A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Assembly and Tracking of Microbial Community Development within a Microwell Array Platform
In This Article
Summary
The development of microbial communities depends on a combination of factors, including environmental architecture, member abundance, traits, and interactions. This protocol describes a synthetic, microfabricated environment for the simultaneous tracking of thousands of communities contained in femtoliter wells, where key factors such as niche size and confinement can be approximated.
Abstract
The development of microbial communities depends on a combination of complex deterministic and stochastic factors that can dramatically alter the spatial distribution and activities of community members. We have developed a microwell array platform that can be used to rapidly assemble and track thousands of bacterial communities in parallel. This protocol highlights the utility of the platform and describes its use for optically monitoring the development of simple, two-member communities within an ensemble of arrays within the platform. This demonstration uses two mutants of Pseudomonas aeruginosa, part of a series of mutants developed to study Type VI secretion pathogenicity. Chromosomal inserts of either mCherry or GFP genes facilitate the constitutive expression of fluorescent proteins with distinct emission wavelengths that can be used to monitor community member abundance and location within each microwell. This protocol describes a detailed method for assembling mixtures of bacteria into the wells of the array and using time-lapse fluorescence imaging and quantitative image analysis to measure the relative growth of each member population over time. The seeding and assembly of the microwell platform, the imaging procedures necessary for the quantitative analysis of microbial communities within the array, and the methods that can be used to reveal interactions between microbial species area all discussed.
Introduction
Microbial communities are shaped by both deterministic factors, such as the structure of the environment, and stochastic processes, which are associated with cell death, division, protein concentration, number of organelles, and mutation1. Within the natural environment, it can be nearly impossible to parse the individual impact of these influences on community composition and activity. Obscured by natural structures and buried within a chemical and biological milieu, identifying community members and further resolving their spatiotemporal distribution within the natural environment is extremely challenging. Nonetheless, recent efforts have underscored the importance of spatial organization on community function and point towards the need to account for both member abundance and organization in ongoing studies2,3,4.
It is clear that the local chemical environment (i.e. the availability of nutrients and secondary metabolites), the physical structure (e.g., soil architecture, plant roots, ocean particles, or the intestinal microvilli), the presence or absence of oxygen, and the introduction of pathogenic species all affect the composition, architecture, and function of microbial communities5,6,7,8,9,10,11. Nonetheless, traditional techniques for cultures that neglect to capture these factors continue to prevail. Community composition (e.g., the presence of co-dependent species), physical attachment, signaling molecule concentration, and direct cell-cell contact are all important factors for shaping a microbial community and can be lost in conventional culture conditions. These properties are difficult to replicate in a bulk liquid culture or on an agar plate. The availability of microfluidic, micropatterning, and nanofabrication techniques that allow for the replication of key physical and chemical features of natural environments has, however, enabled many researchers to build bacterial communities to study their interactions12,13,14 and to develop synthetic environments that mimic natural conditions4,15,16,17,18,19,20.
This protocol describes a method to fabricate a microwell array device and provides detailed experimental procedures that can be used to functionalize the wells in the array and to grow bacteria, both as single-species colonies and in multi-member communities. This work also demonstrates how bacteria modified to produce fluorescent reporter proteins can be used to monitor bacterial growth within wells over time. A similar array was presented previously and showed that it is possible to track the growth of single-species colonies of Pseudomonas aeruginosa (P. aeruginosa) in microwells. By modulating well size and seeding density, the starting conditions of thousands of growth experiments can be varied in parallel to determine how the initial inoculation conditions affect the ability of the bacteria to grow21. The current work uses a slightly modified version of the microwell array that builds on the previous work by enabling the simultaneous comparison of multiple arrays and by using a more robust experimental protocol. The array used in this work contains multiple subarrays, or array ensembles, containing wells of different sizes, ranging from 15 - 100 µm in diameter, that are arranged at three different pitches (i.e. 2x, 3x, and 4x the well diameter). The arrays are etched into silicon, and the growth of the bacteria seeded in the silicon arrays is enabled by sealing the arrays with a coverslip that has been coated with a medium-infused agarose gel. P. aeruginosa mutants designed to study the Type VI secretion system are used in this demonstration.
The results presented here build toward the ultimate goal of analyzing multimember communities within microwell arrays, enabling researchers to monitor the abundance and organization of bacteria in situ while controlling and probing the chemical environment. This should ultimately provide insights into the "rules" that govern community development and succession.
Protocol
1. Silicon Microwell-array Fabrication
- Parylene coating
- Deposit between 1-1.5 µm of parylene N on silicon wafers using a commercially available parylene coating system according to the manufacturer's specifications and instructions (settings: vaporizer set point = 160 °C; furnace set point = 650 °C).
NOTE: Approximately 6 g of parylene N loaded into a chamber yields coatings 1-1.5 µm thick.
- Deposit between 1-1.5 µm of parylene N on silicon wafers using a commercially available parylene coating system according to the manufacturer's specifications and instructions (settings: vaporizer set point = 160 °C; furnace set point = 650 °C).
- Photolithography
- Spin-coat the parylene N-coated wafers with adhesion promoter, 20% hexamethyldisilazane (HMDS), and 80%propylene glycol monomethyl ether acetate (PGMEA) (see the Table of Materials) at 3,000 rpm for 45 s. Fill a 2 mL transfer pipette with adhesion promoter and sprinkle it over the entire wafer. Allow the wafer to sit for approximately 10 s before spinning it dry.
- Fill a 2 mL transfer pipette with positive-tone photoresist (see the Table of Materials) and dispense the photoresist in the center of the wafer. Spin at 3,000 rpm for 45 s to yield a resist coating that is approximately 1.5 µm thick.
- Soft-bake the samples on a hotplate at 115 °C for 1 min.
- Use a contact aligner and photomask with the desired well pattern to expose the sample to ultraviolet light. Expose the spin-coated wafer through the patterned photomask for 6 s, giving an approximate dose of 60-80 mJ/cm2 measured at 365 nm.
- Develop the pattern by submerging the sample in developer (< 3% tetramethyl ammonium hydroxide in water; see the Table of Materials) for 2 min. Rinse with DI water and dry with clean, dry nitrogen.
NOTE: The areas of photoresist exposed to UV should be cleared during development.
- Reactive ion etching
- Use an oxygen plasma etch to remove the exposed parylene all the way to the silicon substrate.
NOTE: The recipe can be modulated to alter the etch rate of the parylene. For parylene thicknesses between 1 and 5 µm, use a recipe with 60 mTorr, 20 °C, 100 sccm O2, 10 W RF, and 2,000 W ICP on a Reactive Ion Etching (RIE) tool. After etching and removing the exposed parylene layer, the patterned area (i.e. the exposed silicon) should look shiny and silver. - Use a deep RIE (DRIE; e.g., Bosch DRIE) etch process to etch into the silicon.
NOTE: The etch rate and duration will determine the well depth. One full cycle of the Bosch process (a 3 s deposition step: 20 mTorr, 15 °C, 140 sccm C4 F8, 10 W RF, and 1,750 W ICP followed by a 10 s etch process: 20 mTorr, 15 °C, 120 sccm SF6, 8 W RF, and 1,750 W ICP) corresponds to approximately 1 µm of etch depth. The wells used in this demonstration range from 3 - 3.5 µm deep. - Verify the etch depth using physical profilometry.
- Load the sample into a physical profilometer (see the Table of Materials).
- Turn on the sample vacuum and press the manual load button.
- Focus the system onto the sample by pressing the "Focus" button. Position an appropriate feature for measurement on the view screen.
- Scan the sample. Level the profile and measure the feature depth.
- Record the etch rate and modulate subsequent etch times to achieve the desired depth.
NOTE: The measurements will include the depth of the silicon well, the thickness of deposited parylene, and the thickness of the photoresist. Verifying the thickness of each layer throughout the procedure is necessary to achieve accurate well depth.
- Use an oxygen plasma etch to remove the exposed parylene all the way to the silicon substrate.
2. Bacterial Culture and Seeding (Figure 1a)
- Start colonies on Luria Broth (LB) agar plates from glycerol stocks and use within two weeks. Pick colonies of the desired strains from LB agar plates and start overnight cultures of P. aeruginosa. Incubate the overnight cultures for approximately 18 h at 37 °C while shaking at 220 rpm in R2A medium.
NOTE: Colonies should be picked within two weeks of plating to ensure that the mutations and fluorescent reporter genes are retained. All P. aeruginosa work should be done under BSL-2 conditions. - Use a diamond scribe to section the silicon wafer into individual chips containing the ensembles of different sizes and pitch-well arrays. Ensure that each chip contains the full complement of well sizes and pitches for the study.
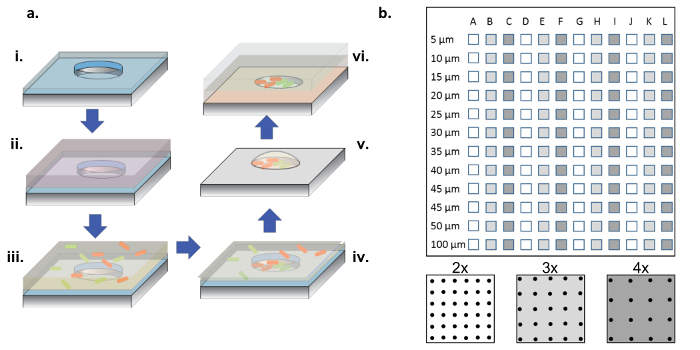
Figure 1: Fabrication and Cell Seeding Procedure. (a) Microwell arrays are etched into silicon wafers coated with a thin layer of parylene (i). To wet the wells and/or functionalize the surface, a protein solution is added in a droplet on top of the arrays (ii). The protein solution is removed, the wafers are dried, and a new solution containing the desired bacteria is added (iii). The bacterial solution is removed after an incubation period, and the wafers are allowed to dry, leaving behind bacteria in the wells and on the surface (iv). The surface-associated bacteria are removed with parylene lift-off, leaving behind bacteria seeded cleanly in the microwells and still viable due to the 2% glycerol medium, which helps to keep the wells hydrated(v). The silicon chips are then placed array-side down on an agarose gel-coated glass coverslip, which feeds bacterial growth in the microwells (vi). (b) Layout of sub-arrays on a single silicon device. Each sub-array contains a set of identical wells. The diameter of the microwells across all sub-arrays range in diameter from 5-100 µm and are organized at 2x, 3x, or 4x the well diameter pitch, which is denoted by the white to dark-gray colors on the bottom panel schematic. When the well depths are shallow (<10 µm), the 5 and 10 µm well diameters are rarely useful, generally because of a lack of cells colonizing these very small wells. In this work, only the data from wells with 15-100 µm diameters were analyzed. Please click here to view a larger version of this figure.
NOTE: As shown in Figure 1b, a complete chip contains sub-arrays of wells, with diameters ranging from 5 to 100 µm, with three different pitches (i.e. 2x, 3x, and 4x the diameter) repeating 4 times.
- Place a 150 µL droplet of 500 µg/mL Bovine Serum Albumin (BSA) in PBS solution on top of the array to wet the microwells. Incubate the BSA solution for 1 h on the chip at RT in a humid chamber.
- Create the chamber by filling the bottom of an empty pipette tip box with Phosphate-Buffered Saline (PBS).
NOTE: Other substances, such as specific lectins, can be used in place of BSA to functionalize the surface of the microwells.
- Create the chamber by filling the bottom of an empty pipette tip box with Phosphate-Buffered Saline (PBS).
- While incubating silicon chips with BSA solution, centrifuge the cultures at 2,500 rpm (corresponding to an average of 950 x g) for 5 min and then resuspend them in 500 µL of fresh R2A medium with 2% glycerol.
- Determine the OD of the culture using a UV-vis spectrometer at 600 nm. Adjust it to an OD of 0.02 using 2% glycerol R2A medium.
NOTE: The glycerol helps to prevent the wells from drying out during parylene lift-off.
- Determine the OD of the culture using a UV-vis spectrometer at 600 nm. Adjust it to an OD of 0.02 using 2% glycerol R2A medium.
- After incubation, remove the BSA solution and rinse 3x with PBS by removing and replacing the liquid droplet covering the silicon microwell array. Dry under nitrogen.
- Add 150 µL of 0.02 OD cultures to each of the dry arrays placed in a humid chamber. Incubate for 1 h at 4 °C to allow the bacteria to adhere to the well walls.
NOTE: Refrigeration is not required for incubation. The 4 °C incubation time can be used to prevent the growth of bacteria before imaging begins so that one can visualize the spatial organization of the communities prior to growth. Room-temperature incubation can also be used. Both protocols result in similar growth curves.
3. Microscope Set-up
- Prior to starting the bacterial incubation on the silicon chips, turn on the stage-top environmental control chamber (see the table of materials) and adjust the settings on the control box so that the humidity (~100%) and temperature (30-32 °C, see step 3.2) can equilibrate prior to adding samples.
- Level the sample holder and line the interior around the sample with PBS-soaked lab wipes (see the Table of Materials) to increase the humidity in the chamber to dew point. Set the temperature of the chamber to 30 °C and that of the chamber lid to 32 °C to reduce condensation on the imaging plane.
NOTE: The slide holder fits into the live-cell chamber with a gasket that is approximately 1 cm thick. The sample holder is leveled with the assistance of a bubble level that is placed on top of the sample holder. The sample holder can be tilted slightly and remain sealed in the gasket to level. - While cultures are incubating on the silicon chips, manually turn on the power switch for the mercury lamp at least 30 min prior to imaging. Manually turn on the camera and automated microscope stage. Open the software used to control the microscope and peripheral equipment and ensure that the equipment is recognized by the software.
NOTE: Magnification is 10X with NA = 0.3.
4. Preparation of Agarose-coated Glass Coverslips
- Microwave previously prepared agarose solutions (i.e. 2% agarose in R2A medium) until a liquid state is reached, approximately 60 s.
- Wet the back of a 75 mm x 22 mm, #1.5 glass coverslip with ethanol and place it lengthwise, centered, across a 2 x 3" (50 x 75 mm) glass slide. Place two PDMS spacers (thickness of ~1 mm) along the long edges of the coverslip and shift the glass coverslip so that roughly 1 mm of the coverslip is overhanging the edge of the slide.
- Pour 5 mL of liquid agarose solution on top of the glass coverslip, just enough to completely cover it, and place a second 2 x 3" glass slide on top of the assembly to "sandwich" the liquid agar between the coverslip and slide.
NOTE: This controls the depth of agarose, making the total thickness of the coverslip and hardened agarose equal in thickness to the PDMS spacers. - Allow the glass slide-coverslip-agarose gel-glass slide sandwich to set until the agarose solution begins to solidify; then, transfer it to a refrigerator. After 15 min, remove the excess solid agarose and cut around the glass coverslip. Move this to a clean dish and place it in a refrigerator until use.
5. Sealing the Wells with an Agarose-coated Coverslip and Imaging
- After the bacteria incubation period is complete, remove the agarose-coated coverslip from the refrigerator and prepare the silicon chips, as follows.
- Dip the silicon chips in ultrapure water, one at a time, for 10 s each. Set them on their edges on a lab wipe or tissue until most of the excess liquid has drained from the edges of the chips.
- Cut a piece of tape to match the edge length of each silicon chip. Place the tape on the parylene that is covering the silicon and use it to quickly peel away the parylene coating.
- Immediately invert each peeled chip and place each chip such that the microwell-array side faces (and makes contact with) the agarose-coated side of an agarose-coated coverslip. Take care not to move or shift the chip after it touches the agarose to prevent the growth of bacteria outside of the wells.
- Place the assembled microwell array/agarose coverslip in the slide holder of the stage-top environmental control chamber on the miscroscope.
- Use ambient light or directed light (e.g., a flashlight) to locate arrays of interest. Use the commercial software controlling the automated stage to save those positions (see the table of materials). Turn off ambient or directed light after the positions are stored.
- In the commercial software, open the "ND Acquisition" panel.
NOTE: This panel includes a menu for automatic saving to a specific directory, as well as programmable image acquisition. For these experiments, the "Time," "XY," and "λ" menus are used. - To save the locations in the software, click on the "XY menu" and then check an empty box on the left-hand side for each position that needs to be saved. Also, click on the "Include Z" button.
- Acquire images over time at the desired wavelengths and 10 magnification using the appropriate fluorescence filter cubes (see the Table of Materials).
- Use the control software and saved array positions to move to each saved location and to focus on the wells. Click on each XY location in the saved list and adjust the focus using the Green Flourescence Protein (GFP) filter. Save the new z-position by clicking the arrow pointing at the z-location.
NOTE: This process can be time-consuming. Consider taking the precaution of increasing the gain and using the neutral density filter to reduce the light intensity to prevent photobleaching. - Determine the z-axis distance between focal planes for each wavelength by noting the difference in the z-axis position when focused on the surface of the array. Choose 2-3 locations from the array with the mixed red/green bacteria population and focus using the Red Fluorescence Protein (RFP) filter.
- Subtract the distance between the focal planes using the GFP and RFP fluorescence filters and add that focal plane adjustment under the "λ" menu.
NOTE: For example, if the array appears focused in the GFP channel at a z-location of 50 µm, and the same array appears focused in the RFP channel at 55 µm, add +5 next to the RFP optical configuration in the "λ" menu.
- Subtract the distance between the focal planes using the GFP and RFP fluorescence filters and add that focal plane adjustment under the "λ" menu.
- Begin time-lapse image acquisition.
NOTE: For the experiments shown here, RFP and GFP images were acquired for every array position at 30 min intervals using multi-dimension image acquisition through a commercial software that controls the camera, shutter, filter wheel, and motorized stage.- Set the "interval" to 30 min and the "duration of the experiment" to 24 h under the "Time" menu. Click "Run Now."
NOTE: With the "Time," "XY," and "λ" boxes checked, running the program will move the stage to image each location (i.e. the saved XYZ locations), take an image in one wavelength, move the z-position to account for focal plane differences (i.e., lambda or wavelength control), take the second image, move on to the next array location (multipoint), and loop this at 30 min intervals (time-lapse).
- Set the "interval" to 30 min and the "duration of the experiment" to 24 h under the "Time" menu. Click "Run Now."
- Use the control software and saved array positions to move to each saved location and to focus on the wells. Click on each XY location in the saved list and adjust the focus using the Green Flourescence Protein (GFP) filter. Save the new z-position by clicking the arrow pointing at the z-location.
- Acquire illumination control images.
NOTE: Use the "ND Acquisition," "Time," and "XY" menus to take images of 4 locations, 25x each.- Take a series of 100 "darkfield" images by turning off all light sources and taking an "image" of a standard slide. These images will capture camera noise. Use the longest exposure time used during the timelapse (step 5.3.3).
- Take a series of 100 "illumination field" images by imaging a standard slide (i.e. uniform RFP or GFP intensity) at a few different locations to capture the uneven illumination at the given experimental conditions. Choose an exposure time that maximizes the signal without reaching saturation.
6. Analysis
- Process the image stacks using an image analysis software (e.g., ImageJ).
- Convert the acquired images to tiff file format using the commercial software. Upload images into the image analysis software by clicking "File"> "Import"> "Image Sequence."
- Create a "Correction Image" by averaging all "darkfield" and "illumination field" images. Subtract the average "darkfield" image from the average "illumination field" image by choosing "Process" > "Image Calculator." Select the two images, "Image1" and "Image2," and then "Subtract" in the "Operation" field. Click "OK."
- For averaging, load the correction (or darkfield) images, click "Image" > "Stacks" > "Z Project" > "Average Projection."
- Perform image registration if necessary. Then, perform background subtraction by clicking "Process" > "Subtract Background." Enter a radius (e.g., 125) in the "radius" field and select "sliding paraboloid."
- Perform illumination correction using "Process" > "Calculator Plus." Choose the following parameters: operation, divide; i1, well image; i2, correction image; k1, correction image mean; and k2, 0. Click "Create New Window."
NOTE: This data set did not require registration, but in other work, the ImageJ Plugin StackReg was used with the "Translation" transformation. For the background subtraction, use the same sliding paraboloid radius for every image set. For example, if the largest wells imaged have a pixel radius of 100, use a radius larger than 100 (e.g., 125) for every image set.
- Determine the growth of each strain in the microwells.
- Select regions of interest (ROIs) around each microwell in the desired arrays using the ImageJ "MicroArray" plugin.
- In the "MAP" menu, click "Reset Grid." Specify rows, columns, and diameter (based on the well size and number on the array; see Figure 1b). Select "circle" from the "ROI shape" menu.
- Hold the "Alt" key while selecting the top-left ROI with the mouse to move the ROI array. Hold the "shift" key while selecting the bottom-left ROI to change the size of the array. Hold the "shift" key while selecting an ROI from the right side of the array, but not at the corners, to change the spacing of the ROIs.
- Use the above commands to fit the ROI array over the wells in an image. Click "Measure RT."
NOTE: The plugin will export the desired measurements from each ROI. Use three ROI sizes, creating concentric rings around the wells to collect local the background signal (i.e., the signal from the middle ring subtracted from the outer ring) and fluorescence measurements (i.e., the signal from the inner ring).
- Collect the data in a spreadsheet software and calculate the background signal. Import it to a custom scripting software for further analysis.
- Select regions of interest (ROIs) around each microwell in the desired arrays using the ImageJ "MicroArray" plugin.
- Data organization and analysis
- Import the data and organize the data collected in ImageJ into a matrix in the following order for all times: column 1, sub-array number; column 2, well row; column 3, well column; column 4, mean intensity; column 5, background intensity; and column 6, mean intensity - background intensity.
- Separate the results of the mCherry and GFP acquisition into different matrices. Store the results from each sub-array and each color in a different cell in a cell array.
NOTE: This organization makes it simpler to move back and forth between image data and measurement results, cleaning the data and ensuring that the measurements accurately represent the data.
- Separate the results of the mCherry and GFP acquisition into different matrices. Store the results from each sub-array and each color in a different cell in a cell array.
- Adjust for the autofluorescence of P. aeruginosa.
NOTE: In experiments involving the co-culture of GFP and mCherry strains, an mCherry-only chip should be analyzed to ascertain the relationship between mCherry and green autofluorescence.- Plot the mCherry-versus-GFP signal from all mCherry ΔretSΔtse/i1-6 wells at all time-points to determine the relationship between the mCherry signal and the autofluorescence in the GFP channel. Subtract the autofluorescence signal from the co-cultures.
- Plot the trajectories and fit a modified logistic equation to each trajectory to extract parameters using least squares fitting in either a spreadsheet software or a custom scripting software.
- Look for correlations between and among GFP and mCherry trajectory parameters.
- Import the data and organize the data collected in ImageJ into a matrix in the following order for all times: column 1, sub-array number; column 2, well row; column 3, well column; column 4, mean intensity; column 5, background intensity; and column 6, mean intensity - background intensity.
Results
The experimental platform presented here is designed for high-throughput and high-content studies of bacterial communities. The design enables thousands of communities, growing in wells of various sizes, to be analyzed simultaneously. With this microwell array design, the dependence of the final community composition on initial seeding densities, well size, and chemical environment can be determined. This work demonstrates the growth of a two-member community in the microwell array and pu...
Discussion
This article presented a microwell array device and experimental protocols designed to enable high-throughput and high-content live-cell imaging-based analysis of bacterial community development. While the focus of the demonstration here was to study the effects of contact-mediated Type VI secretion on community development, the arrays were designed to be flexible and accommodate the study of a broad range of microbial communities and microbe-microbe interactions. The work here focuses solely on the use of bacteria that ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Microwell arrays were fabricated and characterized at the Center for Nanophase Materials Sciences User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy. Financial support for this work was provided through the Oak Ridge National Laboratory Director's Research and Development Fund. The authors would also like to thank the J. Mougous Laboratory (University of Washington, Seattle, WA) for the supply of P. aeruginosa strains used in these studies.
Materials
| Name | Company | Catalog Number | Comments |
| Parylene N | Specialty Coating Systems | CAS NO.:1633-22-3 | |
| Parylene coater | Specialty Coating Systems | Labcoter 2 Parylene Deposition Unit PDS2010 | |
| Silicon Wafer | WRS Materials | 100mm diameter, 500-550μm thickness, Prime, 10-20 resistivity, N/Phos<100>, | |
| adhesion promoter | Shin-Etsu Microsci | MicroPrime P20 adhesion promoter | |
| postive tone photoresist | Rohm and Haas Electronics Materials LLC (Owned by Dow) | Microposit S1818 Positive Photoresist (code 10018357) | |
| Quintel Contact Aligner | Neutronix Quintel Corp | NXQ 7500 Mask Aligner | |
| Reactive Ion Etching Tool | Oxford Instruments | Plasmalab System 100 Reactive Ion Etcher | |
| R2A Broth | TEKnova | R0005 | |
| Bovine Serum Albumin | Sigma | A9647 | |
| Multimode Plate Reader | Perkin Elmer | Enspire, 2300-0000 | |
| Fluorescent Microscope | Nikon | Eclipse Ti-U | |
| Automated Stage | Prior | ProScan III | |
| CCD camera | Nikon | DS-QiMc | |
| Stage-top environmental control chamber | In Vivo Scientific | STEV ECU-HOC | |
| Phosphate Buffered Saline | ThermoFisher Scientific | 14190144 | |
| UltraPure Agarose | ThermoFisher Scientific | 16500500 | |
| 25 x 75 mm No. 1.5 coverslip | Nexterion | High performance #1.5H coverslips | |
| Fluorescence Reference Slides | Ted Pella | 2273 | |
| Physical Stylus Profilometer | KLA Tencor | P-6 | |
| lab wipes | Kimberly Clark | Kimipe KIMTECH SCIENCE Brand, 34155 | |
| commercial software | Nikon | NIS Elements | |
| Zeiss 710 Confocal Microscope | Zeiss | ||
| filter cubes | Nikon | Nikon FITC (96311), Nikon Texas Red(96313) |
References
- Zhou, J., Deng, Y., et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci. 111, E836-E845 (2014).
- Valm, A. M., Welch, J. L. M., et al. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci USA. 108 (10), 4152-4157 (2011).
- Satoh, H., Miura, Y., Tsushima, I., Okabe, S. Layered structure of bacterial and archaeal communities and their in situ activities in anaerobic granules. Appl Environ Microbiol. 73 (22), 7300-7307 (2007).
- Kim, H. J., Boedicker, J. Q., Choi, J. W., Ismagilov, R. F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA. 105 (47), 18188-18193 (2008).
- Nunan, N., Wu, K., Young, I. M., Crawford, J. W., Ritz, K. Spatial distribution of bacterial communities and their relationships with the micro-architecture of soil. FEMS Microbiol Ecol. 44, 203-215 (2003).
- Grundmann, G. L. Spatial scales of soil bacterial diversity - The size of a clone. FEMS Microbiol Ecol. 48, 119-127 (2004).
- Langenheder, S., Lindstrom, E. S., Tranvik, L. J. Structure and Function of Bacterial Communities Emerging from Different Sources under Identical Conditions. Appl Environ Microbiol. 72 (1), 212-220 (2006).
- Camp, J. G., Kanther, M., Semova, I., Rawls, J. F. Patterns and Scales in Gastrointestinal Microbial Ecology. Gastroenterology. 136 (6), 1989-2002 (2009).
- Renner, L. D., Weibel, D. B. Physicochemical regulation of biofilm formation. MRS Bull. 36 (5), 347-355 (2011).
- Wessel, A. K., Hmelo, L., Parsek, M. R., Whiteley, M. Going local: technologies for exploring bacterial microenvironments. Nat Rev Microbiol. 11 (5), 337-348 (2013).
- Stacy, A., McNally, L., Darch, S. E., Brown, S. P., Whiteley, M. The biogeography of polymicrobial infection. Nat Rev Microbiol. 14 (2), 93-105 (2015).
- Hansen, R. R., Shubert, K. R., Morrell-Falvey, J. L., Lokitz, B. S., Doktycz, M. J., Retterer, S. T. Microstructured block copolymer surfaces for control of microbe adhesion and aggregation. Biosensors. 4 (1), 63-75 (2014).
- Hansen, R. R., Hinestrosa, J. P., et al. Lectin-functionalized poly(glycidyl methacrylate)- block -poly(vinyldimethyl azlactone) surface scaffolds for high avidity microbial capture. Biomacromolecules. 14 (10), 3742-3748 (2013).
- Timm, C. M., Hansen, R. R., Doktycz, M. J., Retterer, S. T., Pelletier, D. A. Microstencils to generate defined, multi-species patterns of bacteria. Biomicrofluidics. 9 (6), (2015).
- Keymer, J. E., Galajda, P., Muldoon, C., Park, S., Austin, R. H. Bacterial metapopulations in nanofabricated landscapes. Proc Natl Acad Sci USA. 103 (46), 17290-17295 (2006).
- Zhang, Q., Lambert, G., et al. Acceleration of Emergence of Bacterial Antibiotic Resistance in Connected Microenvironments. Science. 333 (6050), 1764-1767 (2011).
- Friedlander, R. S., Vlamakis, H., Kim, P., Khan, M., Kolter, R., Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci USA. 110 (14), 5624-5629 (2013).
- Zhou, J., Liu, W., et al. Stochastic Assembly Leads to Alternative Communities with Distinct Functions in a Bioreactor Microbial Community. MBio. 4 (2), 1-8 (2013).
- van Vliet, S., Hol, F. J., Weenink, T., Galajda, P., Keymer, J. E. The effects of chemical interactions and culture history on the colonization of structured habitats by competing bacterial populations. BMC Microbiol. 14 (1), 116 (2014).
- Niepa, T. H. R., Hou, L., et al. Microbial Nanoculture as an Artificial Microniche. Sci Rep. 6, 30578 (2016).
- Hansen, R. H., Timm, A. C., et al. Stochastic Assembly of Bacteria in Microwell Arrays Reveals the Importance of Confinement in Community Development. PLoS ONE. 11 (5), e0155080 (2016).
- Hood, R. D., Singh, P., et al. A Type VI Secretion System of Pseudomonas aeruginosa Targets a Toxin to Bacteria. Cell Host Microbe. 7 (1), 25-37 (2010).
- LeRoux, M., Ja De Leon, ., et al. Quantitative single-cell characterization of bacterial interactions reveals type VI secretion is a double-edged sword. Proc Natl Acad Sci. 109 (48), 19804-19809 (2012).
- Whitney, J. C., Beck, C. M., et al. Genetically distinct pathways guide effector export through the type VI secretion system. Mol Microbiol. 92 (3), 529-542 (2014).
- Warrick, J. W., Timm, A., Swick, A., Yin, J. Tools for Single-Cell Kinetic Analysis of Virus-Host Interactions. PLoS ONE. 11 (1), e0145081 (2016).
- Zwietering, M. H., Jongenburger, I., Rombouts, F. M., Van't Riet, K. Modeling of the Bacterial Growth Curve. Appl Environ Microbiol. 56 (6), 1875-1881 (1990).
- Halsted, M., Wilmoth, J. L., et al. Development of transparent microwell arrays for optical monitoring and dissection of microbial communities. J Vac Sci Technol B Nanotechnol Microelectron. 34 (6), 06KI03 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved