. B., Pruitt, B. L. Forces applied during classical touch assays for Caenorhabditis elegans. PLoS One. 12 (5), e0178080 (2017).
- Chronis, N., Zimmer, M., Bargmann, C. I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods. 4 (9), 727-731 (2007).
- Gilleland, C. L., Rohde, C. B., Zeng, F., Yanik, M. F. Microfluidic immobilization of physiologically active Caenorhabditis elegans. Nat. Protoc. 5 (12), 1888-1902 (2010).
- Chuang, H. -. S., Raizen, D. M., Lamb, A., Dabbish, N., Bau, H. H. Dielectrophoresis of Caenorhabditis elegans. Lab Chip. 11 (4), 599 (2011).
- Christensen, A. M., Chang-Yen, D. A., Gale, B. K. Characterization of interconnects used in PDMS microfluidic systems. J. Micromechanics Microengineering. 15 (5), 928-934 (2005).
- Gilpin, W., Uppaluri, S., Brangwynne, C. P. Worms under Pressure: Bulk Mechanical Properties of C. elegans Are Independent of the Cuticle. Biophys. J. 108 (8), 1887-1898 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone