Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Ultrasound-Guided Induced Pluripotent Stem Cell-Derived Cardiomyocyte Implantation in Myocardial Infarcted Mice
W tym Artykule
Podsumowanie
Ultrasound-guided cell delivery around the site of myocardial infarction in mice is a safe, effective, and convenient way of cell transplantation.
Streszczenie
The key objective of cell therapy after myocardial infarction (MI) is to effectively enhance the cell grafted rate, and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are a promising cell source for cardiac repair after ischemic damage. However, a low grafted rate is a significant obstacle for effective cardiac tissue regeneration after transplantation. This protocol shows that multiple hiPSC-CM ultrasound-guided percutaneous injections into an MI area effectively increase cell transplantation rates. The study also describes the entire hiPSC-CM culture process, pretreatment, and ultrasound-guided percutaneous delivery methods. In addition, the use of human mitochondrial DNA help detect the absence of hiPSC-CMs in other mouse organs. Lastly, this paper describes the changes in cardiac function, angiogenesis, cell size, and apoptosis at the infarcted border zone in mice 4 weeks after cell delivery. It can be concluded that echocardiography-guided percutaneous injection of the left ventricular myocardium is a feasible, relatively invasive, satisfactory, repeatable, and effective cellular therapy.
Wprowadzenie
When acute MI occurs, myocardial cells in the infarcted area die quickly due to ischemia and hypoxia. Several inflammatory factors are released after cell death and rupture, while inflammatory cells infiltrate the infarcted site to cause inflammation1. Significantly, fibroblasts and collagen, both without contractility and electrical conductivity, replace the myocardial cells in the infarcted site to form scar tissue. Due to the limited regeneration capacity of cardiomyocytes in adult mammals, viable tissue formed after a large area of infarction is usually not adequate for maintaining sufficient cardiac output2. MI causes heart failure, and in severe cases of heart failure, patients can only rely on heart transplants or ventricular assist devices to maintain normal heart functions3,4.
After MI, the ideal treatment strategy is to replace the dead cardiomyocytes with newly formed cardiomyocytes, forming electromechanical coupling with healthy tissues. However, treatment options have typically adopted myocardial salvage rather than replacement. Currently, stem cell- and progenitor cell-based therapies are among the most promising strategies to promote myocardial repair after MI5. However, the transplantation of these cells has several issues, primarily the inability of adult stem cells to differentiate into cardiomyocytes and their short life span6.
The ethical issues related to the use of embryonic stem (ES) cells can be circumvented by iPSCs, which are a promising source of cells. In addition, iPSCs possess strong self-renewal capabilities and can differentiate into cardiomyocytes7. Studies have shown that hiPSC-CMs transplanted into the MI site can survive and form gap junctions with host cells8,9. However, because these transplanted cells are located in the microenvironment of ischemia and inflammation, their survival rate is extremely low10,11.
Several methods have been established to improve the survival rate of transplanted cells, such as hypoxia and heat shock pretreatment of transplanted cells12,13, genetic modification14,15, and the simultaneous transplantation of cells and capillaries16. Unfortunately, most methods are limited by complexity and high cost. Hence, the present study proposes a reproducible, convenient, relatively invasive, and effective hiPSC-CM delivery method.
Ultrasound-guided intramyocardial cell injection can be carried out with only a high-resolution small veterinary ultrasound machine and a microinjector, regardless of the site. Under ultrasound guidance, directly delivering cells under the xiphoid process from the pericardium into the myocardium in mice is a safe protocol that avoids liver and lung damage. This method can be combined simultaneously with other technologies to significantly improve the survival rate of transplanted cells.
Protokół
All animal experiments in this study were reviewed and approved by the ethics committee of the Second Xiangya Hospital of Central South University. See the Table of Materials for details regarding all the materials and equipment used in this protocol. The timelines for cell injection, imaging and euthansia are as follows: t0- induce infarction, t1 week- image and implant cells, t2 weeks- image and implant cells, t4 weeks- final imaging, euthanasia and tissue collection.
1. hiPSC culture, cardiomyocyte differentiation, and cell purification
- Mix DMEM/F12 and basement membrane matrix stock solution with ice at 4 °C in a ratio of 1.5:1, aliquot, and store the resulting mixture (matrix diluent) at -20 °C. Mix 25 mL of DMEM/F12 culture medium at 4 °C and 1 mL of matrix diluent thoroughly with ice, and spread 1 mL/well in a 6-well plate. Keep the plate upright for 1 h in an incubator at 37 °C, and aspirate the DMEM/F12 supernatant from the wells. Do not touch the bottom of the 6-well plate when performing the procedure.
- Remove the cryopreservation tube containing the hiPSCs from liquid nitrogen and quickly thaw them in a 37 °C water bath. Sterilize the external surface of the cryopreservation tube with 75% alcohol, wipe off the alcohol, and transfer the tube to a sterile ultra-clean table.
- Use a pipette to gently transfer the cryopreservation solution containing hiPSCs into a 15 mL centrifuge tube, add 5 mL of feeder-free ES medium, and centrifuge the suspension at 300 × g for 3 min at room temperature. Discard the supernatant and gently resuspend the cells in 6 mL of the feeder-free ES medium. Count the cells and adjust the cell concentration to 5 × 105 cells/mL with the culture medium.
- Add ROCK inhibitor (Y27632) to the hiPSCs to a final concentration of 5 µM. Transfer the hiPSCs with Y27632 to the 6-well plate pretreated with basement membrane matrix, and gently swirl the 6-well plate, tracing the shape of the number "8" to evenly distribute the hiPSCs. Place the hiPSCs in a 37 °C, humid incubator with 5% CO2 in an upright position for 24 h. Replace the supernatant with the feeder-free ES medium without the Y27632.
- When hiPSCs reach 80% degree of confluence, aspirate the culture supernatant, wash the cells twice with phosphate-buffered saline (PBS), add insulin-free RPMI1640 + B27 containing 10 µM CHIR99021 (GSK3-β inhibitor), and place the hiPSCs in a humid CO2 incubator at 37 °C for 24 h.
- Replace half of the supernatant with RPMI1640 + B27 medium without insulin and incubate the cells for another 24 h.
- Completely replace the culture medium with RPMI1640 + B27 (without insulin). Place the plates in a humid incubator at 37 °C with 5% CO2 in an upright position for 24 h.
- Completely replace the culture medium after 3 days with insulin-free RPMI1640 + B27 + 5 µM IWR1 (Wnt signaling pathway inhibitor) and place the plates in a humid incubator at 37 °C with 5% CO2 for 48 h.
- Replace the culture medium after 5 days with RPMI1640 + B27 without insulin and continue to incubate for 48 h in a humid CO2 incubator at 37 °C.
- Replace the culture medium with the RPMI + B27 (including insulin) medium after 7 days and place the plates in a humidified CO2 incubator for 3 days at 37 °C. Replace the medium with the RPMI + B27 medium every 3 days. Look for the spontaneous beating of cells at 9-12 days of differentiation.
- After observing the pulsating cells, replace the RPMI + B27 medium with glucose-free RPMI 1640 medium containing lactic acid (1 mL of lactic acid added to 500 mL of glucose-free RPMI 1640 medium). Incubate the cells for 72 h in a humid incubator at 37 °C with 5% CO2.
- After 72 h, replace the supernatant with RPMI + B27 medium and incubate for 48 h in a humid incubator at 37 °C with 5% CO2.
- Aspirate the culture supernatant under negative pressure and wash the cells 3x with PBS to remove traces of the culture medium. Use the cell detachment enzyme mixture to dissociate the hiPSC-CMs into individual cells at 37 °C. Collect the obtained cells into a 15 mL centrifuge tube containing 3 mL of cardiomyocyte support solution, centrifuge at 300 × g for 2 min at room temperature, and discard the supernatant.
- Resuspend the cells with RPMI + B27 medium, seed them on basement membrane matrix-coated 6-well plates, and incubate them for 48 h in a humid incubator at 37 °C with 5% CO2.
- Replace the culture supernatant for purification with glucose-free RPMI 1640 medium containing lactic acid and incubate for 72 h in a humid incubator at 37 °C with 5% CO2.
- Replace the culture supernatant with RPMI + B27 medium and change the medium every 3 days.
- Continue the above process for 30 days, replacing the culture supernatant with glucose-free medium containing lactic acid for further purification. Incubate the cells for 3 days with this medium and replace the supernatant with RPMI + B27. Culture the cells continuously for 30 days until the hiPSC-CMs beat stably, and use these cells for intramyocardial injection in mice.
2. Preparation of hiPSC-CMs and the establishment of mouse acute myocardial infarction model
- Vacuum-aspirate the culture supernatant from hiPSC-CMs cultured for 60 days, wash the cells 3x with PBS, and dissociate the hiPSC-CMs into individual cells at 37 °C with the cell detachment enzyme mixture (~3-5 min). Collect the cells in a 15 mL centrifuge tube containing 5 mL of RPMI + B27 medium and mix well before counting the cells to calculate the total number of cells. Centrifuge the cell suspension at 300 × g for 2 min at room temperature. Discard the supernatant and add cardiomyocyte support medium to resuspend to a concentration of 0.6 × 105 cells/µL.
- Keep the centrifuge tube containing the resuspended hiPSC-CMs ready at 37 °C for injection14,17,18,19,20.
- Place the mice in an anesthesia box connected with isoflurane to induce anesthetic inhalation followed by the use of vet ointment on the eyes to prevent dryness while under anesthesia.
NOTE: The isoflurane concentration was 5%. Pay attention to laboratory ventilation during the experiment. - Look for loss of reflexes in the feet and tail region after pinching when the mouse is laid flat on the operating table, indicating sufficient anesthesia. Administer buprenorphine (0.1 mg/kg) intraperitoneally.
- Smear the hair on the middle of the neck and the left chest of the mice with a depilatory cream, and wipe off the applied cream and hair with gauze after 5 min.
- Fix the limbs and the mouse tail with tape. Fix the mouse incisors with a 2-0 silk thread and tape. Sterilize the surgical area (median neck and left chest) with three alternating rounds of betadine followed by alcohol.
- Place a surgical drape around the sterilized surgical area. Then, make a median neck incision using fine anatomical scissors and fine dissecting forceps to expose the trachea fully. If necessary, cut off a few anterior cervical muscles for stabilization.
NOTE: The operators were blinded to the animal groups. - Insert a tracheal tube (20 G catheter puncture needle) through the mouth.
- Connect the tracheal tube to the ventilator and check for thoracic movement to ensure both lungs are well ventilated.
- Adjust the breathing parameters (breathing rate: 100-150 bpm, tidal volume 250-300 µL). After that, refix the left hind limb to the lower right side, loosen the tape around the left upper limb, and perform a thoracotomy at the left thorax's 3-4 intercostal space using fine dissecting scissors and forceps for maximal heart exposure.
- Using forceps, peel off the pericardium under a microscope to visualize the left anterior descending coronary artery (LAD) at the lower edge of the left atrial appendage.
- Use a 6-0 silk thread to ligate the proximal end of the LAD. After ensuring that the acute MI model is adequately established (when the distal LAD at the ligation site changes from red to white), close the chest layer by layer and suture the skin with 4-0 threaded intermittent stitches. Perform all the above-mentioned surgical procedures for MI induction for the sham group of animals except for ligation.
- Turn off the inhalation anesthesia, remove the cannula after the mouse resumes spontaneous breathing, and return it to its cage. Monitor the animal until it has regained sufficient consciousness to maintain sternal recumbency. Do not return it to the company of other animals until it has fully recovered. Administer intraperitoneal injections of buprenorphine (0.1 mg/kg x 3 days) and carprofen (5 mg/kg x 1 day) every 12 h postoperatively.
- Inject cyclosporin A (10 mg·kg-1·day-1) into the intraperitoneal cavity of mice 3 days before administering hiPSC-CMs to prevent the rejection of the transplanted cells. Inject cyclosporin A continuously for 1 month after transplantation.
3. hiPSC-CM injection under ultrasound guidance
- After setting up the MI model, assign the MI model mice (12-week-old C57BL/6 mice; 22g to 24g in weight, 50% male and 50% female) to single-dose (SD, n = 8 in this study) and multiple-dose (MD, n = 8 in this study) groups on the first post-MI day and place them in the first and second weeks following MI in an anesthesia box connected with 5% isoflurane for the induction of inhalation anesthesia followed by tracheal intubation.
- Smear the hair on the chest and upper abdomen of the mice with depilatory cream and wipe the cream off with gauze 5 min later. Monitor the heart rate of the mouse on the operation board and administer inhalation isoflurane until the heart rate is maintained at 400-500 bpm.
- Fix the mouse limbs and tail with tape on the detection console and set the platform temperature at 37 °C.
- Apply a special ultrasound jelly on the upper abdomen and use a smaller high-resolution veterinary ultrasound probe to obtain mouse liver images.
- Decrease the ventilator rate to 50 bpm. Use a 5 µL microsyringe to draw ~3 × 105 cells (from step 2.1). Holding the syringe with a micromanipulator, insert a 3 mm needle at the left para-xiphoid process (Figure 1A). Follow the upper edge of the diaphragm under ultrasound guidance (Figure 1B), observe the respiratory rhythm and range (Figure 1C,D), and enter the pericardium at the end of the expiratory cycle avoiding damage to the liver and lung (Figure 1D).
- After the microinjector needle enters the pericardial cavity, adjust the breathing rate to the original parameter (150 bpm). Acquire the parasternal long-axis image of the mouse heart by M-mold echocardiography according to the manufacturer's instructions17 (Figure 1E). Under ultrasonic guidance, inject 3 × 105 cells (Figure 1F) into three marginal areas (1 × 105 cells per injected site) of the infarct site.
- Remove the special ultrasound probe and rinse off the jelly. Wean the mouse off the inhalation anesthesia, and return it to its cage following full consciousness.
- Repeat the above ultrasound-guided cell injection procedures 1 and 2 weeks after establishing MI for the MD group mice.
4. Evaluation of heart function, fluorescence labeling, transplanted cell count, myocardial infarcted area, and organ human mitochondria detection in mice 30 days after left anterior descending branch ligation
- Heart function assessment
- Place the mouse in an isoflurane-connected anesthesia box for induction-inhalation anesthesia followed by the use of vet ointment on the eyes to prevent dryness while under anesthesia. Remove the hair on the left chest of the mouse with depilatory cream. Lay the mouse on the operating panel to monitor the heart rate and adjust the isoflurane inhalation. Maintain the mouse heart rate at 400-500 bpm. The experimental endpoint is day 30 (after the MI induction).
- Fix the mouse limbs and tail with tape on the detection console and set the platform temperature at 37 °C.
- After applying a special ultrasound gel to the anterior heart area, use a (veterinary) high-resolution cardiac ultrasound probe to acquire parasternal long-axis and two-dimensional short-axis images under echocardiography of M-mold and B-mold according to the manufacturer's instructions17.
- After the ultrasonic detection is completed, rinse off the special ultrasonic gel, turn off the inhalation anesthesia, and return the mouse to its cage separately following full consciousness.
- Analyze the obtained data and calculate the left ventricular ejection fraction (EF) and fractional shortening (FS) using the analysis software. Ensure that the operator is blinded to the experimental groups17.
- Fluorescence labeling of sections
- Wash the isolated heart with PBS, immerse it in 4% paraformaldehyde at 4 °C for 12 h. Take the heart out and immerse it in 30% sucrose for 24 h to dehydrate.
- Embed the dehydrated heart with the optimal cutting temperature (OCT) compound on dry ice. Slice the heart from the bottom to the apex with a cryostat thickness of 10 µm and place the sections on slides and store the slides at -20 °C.
- Wash the slides with the heart tissue with PBS for 3 min.
- Permeabilize the tissue on slides with 0.5% Triton X-100 at room temperature for 8 min and wash them 3x with PBS for 5 min per wash.
- After covering the tissue with 10% donkey serum and 90% PBS (blocking solution), block the sections for 1 h at room temperature.
- After removing the blocking solution, incubate the sections with the primary antibody (diluted to 1:100 with the blocking solution) overnight at 4 °C. Wash them 3x with PBS for 5 min per wash.
- Incubate the tissue on slides with fluorophore-conjugated secondary antibody (diluted to 1:100 with the blocking solution) for 1 h at room temperature.
NOTE: Avoid exposing the tissues with the fluorophore-conjugated secondary antibody to light from this point onward. - Wash the slides 3x with PBS for 5 min per wash.
- Mount the slides using 4',6-diamidino-2-phenylindole (DAPI)-containing mounting medium and photograph under a fluorescence microscope17.
- Transplanted hiPSC-CMs count17
- Perform tissue immunofluorescence staining on frozen sections of the heart for pedestrian-specific troponin T (hcTnT), human nuclear antigen (HNA), and nonspecific Sarcomeric Alpha Actinin.
- After the whole heart was serially sectioned, take one section for every 50 sections for fluorescence labeling.
- Randomly capture five high-magnification images per slice. Count the grafted number of fields-of-view, calculate the average grafted cell numbers per unit area, and set them as A1/µm2. Calculate the total grafted area of the slice using ImageJ (https://imagej.nih.gov/ij/) and set it as B1μm2 so that the grafted number in this slice = A1 × B121,22.
- Calculate the total number of grafted cells per mouse heart using Eq (1) and the percent engraftment rate using Eq (2), given that one section was chosen from every 50 sections21,22.
- Total number of grafted cells per mouse heart = (A1 × B1 + A2 × B2 +...+ An × Bn) × 50 (1)
engraftment rate =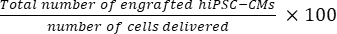 (2)
(2)
NOTE: The number of deliveries were 3 × 105 for the SD group and 3 × 3 × 105 for the MD group.
- Assessing the myocardial infarcted area
- Cut a cross-sectional frozen section (short axis) of the isolated heart, from the apex to the base of the heart with a thickness of 10 µm.
- After the whole heart has been serially sectioned, take one section slide for every 30 sections and fix it in prewarmed Bouin solution at 58 °C. Stain the section with 0.04% Direct Red and 0.1% Fast Green collagen stains (diluted in PBS) according to the manufacturer's instructions17.
- Use software (e.g., ImageJ) to analyze the images after cross-sectional whole-body photography of the heart under a microscope using Eq (3):14,17
Infarct area % =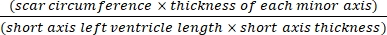 × 100% (3)
× 100% (3)
- Detection of human mitochondrial DNA in various organs
- Anesthetize the mice with 5% isoflurane and sacrifice them by posterior cervical dislocation. Dissect the mice 4 weeks after cell transplantation. Harvest the organs (liver, lung, brain, kidney, spleen) and part of the myocardium (n = 3 in this section).
- Grind each organ tissue in liquid nitrogen and extract DNA from the tissue using the referenced kit (see the Table of Materials) according to the manufacturer's instructions.
- Follow the manufacturer's instructions to use the referenced kit and primers (see the Table of Materials) to detect human mitochondrial DNA in the above-mentioned tissues (from step 4.5.1).
Wyniki
Echocardiography for evaluation of the left ventricular function of the mice in each group revealed that the MI injuries were effectively reversed in the MD group (Figure 2A). Compared with the MI group, the SD group showed increased ejection fraction (EF) (from 30% to 35%; Figure 2B) and fraction shortening (FS) (from 18% to 22%; Figure 2C) after MI. However, it is even more crucial to note that multiple injections of the hiPSC-CMs...
Dyskusje
The critical steps of this study include hiPSC culture, cardiomyocyte differentiation, hiPSC-CM purification, and hiPSC-CM transplantation into the mouse myocardial infarction site. The key is to use cardiac ultrasound to transcutaneously guide treatment toward the infarct site at the edge of the infarction where hiPSC-CMs were injected into the area.
With the prolongation of culture time, the hiPSC-CM phenotype changes in morphology (larger cell size), structure (muscle, fibril density, arran...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (No. 91539111to JY), Key Project of Science and Technology of Hunan Province (No. 2020SK53420 to JY) and The Science and Technology Innovation Program of Hunan Province (2021RC2106 to CF).
Materiały
| Name | Company | Catalog Number | Comments |
| Antibody | |||
| Cardiac troponin T | Abcam | ab8295 | |
| Donkey Anti-Mouse IgG H&L (Alexa Fluor 488) | Abcam | ab150105 | |
| Donkey Anti-Mouse IgG H&L (Alexa Fluor 555) | Abcam | ab150110 | |
| Donkey Anti-Rabbit IgG H&L (Alexa Fluor 488) | Abcam | ab150073 | |
| Donkey Anti-Rabbit IgG H&L (Alexa Fluor 555) | Abcam | ab150062 | |
| Human cardiac troponin T | Abcam | ab91605 | |
| Isolectin B4 | Vector | FL-1201 | |
| Sarcomeric alpha actinin | Abcam | ab9465 | |
| Wheat germ agglutinin | Thermo Fisher Scientific | W11261 | |
| Reagent | |||
| Accutase | Thermo Fisher Scientific | 00-4555-56 | |
| B27 Supplement(minus insulin) | Thermo Fisher Scientific | A1895601 | |
| B27 Supplement(serum free) | Thermo Fisher Scientific | 17–504-044 | |
| Bouin's solution | Thermo Fisher Scientific | SDHT10132 | |
| CHIR99021 | Selleck | CT99021 | |
| cyclosporin A | Medchemexpress | HY-B0579 | |
| DIRECT RED | Sigma-Aldrich | 365548-25G | |
| DMEM/F12 | Thermo Fisher Scientific | 11320033 | |
| DNeasy Blood & Tissue Kit | Qiagen | 69504 | |
| FAST GREEN FCF | Sigma-Aldrich | F7252-5G | |
| Glucose-free RPMI 1640 | Thermo Fisher Scientific | 11879020 | |
| IWR1 | Selleck | S7086 | |
| lactic acid | Sigma-Aldrich | L6661 | |
| Matrigel | BD Biosciences | BD356234 | |
| mTeSR1 | Stem Cell Technologies | 72562 | |
| O.C.T. Compound | SAKURA | 4583 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| PowerUP SYBR Green MasterMix kit | Thermo Fisher Scientific | A25742 | |
| RPMI1640 | Thermo Fisher Scientific | 11875119 | |
| STEMdif Cardiomyocyte Freezing Medium/STEMdiff | Stem Cell Technologies | 5030 | |
| STEMdiff Cardiomyocyte Support Medium | Stem Cell Technologies | 5027 | |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| ultrasound coupling agent | CARENT | 22396269389 | |
| Y-27632 | Selleck | S6390 | |
| Equipment and Supplies | |||
| Applied Biosystems | Thermo Fisher Scientific | 7500 Real-Time PCR | |
| cryostat | Leica | CM1950 | |
| fluoresence microscope | Olympus | IX83 | |
| fine anatomical scissors | Fine Science Tools | 15000-08 | |
| fine dissecting forceps | Fine Science Tools | 11255-20 | |
| Micro syringe | Hamilton | 7633 | |
| Small animal anesthesia machine | MATRX | VMR | |
| Ultra-high resolution small animal ultrasound imaging system | VisualSonics | Vevo 2100 | |
| Software | |||
| Statistical Product and Service Solutions | IBM | 21 | |
| Image J | NIH | 1.48 | |
| Human mitochondrial DNA primers | |||
| the forward primer sequence | CCGCTACCATAATCATCGCTAT | ||
| the reverse primer sequence | TGCTAATACAATGCCAGTCAGG |
Odniesienia
- Leuschner, F., et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. The Journal of Experimental Medicine. 209 (1), 123-137 (2012).
- Lázár, E., et al. Cardiomyocyte renewal in the human heart: insights from the fall-out. European Heart Journal. 38 (30), 2333-2342 (2017).
- Davis, M. K., et al. State of the art: cardiac transplantation. Trends in Cardiovascular Medicine. 24 (8), 341-349 (2014).
- Mancini, D., Colombo, P. C. Left ventricular assist devices: A rapidly evolving alternative to transplant. Journal of the American College of Cardiology. 65 (23), 2542-2555 (2015).
- Zimmermann, W. H. Translating myocardial remuscularization. Circulation Research. 120 (2), 278-281 (2017).
- Hu, X., et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: Paracrine activity without remuscularization. Circulation Research. 118 (6), 970-983 (2016).
- Kempf, H., et al. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nature Protocols. 10 (9), 1345-1361 (2015).
- Chong, J. J., et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 510 (7504), 273-277 (2014).
- Shiba, Y., et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 538 (7625), 388-391 (2016).
- Nguyen, P. K., et al. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: A review. JAMA Cardiology. 1 (8), 953-962 (2016).
- Zimmermann, W. H. Remuscularization of the failing heart. The Journal of Physiology. 595 (12), 3685-3690 (2017).
- Hu, X., et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. The Journal of Thoracic and Cardiovascular Surgery. 135 (4), 799-808 (2008).
- Feng, Y., et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells. 32 (2), 462-472 (2014).
- Fan, C., et al. Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study. Journal of Molecular and Cellular Cardiology. 137, 25-33 (2019).
- Lou, X., et al. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovascular Research. 116 (3), 671-685 (2020).
- Sun, X., et al. Transplanted microvessels improve pluripotent stem cell-derived cardiomyocyte engraftment and cardiac function after infarction in rats. Science Translational Medicine. 12 (562), (2020).
- Wu, X., et al. Cardiac repair with echocardiography-guided multiple percutaneous left ventricular intramyocardial injection of hiPSC-CMs after myocardial infarction. Frontiers in Cardiovascular Medicine. 8, 768873 (2021).
- Kannappan, R., et al. Functionally competent DNA damage-free induced pluripotent stem cell-derived cardiomyocytes for myocardial repair. Circulation. 140 (6), 520-522 (2019).
- Lou, X., et al. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovascular Research. 116 (3), 671-685 (2020).
- Zhao, M., et al. Y-27632 preconditioning enhances transplantation of human-induced pluripotent stem cell-derived cardiomyocytes in myocardial infarction mice. Cardiovascular Research. 115 (2), 343-356 (2019).
- Zhao, M., et al. Enhancing the engraftment of human induced pluripotent stem cell-derived cardiomyocytes via a transient inhibition of rho kinase activity. Journal of Visualized Experiments: JoVE. (149), e59452 (2019).
- O'Brien, J., Hayder, H., Peng, C. Automated quantification and analysis of cell counting procedures using ImageJ plugins. Journal of Visualized Experiments: JoVE. (117), e54719 (2016).
- Kain, V., Prabhu, S. D., Halade, G. V. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Research in Cardiology. 109 (6), 444 (2014).
- Rainer, P. P., et al. Cardiomyocyte-specific transforming growth factor β suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circulation Research. 114 (8), 1246-1257 (2014).
- Yu, Y., et al. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Research & Therapy. 10 (1), 167 (2019).
- Iwanaga, K., et al. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochemical and Biophysical Research Communications. 325 (4), 1353-1359 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone