Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Lethality Bioassay Using Artemia salina L.
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This work aims to evaluate and review the Artemia salina lethality bioassay procedure, also identified as brine shrimp lethality assay. This simple and cheap method gives information about the general toxicity (considered as a preliminary toxicity evaluation) of samples, namely, natural products.
Streszczenie
Natural products have been used since ancient times to produce medicines. Nowadays, there are plenty of chemotherapeutic drugs obtained from natural sources and used against a plethora of diseases. Unfortunately, most of these compounds often display systemic toxicity and adverse effects. In order to better evaluate the tolerability of selected potentially bioactive samples, brine shrimp (Artemia salina) is generally used as a model in lethality studies. The A. salina test is based on the ability of the studied bioactive compounds to kill the microcrustaceans in their larval stage (nauplii). This method represents a convenient starting point for cytotoxicity studies, as well as for the general toxicity screening of synthetic, semisynthetic, and natural products. It can be considered a simple, quick, and low-cost assay, compared to many other assays (in vitro cells or yeast strains, zebrafish, rodents) generally suitable for the aforementioned purposes; moreover, it can be easily performed even without any specific training. Overall, A. salina assay represents a useful tool for the preliminary toxicity evaluation of selected compounds and the bio-guided fractionation of natural product extracts.
Wprowadzenie
Natural products from plants, animals, or microorganisms have been a growing area of interest over the years in the development of new bioactive molecules because of their varied range of biological and pharmacological activities1. However, the associated side effects, drug resistance, or inadequate specificity of the agents, especially when used as anticancer drugs, represent the major factors that can lead to ineffective treatment1,2.
Over the last few decades, several plant-derived cytotoxic agents have been discovered, some of them used as anticancer agents1,2,3. In this context, paclitaxel is reported as one of the best-known and most active chemotherapeutic drugs of natural origin3,4. Currently, it is estimated that more than 35% of all medicines on the market are derived from or are inspired by natural products5. The potential high toxicity of these compounds requires consideration during all of the study phases, since different types of contaminants or even metabolic components of the plant itself can cause toxic effects. For this reason, pharmacological and toxicological profiles should be undertaken in the preliminarily phase, to assess the biological activity and safety of new potential plant-based treatments. To evaluate the toxicity of new bioactive samples, invertebrate animals can be considered as the best models to study. They demand minimal ethical requirements and allow preliminary in vitro assays, to prioritize the most promising products for the next round of testing in vertebrates1,6.
Commonly known as brine shrimp, A. salina is a small halophilic invertebrate belonging to the genus Artemia (family Artemiidae, order Anostraca, subphylum Crustacea; Figure 1). In marine and aquatic saline ecosystems, brine shrimps play an important nutritional role as they feed on microalgae and are constituents of the zooplankton used to feed fish. Moreover, their larvae (known as nauplii) are widely used in the assessment of general toxicity during preliminary studies1,3,7.
Artemia spp. are widely used in lethality studies and are also a convenient starting point for toxicity assessments, by tracking the toxicity of potentially bioactive compounds based on their ability to kill nauplii grown in the laboratory1,8. For this reason, the use of A. salina gained attraction in general toxicity studies, because it is a very efficient and easy-to-use method, compared to other tests on animal models9.
Owing to their simple anatomy, tiny size and short life cycle, a vast number of invertebrates can be studied in a single experiment. As such, they combine genetic amenability and low-cost compatibility with large-scale screenings1. In this context, the use of brine shrimp in a general toxicity assay shows several advantages, such as fast growth (28-72 h is needed from hatching to the first results), cost-effectiveness, and long shelf-life of commercial eggs, that can be used all year round3,10. On the other hand, since invertebrates have a primitive organ system and lack an adaptive immune system, they do not represent a perfect and reliable model for human cells1.
However, it provides a preliminary evaluation method for the general toxicity of selected samples. Since it is widely used as a lethality assay, it can provide provisional indications about the toxic effects of potential anticancer agents. It is often also used to obtain feedback about the general toxicity of compounds endowed with any other biological activities for which it is essential to show the lowest mortality rate possible among the Artemia shrimps.
In an ongoing study from our group, different extracts from Plectranthus species showed antioxidant and antimicrobial activities (unpublished results). In parallel, isolated compounds were obtained by purification of the extracts and were then chemically modified. The extracts, pure compounds, and semisynthetic derivatives were then tested in terms of general toxicity. In this context, the present work aims to give an overview of the use of the Artemia lethality bioassay for the evaluation of general toxicity and potential cytotoxic activity of bioactive extracts and isolated compounds from different plants of the genus Plectranthus11.

Figure 1: Artemia salina under the microscope. Newly hatched nauplii of A. salina as seen under the microscope (magnification 12x). Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Equipment preparation
- Acquire commercially available hatching equipment. Select a suitable place to set up the hatching equipment (Figure 2A). Place the funnel-shaped container in the black support (included in the set) and turn the funnel in a suitable direction to see the level mark and the tap.
- To make hand-made migration equipment, cut the top of two 0.5 L (5.8 cm diameter) plastic bottles to obtain a final height of 12 cm. Create a hole of 1.5 cm diameter on one side at 7 cm from the bottom in each bottle and insert a 13 cm rubber tube (1.3 cm outer and 0.9 cm inner diameter) between the two openings. Seal the openings with hot glue (Figure 2B) and leave to dry for 15 min; put the bottles on a flat surface and fill them with water to verify that there is no leakage.
2. Preparation of artificial salt solution
- In a glass beaker, prepare an artificial salt solution (brine shrimp salt) at a concentration of 35 g/L. To do this, add 28 g of the salt to 800mL of tap water, according to the manufacturer's instructions. Mix it with a stirring rod until all the salt is thoroughly dissolved.
NOTE: Adjust the volume of the prepared saline solution according to the size of the available containers.
3. Sample preparation
- Prepare all the samples in a microcentrifuge tube by dissolving a suitable amount of extracts (Plectranthus extracts, Pa- P. ambigerus; Pb- P. barbatus; Pc- P. cylindraceus; and Pe- P. ecklonii) or compounds 1-5 (two natural compounds [1 and 2] obtained from Plectranthus spp. and three semi-synthetic derivatives [3, 4, 5]; Figure 3) in dimethyl sulfoxide (DMSO)12, so as to obtain a final concentration of 10 mg/mL (if the sample is water-soluble, use of DMSO is not necessary).
- Dilute 10 µL of each sample (and DMSO for the negative control) in a new microcentrifuge tube using 990 µL of artificial saline solution prepared in step 2.1, to obtain a final concentration of 0.1 mg/mL.
- Under a fume hood, in an Erlenmeyer flask, prepare a solution of potassium dichromate (K2Cr2O7) in distilled water at a concentration of 1 mg/mL13,14,15.
4. Brine shrimp lethality bioassay
NOTE: This assay is developed from the works of several authors with modifications1,16,17,18,19.
- Fill the hatching vessel with the medium prepared in step 2.1 up to the level mark (500 mL) (Figure 2C).
- Place one spoon (approximately 0.75 g) of brine shrimp cysts in the salt solution, and then close the container. Place a lamp (table lamp, 40 W, 230 V, 50 Hz, with a LED light bulb of 8 W, 4,000 K, 830 lm) pointing directly toward the equipment (Figure 2A) and turn it on.
- Attach the air supplier system (3 W output, 50 Hz, 230 V) to the connector placed on the top of the equipment and turn the pump on.
- Keep the room temperature at 25 ± 3 °C. Brine shrimp cysts hatch in the artificial salt solution, under vigorous aeration, continuous lighting, and stable temperature, after 24 h to 48 h.
NOTE: Alternatively, a vertical incubator can be used. - Once the eggs have hatched, turn the air pump off and wait until the nauplii (moving toward the bottom of the funnel) are separated from the empty egg cases (floating at the top).
- In order to separate the unhatched eggs from alive nauplii, open the outlet tap at the bottom and discharge the content of the funnel in one of the containers of the hand-made migration equipment container (described in step 1.2). Ensure that the solution containing the nauplii and the residual unhatched eggs is below the level of the tube. In the second container, add the residual salt solution from step 2.1 above the height of the tube.
- Cover the container with the nauplii and the residual unhatched eggs using aluminum foil. Place the lamp on the second container with just the salt solution. The brine shrimp will be attracted by the light and migrate from one container to the other (harvesting container), leading to efficient separation between eggs (slowly sedimented to the bottom) and alive Artemia.
- Then, place the equipment in the incubator under the same conditions used in step 4.4 for 4 h (Figure 2E). From the harvesting container, collect 900 µL of saline solution containing 10 to 15 nauplii. Place the saline solution with nauplii in each well of a 24-well plate (Figure 2F); all the samples are tested in quadruplicates.
- Add 100 µL each of the negative control (DMSO), the positive control (K2Cr2O7, potassium dichromate), the artificial salt solution, and each of the samples to respective well (Figure 2F)13,14.
NOTE: The samples in each well will be at a concentration of 0.01 mg/mL. The final concentration of the positive control in salt solution will be 0.1 mg/mL, to be sure that all the nauplii in the well are exposed to the toxic effect of potassium dichromate and die. The artificial salt solution will act as blank. - Incubate the plate at 25 ± 3 °C under illumination for 24 h (Figure 2G). After 24 h, register the number of dead larvae (non-mobile nauplii for 5 s) in each well under a binocular microscope (12x)20 (Figure 2H). Alternatively, use a hand lens.
- Add 100 µL of potassium dichromate solution, to induce the death of the remaining living larvae, and wait for 6 h. Count the total dead larvae in each well under a microscope. Determine the mortality rate according to the following equation.
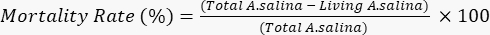
- Perform all the assay in triplicate. Calculate standard deviations (SD), and express the results as the mean of three independent experiments, each with internal quadruplicates (n = 12), ± SD. As mentioned by Meyer et al., consider crude extracts and pure compounds with LC50< 1,000 µg/mL as toxic; also, take into account that the mortality rate of brine shrimp is proportional to the concentration of the tested samples21.

Figure 2: Artemia salina lethality bioassay method. (A) Commercially available equipment employed for the hatching of brine shrimp cysts; (B) Hand-made migration equipment; (C) Hatching vessel filled with saline solution; (D) Collection of unhatched eggs and nauplii; (E) Hand-made equipment in the incubator during the migration step. The container far from the lamp should be covered with aluminum foil; however, for a better view of the set installation here it was removed; (F) Harvesting of Artemia in wells prior to performing the assay. The compounds should be placed as shown: - refers to the negative control (DMSO), + to the positive control (K2Cr2O7), salt to the artificial salt solution, and 1 to 3 to the samples to test (in this case compounds 1-3); (G) Incubation of the 24-well plate containing Artemia and the selected samples; (H) Artemia count under the binocular microscope. Please click here to view a larger version of this figure.

Figure 3: Structures of selected compounds. Structure of compounds 1-2, extracted from Plectranthus species, and compounds 3-5, obtained by semi-synthesis. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The general toxicity of some natural products recently studied by our group was evaluated through the brine shrimp lethality bioassay. Four extracts (Pa- P. ambigerus; Pb- P. barbatus; Pc- P. cylindraceus; and Pe- P. ecklonii) from Plectranthus genus, known for their antioxidant activity (unpublished results), were tested. Additionally, two natural compounds (1 and 2) obtained from Plectranthus spp., and three semi-synthetic derivatives (3, 4, 5; ...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
During the last years, the scientific community has increased its attention toward alternative models for toxicity screenings21. Beside A. salina lethality bioassay, other methodologies are usually performed for the evaluation of sample tolerability and include vertebrate bioassays (such as rodents), invertebrates (such as zebrafish), in vitro methods using yeast strains or cells, and in silico methods22,23,...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors declare no conflicts of interest, financial or otherwise.
Podziękowania
In memory of Professor Amilcar Roberto.
This work was financially supported by Fundação para a Ciência e a Tecnologia (FCT, Portugal) under projects UIDB/04567/2020 and UIDP/04567/2020 attributed to CBIOS and PhD grant SFRH/BD/137671/2018 (Vera Isca).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 24-well plates | Thermo Fisher Scientific, Denmark | 174899 | Thermo Scientific Nunc Up Cell 24 multidish |
| Aluminium foil | Albal | - | Can be purchased in supermarket |
| Artemio Set | JBL GmbH and Co. KG, D-67141, Neuhofen Germany | 61066000 | Can be purchased in pet shops |
| Binocular microscope | Ceti, Belgium | 1700.0000 | Flexum-24AED, 220-240 V, 50 Hz |
| Bottles | - | - | 0.5 L Diameter: 5.8 cm; Height: 12 cm |
| Brine shrimp cysts | JBL GmbH and Co. KG, D-67141, Neuhofen Germany | 3090700 | Can be purchased in pet shops |
| Brine shrimp salt | JBL GmbH and Co. KG, D-67141, Neuhofen Germany | 3090600 | Can be purchased in pet shops |
| Dimethyl sulfoxide (DMSO) | VWR chemicals | CAS: 67-68-5 | 99% purity |
| Discartable tips | Diamond | F171500 | Volume range: 100 - 1000 µL |
| Eppendorf microtubes | BRAND | 7,80,546 | Microtubes, PP, 2 mL, BIO-CERT PCR QUALITY |
| Erlenmeyer flask | VWR chemicals | 4,47,109 | volume: 100 mL |
| Glass beaker | Normax | 3.2111654N | Volume: 1000 mL |
| Gloves | Guantes Luna | GLSP3 | - |
| GraphPad Prism | GraphPad Software, San Diego, CA, USA | - | GraphPad Prism version 5.00 for Windows, www.graphpad.com, accessed on 5 February 2021; commercial statistical analysis software |
| Home-made A. salina Grower | - | - | Home made: two plastic bottles connected by a hose |
| Hot glue | Parkside | PHP500E3 | 230 V, 50 Hz, 25 W |
| Incubator | Heidolph Instruments, Denmark | - | One Heidolph Unimax 1010 equipment and one Heidolph Inkubator 1006 |
| Light | Roblan | SKYC3008FE14 | LED light bulb |
| Micropipettes | VWR chemicals | 613-5265 | Volume range: 100 - 1000 µL |
| Potassium dichromate (K2Cr2O7) | VWR chemicals | CAS: 7778-50-9 | 99% purity |
| Pump ProAir a50 | JBL GmbH and Co. KG, D-67141, Neuhofen Germany | - | Included in the Artemio Set+1 kit |
| Rubber tube | - | - | 1.3 cm outer and 0.9 cm inner diameter |
| Stirring rod | VWR chemicals | 441-0147 |  6 mm, 250 mm 6 mm, 250 mm |
| Termometer | VWR chemicals | 620-0821 | 0 - 100 °C |
Odniesienia
- Ntungwe, N. E., et al. Artemia species: An important tool to screen general toxicity samples. Current Pharmaceutical Design. 26 (24), 2892-2908 (2020).
- Cragg, G. M., Newman, D. J. Natural products: A continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA) - General Subjects. 1830 (6), 3670-3695 (2013).
- Ntungwe, E., et al. General toxicity screening of Royleanone derivatives using an artemia salina model. Journal Biomedical and Biopharmaceutical Research. 18 (1), 114(2021).
- Seca, A., Plant Pinto, D. secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. International Journal of Molecular Sciences. 19 (1), 263(2018).
- Calixto, J. B. The role of natural products in modern drug discovery. Anais da Academia Brasileira de Ciências. 91 (3), 1-7 (2019).
- Mandrell, D., et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. Journal of Laboratory Automation. 17 (1), 66-74 (2012).
- Zhang, Y., Mu, J., Han, J., Gu, X. An improved brine shrimp larvae lethality microwell test method. Toxicology Mechanisms and Methods. 22 (1), 23-30 (2012).
- Domínguez-Villegas, V., et al. antioxidant and cytotoxicity activities of methanolic extract and prenylated flavanones isolated from leaves of eysehardtia platycarpa. Natural Product Communications. 8 (2), 177-180 (2013).
- Hamidi, M. R., Jovanova, B., Panovska, T. K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Macedonian Pharmaceutical Bulletin. 60 (01), 9-18 (2014).
- Libralato, G., Prato, E., Migliore, L., Cicero, A. M., Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecological Indicators. 69, 35-49 (2016).
- Mendes Hacke, A. C., et al. Cytotoxicity of cymbopogon citratus (DC) Stapf fractions, essential oil, citral, and geraniol in human leukocytes and erythrocytes. Journal of Ethnopharmacology. 291, 115147(2022).
- Thangapandi, V., Pushpanathan, T. Comparison of the Artemia salina and Artemia fransiscana bioassays for toxicity of Indian medicinal plants. Journal of Coastal Life Medicine. 2 (6), 453-457 (2014).
- Syahmi, A. R. M., et al. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq., (Oil Palm Leaf) methanol extract. Molecules. 15 (11), 8111-8121 (2010).
- Sasidharan, S., et al. Acute toxicity impacts of Euphorbia hirta L extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacognosy Research. 4 (3), 170(2012).
- Libralato, G. The case of Artemia spp. in nanoecotoxicology. Marine Environmental Research. 101, 38-43 (2014).
- Okumu, M. O., et al. Artemia salina as an animal model for the preliminary evaluation of snake venom-induced toxicity. Toxicon: X. 12, 100082(2021).
- Rajabi, S., Ramazani, A., Hamidi, M., Naji, T. Artemia salina as a model organism in toxicity assessment of nanoparticles. DARU Journal of Pharmaceutical Sciences. 23 (1), 20(2015).
- Svensson, B. -M., Mathiasson, L., Mårtensson, L., Bergström, S. Artemia salina as test organism for assessment of acute toxicity of leachate water from landfills. Environmental Monitoring and Assessment. 102 (1), 309-321 (2005).
- Banti, C., Hadjikakou, S. Evaluation of toxicity with brine shrimp assay. Bio-Protocol. 11 (2), 3895(2021).
- Pecoraro, R., et al. Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microscopy Research and Technique. 84 (3), 531-536 (2021).
- Lillicrap, A., et al. Alternative approaches to vertebrate ecotoxicity tests in the 21st century: A review of developments over the last 2 decades and current status. Environmental Toxicology and Chemistry. 35 (11), 2637-2646 (2016).
- Ribeiro, I. C., et al. Yeasts as a model for assessing the toxicity of the fungicides Penconazol, Cymoxanil and Dichlofulanid. Chemosphere. (10), 1637-1642 (2000).
- Armour, C. D., Lum, P. Y. From drug to protein: using yeast genetics for high-throughput target discovery. Current Opinion in Chemical Biology. 9 (1), 20-24 (2005).
- Modarresi Chahardehi, A., Arsad, H., Lim, V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants. 9 (10), 1345(2020).
- Fischer, I., Milton, C., Wallace, H. Toxicity testing is evolving. Toxicology Research. 9 (2), 67-80 (2020).
- de Araújo, G. L., et al. Alternative methods in toxicity testing: the current approach. Brazilian Journal of Pharmaceutical Sciences. 50 (1), 55-62 (2014).
- Toussaint, M., et al. A high-throughput method to measure the sensitivity of yeast cells to genotoxic agents in liquid cultures. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 606 (1), 92-105 (2006).
- Horzmann, K. A., Freeman, J. L. Making waves: New developments in toxicology with the zebrafish. Toxicological Sciences. 163 (1), 5-12 (2018).
- Avdesh, A., et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. Journal of Visualized Experiments. (69), e4196(2012).
- Cunliffe, V. T. Zebrafish: A Practical Approach. Nüsslein-Volhard, C., Dahm, R. , Oxford University Press. (2002).
- Sitarek, P., et al. Insight the biological activities of selected Abietane Diterpenes isolated from Plectranthus spp. Biomolecules. 10 (2), 194(2020).
- Matias, D., et al. Cytotoxic activity of Royleanone Diterpenes from Plectranthus madagascariensis Benth. ACS Omega. 4 (5), 8094-8103 (2019).
- Garcia, C., et al. Royleanone derivatives from Plectranthus spp. as a novel class of P-glycoprotein inhibitors. Frontiers in Pharmacology. 11, (2020).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone