A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Three Different Protocols of Corneal Collagen Crosslinking in Keratoconus: Conventional, Accelerated and Iontophoresis
In This Article
Summary
Corneal collagen cross-linking (CXL) is the only conservative treatment currently available to halt keratoconus progression by improving the biomechanical rigidity of the corneal stroma. The aim of this manuscript is to highlight the methods of three different protocols of CXL: conventional CXL (C-CXL), accelerated CXL (A-CXL), and iontophoresis CXL (I-CXL).
Abstract
Keratoconus is a bilateral and progressive corneal ectasia. In order to slow down its progression, corneal collagen cross-linking (CXL) has recently been introduced as an efficient treatment option. In biological and chemical sciences, crosslinking refers to new chemical bonds formed between reactive molecules. Hence, the aim of corneal collagen CXL is to synthetically increase the formation of crosslinks between collagen fibrils in the corneal stroma. Despite the fact that the efficiency of the conventional CXL (C-CXL) protocol has already been shown in several clinical studies, it might benefit from improvements in duration of the procedure and removal of corneal epithelium. Hence, in order to provide a coherent evaluation of two new and optimized CXL protocols, we studied keratoconus patients who had undergone one of the three CXL treatments: iontophoresis (I-CXL), accelerated CXL (A-CXL), and conventional CXL (C-CXL). A-CXL is a 6 time faster CXL procedure using a ten time higher UVA irradiance but still including an epithelium removal. Iontophoresis is a transepithelial non-invasive technique in which a small electric current is applied to improve riboflavin penetration throughout the cornea. Using anterior segment optical coherence tomography (AS OCT) and in vivo confocal microscopy (IVCM), we conclude that regarding the depth of treatment penetration, conventional CXL protocol remains the standard for treating progressive keratoconus. Accelerated CXL seems to be a quick, effective and safe alternative to treat thin corneas. The use of iontophoresis is still being investigated and should be considered with greater caution.
Introduction
Keratoconus is a bilateral and progressive corneal ectasia usually reported in 1 in 2,000 in the general population1 resulting in modification of the corneal shape and thus decreased vision2. Keratoconus is usually present in early puberty and progresses until the third to fourth decade of life when the disease typically tends to stabilize, although progression can be variable throughout a patient's life. By halting keratoconus progression, cross linking aims at postpone or avoid keratoplasty.
To date, the only efficient and safe treatment of progressive keratoconus proven in clinical studies is the conventional corneal collagen cross-linking (C-CXL) protocol, which aims to increase stiffness and hence halt keratoconus progression3-8. In order to reduce operation time and other possible risk factors of C-CXL, such as infectious keratitis or stromal haze9, several improved protocols have been described. First, in accelerated CXL (A-CXL), a higher irradiance of UVA is delivered to the cornea over a reduced time10. Secondly, to avoid the necessity for epithelial debridement, transepithelial approaches have been employed. Unfortunately, they have limited success when compared to the conventional protocol11. The most recent transepithelial method for corneal riboflavin delivery during CXL is iontophoresis (I-CXL), but rigorous evaluation of this treatment has not yet been performed12. Iontophoresis is a non-invasive technique in which a small electric current is applied to improve an ionized drug's penetration through a tissue. In CXL by iontophoresis, the riboflavin is ionized to penetrate the cornea through the epithelium.
In vivo confocal microscopy (IVCM) is a method of imaging the cornea that can highlight the cellular changes of abnormal corneas in diseases such as keratoconus13. Indeed, IVCM has demonstrated alterations to all layers of the cornea in keratoconus with a particular reduction in density of the sub-basal nerve plexus and stromal keratocytes13-15. Plus, IVCM has proven to be highly convenient for microstructural analysis of the cornea after C-CXL16.
The corneal demarcation line is described as a hyperreflective line seen in anterior segment optical coherence tomography (AS OCT) 1 month after C-CXL at a depth of 300 µm17,18. IVCM following C-CXL provides information about corneal structural alterations, including the absence of corneal keratocytes to a depth of 300 µm. The depth of this acellular zone, as well as the depth of the demarcation line within the corneal stroma revealed on AS OCT, seems to be associated with the effective depth of CXL treatment19, and measurement of the corneal demarcation line depth in AS OCT 1 month after CXL has been proposed as an efficient clinical method for evaluation of CXL effectiveness18.
In the present study we investigate the efficiency of three different protocols of corneal collagen crosslinking (conventional, accelerated, and iontophoresis) using measurement of the corneal stromal demarcation line by AS OCT and confocal microscopy. We furthermore used IVCM to quantitatively analyze corneal microstructure changes after the three treatments.
Protocol
These protocols follow the guidelines of our institution's human research ethics committee.
1. Conventional Corneal Collagen CXL (C-CXL)
1. Preparation of the Patient
- 5 days before the surgery, put 1% pilocarpine drops twice a day in the treated eye.
- In the operating room, in aseptic conditions, lie the patient on his/her back.
- Administer topical anesthesia such as oxybuprocaine 0.4%.
- Clean the eye and the skin around the eye with iodine antiseptic twice.
- Use a lid speculum to keep the eye open.
2. Epithelial Removal
- Mark the central 9.0 mm of the cornea with a circle corneal marker.
- Remove the central 7.0 to 9.0 mm of corneal epithelium by mechanical debridement using a blunt spatula.
3. Riboflavin Application
- Apply 0.1% riboflavin with 20% Dextran on the cornea every min for 20 min.
4. UVA Irradiation
- Irradiate the cornea with a 370 nm wavelength UVA light at an irradiance of 3 mW/cm2 (5.4 J/cm2 surface dose) and at a 5 cm working distance for 30 min.

Figure 1: UVA irradiation in C-CXL. The cornea is irradiated with a 370 nm wavelength UVA light at an irradiance of 3 mW/cm2 (5.4 J/cm2 surface dose) and at a 5 cm working distance for 30 minutes. Please click here to view a larger version of this figure.
- During irradiation, apply drops of riboflavin to the cornea every 5 min.
- During irradiation, add topical anesthesia (oxybuprocaine 0.4%) if necessary.
5. End of the Surgery
- Put antibiotic drops (tobramycin 0.3%) and artificial tears (hyaluronate drops 0.18%) into the operated eye.
- Place a soft bandage contact lens at the end of surgery until re-epithelialization is complete. Re-epithelialization usually takes 3 days.
- Prescribe analgesics such as paracetamol (500 mg) plus codeine (30 mg), 6 pills a day.
- After corneal re-epithelialization, initiate topical therapy with steroids (topical dexamethasone 1 mg/ml) and continue for 3-4 weeks. Plus, use artificial tears 4 times a day for 1 month.
2. Accelerated Corneal Collagen CXL (A-CXL)
1. Preparation of the Patient
- 5 days before the surgery, put 1% pilocarpine drops twice a day in the treated eye.
- In the operating room, in aseptic conditions, lie the patient on his/her back.
- Administer topical anesthesia such as oxybuprocaine 0.4%.
- Clean the eye and the skin around the eye with iodine antiseptic twice.
- Use a lid speculum to keep the eye open.
2. Epithelial Removal
- Mark the central 9.0 mm of the cornea with a circle corneal marker
- Remove the central 7.0 to 9.0 mm of corneal epithelium by mechanical debridement using a blunt spatula.
3. Riboflavin Application
- Apply 0.1% riboflavin without Dextran on the cornea every 2 min for 10 min.
4. UVA Irradiation
- Irradiate the cornea with a 370 nm wavelength UVA light at an irradiance of 30 mW/cm2 (5.4 J/cm2 surface dose) and at a 5 cm working distance for 3 min.
- During the irradiation, add topical anesthesia (oxybuprocaïne 0.4%) if necessary.
5. End of the Surgery
- Place antibiotic drops (tobramycin 0.3%) and artificial tears (hyaluronate drops 0.18%) into the operated eye.
- Place a soft bandage contact lens at the end of surgery until re-epithelialization is complete. Re-epithelialization usually takes 3 days.
- Prescribe analgesics such as paracetamol (500 mg) plus codeine (30 mg), 6 pills a day.
- After corneal re-epithelialization, initiate topical therapy with steroids (topical dexamethasone 1 mg/ml) and continue for 3-4 weeks. Plus, use artificial tears 4 times a day for 1 month.
3. Iontophoresis (I-CXL)
1. Preparation of the Patient
- 5 days before the surgery, put 1% pilocarpine drops twice a day in the treated eye.
- In the operating room, in aseptic conditions, lie the patient on his/her back.
- Administer topical anesthesia such as oxybuprocaine 0.4%.
- Clean the eye and the skin around the eye with iodine antiseptic twice.
- Use a lid speculum to keep the eye open.
2. Position the Iontophoresis Device.
- Apply the sticky passive electrode on the forehead under the operative field.
- Apply the active electrode, a suction ring, to the open eye. Center the suction ring on the periphery of the cornea before releasing the suction.

Figure 2. Iontophoresis device. The passive electrode is applied on the forehead under the operative field and the active electrode, a suction ring, is applied to the open eye. Please click here to view a larger version of this figure.
3. Riboflavin Application
- Fill the suction ring with hypoosmolar 0.1% riboflavin without Dextran.
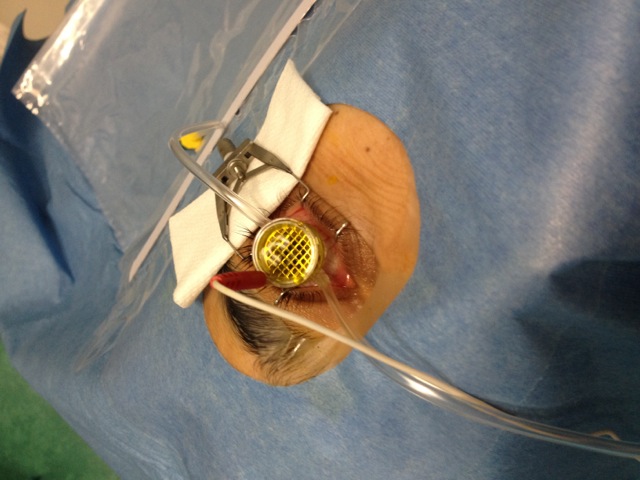
Figure 3. Riboflavin application in I-CXL. The suction ring is filled with hypoosmolar 0.1% riboflavine without Dextran. Please click here to view a larger version of this figure.
- Start the electrical current at 0.2 mA and gradually increase to 1.0 mA for a total iontophoresis time of 5 min (Figure 4).
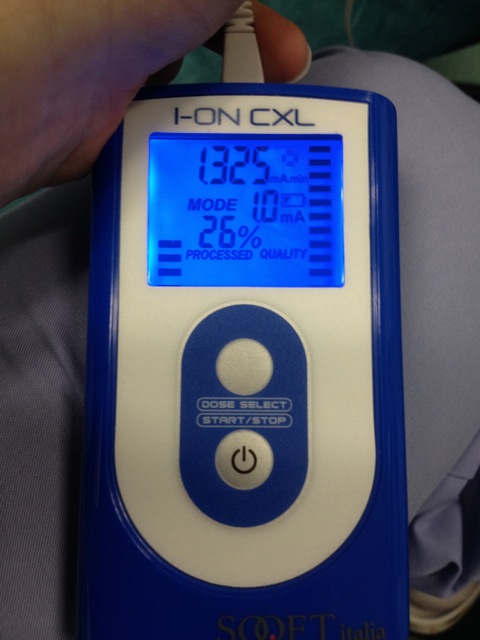
Figure 4. Iontophoresis device for riboflavin penetration. The electrical current is initially 0.2 mA and gradually increased to 1.0 mA. Total iontophoresis time is 5 minutes. Please click here to view a larger version of this figure.
4. UVA Irradiation
- Irradiate the cornea with a 370 nm wavelength UVA light at an irradiance of 10 mW/cm2 (5.4 J/cm2 surface dose) and at a 5 cm working distance for 9 min.
- During the irradiation, add topical anesthesia (oxybuprocaïne 0.4%) if necessary.
5. End of the Surgery
- Place antibiotic drops (tobramycin 0.3%) and artificial tears (hyaluronate drops 0.18%) into the operated eye.
- Prescribe analgesics such as paracetamol (500 mg) plus codeine (30 mg), 6 pills a day.
- After surgery, initiate topical therapy with steroids (topical dexamethasone 1mg/ml) and continue for 3-4 weeks. Plus, use artificial tears 4 times a day for 1 month.
Results
The corneal demarcation line was visible in AS OCT in 92% of cases at a mean depth of 301.6 µm (SD, 73.6)
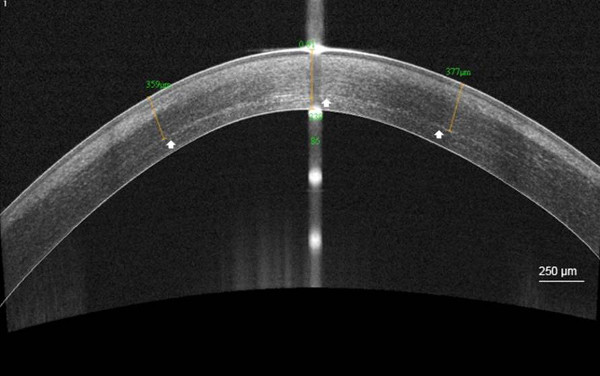
Figure 5. Demarcation line after C-CXL. High-resolution corneal anterior segment optical coherence tomography scan (AS OCT) visualizing the corneal stromal demarcation line at a mean depth of 358 µm (white arrow), 1 month after conventional corneal colla...
Discussion
CXL using UVA irradiation and riboflavin is the standard treatment for arresting the progression of keratoconus. Riboflavin is a photosensitizer which induces chemical covalent bonds (cross-links) when irradiated with UVA3. In the cornea, this phenomenon creates cross-links between collagen fibrils that increase corneal stiffness. Although this phenomenon is well described, until now there has been no direct evidence of intracorneal cross-links. Nonetheless, several studies have reported a stabilization of the...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Name | Company | Catalog Number | Comments |
| Riboflavin Product number | |||
| C-CXL | Sooft SPA, Montegiorgio, Italy | Ricrolin 468465-6 | |
| A-CXL | Avedro Inc, Waltham, Massachusetts | VibeX 520-01863-006 | |
| I-CXL | Sooft SPA, Montegiorgio, Italy | Ricrolin+ 975481-6 | Passive electrode: PROTENS ELITE 4848LE/ Active electrode: IONTOFOR CXL |
| UVA Machine | |||
| X-Vega | UVA: 3 mW/cm2 30 min | ||
| KXL System | UVA: 30 mW/cm2 10 min | ||
| X-Vega | UVA: 10 mW/cm2 9 min |
References
- Rabinowitz, Y. S. Keratoconus. Surv Ophthalmol. 42 (4), 297-319 (1998).
- Tuori, A. J., et al. The immunohistochemical composition of corneal basement membrane in keratoconus. Curr Eye Res. 16 (8), 792-801 (1997).
- Wollensak, G., Spoerl, E., Seiler, T. Riboflavin/ultraviolet-A-induced collagen cross-linking for the treatment of keratoconus. Am J Ophthalmol. 135 (5), 620-627 (2003).
- Raiskup-Wolf, F., Hoyer, A., Spoerl, E., Pillunat, L. E. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 34 (5), 796-801 (2008).
- Vinciguerra, P., et al. topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 116 (3), 369-378 (2009).
- Caporossi, A., Mazzotta, C., Baiocchi, S., Caporossi, T. Long-term results of riboflavin ultraviolet-A corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 149 (4), 585-593 (2010).
- Greenstein, S. A., Fry, K. L., Hersh, P. S. Corneal topography indices after corneal collagen cross-linking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 37 (7), 1282-1290 (2011).
- Ghanem, R. C., Santhiago, M. R., Berti, T., Netto, M. V., Ghanem, V. C. Topographic corneal wavefront, and refractive outcomes 2 years after collagen cross-linking for progressive keratoconus. Cornea. 33 (1), 43-48 (2014).
- Koller, T., Mrochen, M., Seiler, T. Complication and failure rates after corneal cross-linking. J Cataract Refract Surg. 35 (8), 1358-1362 (2009).
- Rocha, K. M., Ramos-Esteban, J. C., Qian, Y., Herekar, S., Krueger, R. R. Comparative study of riboflavin-UVA cross-linking and “flash-linking” using surface wave elastometry. J Refract Surg. 24 (7), 748-751 (2008).
- Caporossi, A., et al. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 39 (8), 1157-1163 (2013).
- Bikbova, G., Bikbov, M. Transepithelial corneal collagen cross-linking by iontophoresis of riboflavin. Acta Ophthalmol. 92 (1), 30-34 (2014).
- Efron, N., Hollingsworth, J. G. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 91 (1), 34-55 (2008).
- Patel, D. V., McGhee, C. N. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 47 (4), 1348-1351 (2006).
- Ku, J. Y., Niederer, R. L., Patel, D. V., Sherwin, T., McGhee, C. N. Laser scanning in vivo confocal analysis of keratocyte density in keratoconus. Ophthalmology. 115 (5), 845-850 (2008).
- Mazzotta, C., et al. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 146 (4), 527-533 (2008).
- Seiler, T., Hafezi, F. Corneal cross-linking-induced stromal demarcation line. Cornea. 25 (9), 1057-1059 (2006).
- Doors, M., et al. Use of anterior segment optical coherence tomography to study corneal changes after collagen cross-linking. Am J Ophthalmol. 148 (6), 844-851 (2009).
- Mazzotta, C., et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 26 (4), 390-397 (2007).
- Kymionis, G. D., et al. Correlation of the corneal collagen cross-linking demarcation line using confocal microscopy and anterior segment optical coherence tomography in keratoconic patients. Am J Ophthalmol. 157 (1), 110-115 (2014).
- Yam, J. C., Chan, C. W., Cheng, A. C. Corneal collagen cross-linking demarcation line depth assessed by Visante OCT After CXL for keratoconus and corneal ectasia. J Refract Surg. 28 (7), 475-481 (2012).
- Jordan, C., Patel, D. V., Abeysekera, N., McGhee, C. .. N. .. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmology. 121 (2), 469-474 (2014).
- Touboul, D., et al. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg. 28 (11), 769-776 (2012).
- Bouheraoua, N., et al. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 55 (11), 7601-7609 (2014).
- Hafezi, F., Mrochen, M., Iseli, H. P., Seiler, T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 35 (4), 621-624 (2009).
- Cınar, Y., et al. Comparison of accelerated and conventional corneal collagen cross-linking for progressive keratoconus. Cutan Ocul Toxicol. 33 (3), 218-222 (2013).
- Cingü, A. K., et al. Transient corneal endothelial changes following accelerated collagen cross-linking for the treatment of progressive keratoconus. Cutan Ocul Toxicol. 33 (2), 127-131 (2013).
- Spoerl, E., Mrochen, M., Sliney, D., Trokel, S., Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 26 (4), 385-389 (2007).
- Gokhale, N. S. Corneal endothelial damage after collagen cross-linking treatment. Cornea. 30 (12), 1495-1498 (2011).
- Rootman, D. S., et al. Pharmacokinetics and safety of transcorneal iontophoresis of tobramycin in the rabbit. Invest Ophthalmol Vis Sci. 29 (9), 1397-1401 (1998).
- Vinciguerra, P., et al. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus: initial clinical outcomes. J Refract Surg. 30 (11), 746-753 (2014).
- Caporossi, A., et al. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 31 (3), 227-231 (2012).
- Buzzonetti, L., Petrocelli, G., Valente, P., Larossi, G., Ardia, R., Petroni, S. Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea. 34 (5), 512-515 (2015).
- Baiocchi, S., Mazzotta, C., Cerretani, D., Caporossi, T., Caporossi, A. Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 35 (5), 893-899 (2009).
- Wollensak, G., Iomdina, E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 35 (3), 540-546 (2009).
- Soeters, N., Wisse, R. P., Godefrooij, D. A., Imhof, S. M., Tahzib, N. G. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 159 (5), 821-828 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved