A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measuring Glutathione-induced Feeding Response in Hydra
In This Article
Summary
Here we describe a simple assay for the quantification of the feeding response in hydra induced by the reduced form of glutathione. This assay relies on measuring the distance between the apical end of the tentacle and mouth of hydra.
Abstract
Hydra is among the most primitive organisms possessing a nervous system and chemosensation for detecting reduced glutathione (GSH) for capturing the prey. The movement of prey organisms causes mechanosensory discharge of the stinging cells called nematocysts from hydra, which are inserted into the prey. The feeding response in hydra, which includes curling of the tentacles to bring the prey towards the mouth, opening of the mouth and consequent engulfing of the prey, is triggered by GSH present in the fluid released from the injured prey. To be able to identify the molecular mechanism of the feeding response in hydra which is unknown to date, it is necessary to establish an assay to measure the feeding response. Here, we describe a simple method for the quantitation of the feeding response in which the distance between the apical end of the tentacle and mouth of hydra is measured and the ratio of such distance before and after the addition of GSH is determined. The ratio, called the relative tentacle spread, was found to give a measure of the feeding response. This assay was validated using a starvation model in which starved hydra show an enhanced feeding response in comparison with daily fed hydra.
Introduction
Hydra is the most primitive organism possessing a nervous system and chemosensation for detecting reduced glutathione (GSH) for capturing the prey1. It feeds on a variety of animals such as nematode, crustacea, insect larvae, tadpoles and newly hatched fish1. The movement of these prey organisms causes mechanosensory discharge of the stinging capsules called nematocysts from hydra, which are inserted into the prey2. GSH present in the fluid released from the injured prey subsequently activates the feeding response in hydra which includes curling of the tentacles to bring the prey towards the mouth, opening of the mouth, and consequent engulfing of the prey. Multiple molecules, such as dopamine3, glutamate4, GABA, glycine5, NMDA receptors6, and allatotropin7, have been shown to be involved in the feeding response in hydra. It has also been shown that the chemosensory response induced by GSH is modulated by the feeding status of the animal such that starved hydra exhibited enhanced feeding response1. Such an increase in the GSH sensitivity is biologically relevant since under starvation hydra need to find its prey at higher sensitivity.
Although the feeding response induced by GSH can be clearly observed under microscope, the methods typically used for measuring the feeding response observations are non-quantitative. In most of the cases, the time during which the mouth of the hydra remains open was taken as a measure of the feeding response8,9; whereas in another case, quantitation was based on the number of hydra out of a population showing the feeding response10. However, observing the mouth opening time of the hydra polyps is cumbersome and subject to variation induced by uncontrollable parameters such as the direction of the mouth orientation during observations. Similarly, since the feeding response is a quantitative parameter, population-based approaches are subject to variations/errors caused by the opinion or observational bias of the individual observer. To circumvent these issues we have developed a method for the relative quantification of the feeding response in hydra (Hydra vulgaris Ind-Pune11) based on the distance of the apical end of the tentacle from the mouth of the hydra polyp.
Protocol
1. Hydra Culture and Measurement of the Feeding Response
- Maintain hydra polyps in culture by feeding them daily with artemia and keeping them in a medium (1 mM Tris-HCl buffer, pH 7.6, 1 mM NaCl, 1 mM CaCl2, 0.1 mM KCl, and 0.1 mM MgSO4) contained in a glass bowl at 18 °C under 12 hr light-12 hr dark cycles as described earlier12.
- For measuring the feeding response, transfer one mature hydra polyp having 5 to 6 tentacles to a single well of a 24-well plate. Remove the residual medium from the well by tilting it, and then immediately add 500 µl of fresh medium.
- Prepare 9 µM glutathione solution in hydra medium. Since the glutathione solution is prone to oxidation, always use freshly prepared glutathione solution for each experiment.
- Transfer the plate to the imaging platform of a microscope having provisions for image recording. Use a dark background such that the behavior of hydra polyp can be clearly imaged against the contrasting background.
- The room used for observing and imaging the behavior of hydra free from lights of fluctuating intensities, air currents and noise. Such disturbances could also cause the hydra polyp to show contraction of the tentacles - even in the absence of glutathione.
- Allow the polyp to relax for 5 min.
- Make sure that the polyp is located along the central region of the well such that the behavior can be imaged clearly. If the polyp is at the edge of the well, bring it to the center by flushing the medium using a pipette and again allow it to relax.
- Capture an image of hydra in the relaxed state. This will be the zero-time point observation.
- Quickly add 9 µM glutathione solution to reach a final concentration of 3 µM in the well. Depending on the purpose of the experiment and the response shown by hydra, test a range of different concentrations of glutathione and choose the appropriate concentration required.
- Start the timer immediately after adding glutathione solution and capture images after every 15-30 sec for 4-5 min. Do not change the magnification settings during the time-lapse imaging.
- Add the glutathione solution gently and with uniform flow such that the position of the animal in the well would be minimally disturbed in the field of view of the microscope. However, if the polyp moves extensively after adding glutathione solution, move the plate very gently to bring the polyp in the field of view for image capture.
- In the control experiment, use medium lacking glutathione while keeping all the other parameters identical.
- Make sure to perform all of the above experimental steps during the first half of the day - before 1 P.M. to avoid the possible effect of circadian rhythm on the extent of feeding response.
- Open each of the captured images using the GNU Image Manipulation Program (GIMP).
- Use the “Measure” tool available from Menu > Tools > Measure to determine the distance between apical end of each of the tentacles and hypostome. If the mouth opening is observed in any of the images, determine the distance between the center of the opened mouth and the apical end of the tentacle. Refer to this distance as the tentacle spread.
- Calculate the average tentacle spread for each polyp before and after glutathione exposure. Calculate the ratio of average tentacle spread at zero-time point to that at each of the subsequent time-points. This ratio will be called relative tentacle spread.
- Repeat measurements for at least 20 polyps.
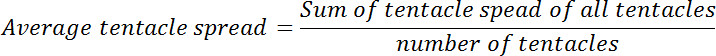

2. Method Validation using the Starvation Model
- For starvation, transfer a few hydra polyps to a separate glass bowl and do not feed them for 5 days. Feed the control group of a few polyps daily with artemia in a similar-sized bowl. Change the medium from both experimental bowls daily to avoid fungal growth in the medium.
- On the day of the experiment, feed the control group of hydra with artemia for 1 hr and use these hydra for the subsequent experiments after removing all uneaten and dead artemia from the medium.
- Measure the feeding response of the starved hydra in comparison with the hydra from control group by the method described earlier in step 1. To avoid any bias due to time of observation, alternate the measurements of each of the starved and control hydra polyps.
Results
Glutathione causes hydra to exhibit curling of the tentacles towards the mouth for the purpose of engulfing the prey. Such curling of tentacles brings apical ends of the tentacles closer to the hypostome. This results in the reduction in the tentacle spread, or the linear distance between apical end of the tentacle and hypostome (Figure 1). The relative tentacle spread, or the ratio of average tentacle spread before and after adding glutathione, averaged across multiple polyps reduces over time. The rela...
Discussion
Feeding behavior in hydra represents one of the most ancestral chemosensory systems in the metazoans. Although the presence of GSH in the crustacean fluid released after nematocyst-assisted prey capture was detected long ago1, neither the GSHR protein nor the putative encoding gene/s have been characterized from hydra to date. Few attempts have been made to characterize GSH binding proteins in hydra8, 14, 15. However, the identity of these putative receptor proteins remains obscure and very few othe...
Disclosures
The authors declare no competing financial interests.
Acknowledgements
Authors are thankful to K. P. Madhu, Nita Beliappa and staff of the Media Centre of Indian Institute of Science Education and Research, Pune for their help in the video production. The work was supported by funding under the Centre of Excellence program of Department of Biotechnology, Government of India to SG and postdoctoral fellowship by Department of Science and Technology, Government of India to RK.
Materials
| Name | Company | Catalog Number | Comments |
| Cooled Incubator | Panasonic | MIR-254-PE | |
| Microscope | Leica | S8AP0 | |
| Camera for the microscope | Leica | EC3 | |
| Reduced glutathione | Sigma | G4251 | Stored at 4 °C. Bring the bottle to room temperature before opening to avoid oxidation |
| Image editing program | GIMP | Version 2.8 |
References
- Loomis, W. F. Glutathione control of the specific feeding reactions of hydra. Ann. Ny. Acad. Sci. 62, 209-228 (1955).
- Beckmann, A., Ozbek, S. The Nematocyst: a molecular map of the Cnidarian stinging organelle. Int. J. Dev. Biol. 56, 577-582 (2012).
- Venturini, G., Carolei, A. Dopaminergic receptors in Hydra. Pharmacological and biochemical observations. Comp. Biochem. Phys. C. 102, 39-43 (1992).
- Kass-Simon, G., Scappaticci, A. A. Glutamatergic and GABAnergic control in the tentacle effector systems of Hydra vulgaris. Hydrobiologia. 530-531, 67-71 (2004).
- Pierobon, P., Tino, A., Minei, R., Marino, G. Different roles of GABA and glycine in the modulation of chemosensory responses in Hydra vulgaris (Cnidaria, Hydrozoa). Hydrobiology. 178, 59-66 (2004).
- Pierobon, P., Sogliano, C., Minei, R., Tino, A., Porcu, P., Marino, G., Tortiglione, C., Concas, A. Putative NMDA receptors in Hydra: a biochemical and functional study. Eur. J. Neurosci. 20, 2598-2604 (2004).
- Alzugaray, M. E., Adami, M. L., Diambra, L. A., Hernandez-Martinez, S., Damborenea, C., Noriega, F. G., Ronderos, J. R. Allatotropin: An ancestral myotropic neuropeptide involved in feeding. PLoS ONE. 8, (2013).
- Bellis, S. L., Laux, D. C., Rhoads, D. E. Affinity purification of Hydra glutathione binding proteins. FEBS Lett. 354, 320-324 (1994).
- Lenhoff, H. M., Shaw, C. A. The discovery of the GSH receptor in Hydra and its evolutionary significance. Glutathione in the Nervous System. , 25-43 (1998).
- Venturini, G. The hydra GSH receptor. Pharmacological and radioligand binding studies. Comp. Biochem. Phys. C. 87, 321-324 (1987).
- Reddy, P. C., Barve, A., Ghaskadbi, S. Description and phylogenetic characterization of common hydra from India. Curr. Sci. 101, 736-738 (2011).
- Horibata, Y., et al. Unique catabolic pathway of glycosphingolipids in a hydrozoan, Hydra magnipapillata. Involving endoglycoceramidase. J. Biol. Chem. 279, 33379-33389 (2004).
- Koizumi, O., Maeda, N. Rise of feeding threshold in satiated Hydra. J. Comp. Physiol. 142, 75-80 (1981).
- Bellis, S. L., Kass-Simon, G., Rhoads, D. E. Partial characterization and detergent solubilization of the putative glutathione chemoreceptor from hydra. Biochemistry. 31, 9838-9843 (1992).
- Morita, H., Hanai, K. Taste receptor proteins in invertebrates - with special reference to glutathione receptor of hydra. Chem. Senses. 12, 245-250 (1987).
- Colasanti, M., Venturini, G., Merante, A., Musci, G., Lauro, G. M. Nitric oxide involvement in Hydra vulgaris very primitive olfactory- like system. Journal of Neurosci. 17, 493-499 (1997).
- Kuhn, A., Tsiairis, C. D., Williamson, M., Kalbacher, H., Grimmelikhuijzen, C. J., Holstein, T. W., Gründer, S. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J. Biol. Chem. 285, 11958-11965 (2010).
- Wang, M., Yao, Y., Kuang, D., Hampson, D. R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 281, 8864-8870 (2006).
- Ruggieri, R. D., Pierobon, P., Kass-Simon, G. Pacemaker activity in hydra is modulated by glycine receptor ligands. Comp. Biochem. Phys. C. 138, 193-202 (2004).
- Ramazani, R. B., Krishnan, H. R., Bergeson, S. E., Atkinson, N. S. Computer automated movement detection for the analysis of behavior. J. Neurosci. Meth. 162, 171-179 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved