A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Selection-dependent and Independent Generation of CRISPR/Cas9-mediated Gene Knockouts in Mammalian Cells
In This Article
Summary
Recent advances in the ability to genetically manipulate somatic cell lines hold great potential for basic and applied research. Here, we present two approaches for CRISPR/Cas9 generated knockout production and screening in mammalian cell lines, with and without the use of selectable markers.
Abstract
The CRISPR/Cas9 genome engineering system has revolutionized biology by allowing for precise genome editing with little effort. Guided by a single guide RNA (sgRNA) that confers specificity, the Cas9 protein cleaves both DNA strands at the targeted locus. The DNA break can trigger either non-homologous end joining (NHEJ) or homology directed repair (HDR). NHEJ can introduce small deletions or insertions which lead to frame-shift mutations, while HDR allows for larger and more precise perturbations. Here, we present protocols for generating knockout cell lines by coupling established CRISPR/Cas9 methods with two options for downstream selection/screening. The NHEJ approach uses a single sgRNA cut site and selection-independent screening, where protein production is assessed by dot immunoblot in a high-throughput manner. The HDR approach uses two sgRNA cut sites that span the gene of interest. Together with a provided HDR template, this method can achieve deletion of tens of kb, aided by the inserted selectable resistance marker. The appropriate applications and advantages of each method are discussed.
Introduction
Stable genetic alterations provide an advantage over transient methods of cellular perturbation, which can be variable in their efficiency and duration. Genomic editing has become increasingly common in recent years due to the development of target specific nucleases, such as zinc-finger nucleases1,2,3,4,5, transcription activator-like effector nucleases (TALENs)6,7,8,9 and RNA-guided nucleases derived from the clustered, regularly interspaced short palindromic repeats (CRISPR) system10.
The CRISPR/Cas9 editing machinery is adapted from an immune system that bacteria and archaea use to defend against viral infections11,12,13. In this process, short, 20-30 nt fragments of invading viral sequence are incorporated into a genomic locus as "spacers" flanked by repeating units14,15. Subsequent transcription and RNA processing generates small CRISPR-associated RNAs16 (crRNAs) that, together with a trans-activating crRNA17 (tracrRNA), assemble with the effector Cas9 endonuclease. The crRNAs thus provide specificity to Cas9 targeting, guiding the complex to cleave complementary viral DNA sequences and preventing further infections18,19. Any "protospacer" sequence in the targeted DNA can serve as the source of the crRNA, as long as it is directly 5' to a short protospacer adjacent motif (PAM), NGG in the case of S. pyogenes Cas920. The absence of the PAM sequence near the spacer in the host's CRISPR locus distinguishes between self and non-self, preventing targeting of the host. Because of its universality and flexibility, this biological system has been powerfully adapted for genomic editing, such that nearly any PAM-adjacent DNA site can be targeted. In this version, a further modification fused the crRNA and tracrRNA into a single guide RNA (sgRNA) component that is loaded into the Cas9 protein21.
Upon expression of Cas9 and an sgRNA in eukaryotic cells, the Cas9 protein cleaves both DNA strands at the targeted locus. In the absence of a suitable region of homologous sequence, the cell fixes this break via non-homologous end joining (NHEJ)22,23,24, which typically introduces small deletions or, rarely, insertions. When targeting an open reading frame, the repair likely leads to a translational frameshift that produces a non-functional protein product. In contrast, when provided with an exogenous template with large regions of homology, the cell may fix the double-strand break by homology directed repair25,26. This route allows for larger precise deletions, replacements or insertions in the genome, coupled with the introduction of excisable selection markers27.
Here, we present protocols for generating knockout cell lines by either of these two CRISPR/Cas9 methods (Figure 1A). The NHEJ approach uses a single sgRNA cut site and selection-independent screening, and thus requires little upfront preparation. When using this method, guide RNAs complementary to exons near the 5' end of the transcript, which are most likely to produce a knockout, must be designed. Since the modifications to the genome in this case are small, screening for knockout clones is based on dot blots, where the protein product is assessed in a high-throughput manner. We use the generation of ELAV-like 1 protein (ELAVL1) knockout lines as an example. The second approach relies on homology directed repair (HDR) and uses two sgRNA cut sites that span the gene or region of interest, allowing for deletions of tens of kb. A plasmid with two regions of homology that flank the cleavage sites provides a replacement template (Figure 1B), introducing a selectable resistance marker that increases efficiency of knockout generation. This method can also be adapted to introduce gene modifications with properly designed homology arms. In this case, the integration of a new DNA fragment allows for PCR based screening (Figure 1C). Here, we use the generation of Pumilio RNA binding family member 2 (PUM2) knockout lines as an example.
Protocol
1. Identification of Homology Regions Around the Desired Deletion
NOTE: Only necessary if using selection-based editing.
- Select two regions, initially 1.5-2 kb, on either side of the desired deletion locus, which will serve as homology arms in the HDR template (Figure 1A). Identify regions that lack BsaI recognition sites on either strand (GGTCTC) to facilitate cloning. If BsaI sites are unavoidable, use an alternate type IIs restriction enzyme (BsmBI, SapI, BbsI) and modify the corresponding sites in the recipient plasmid (pUC19-BsaI), resistance marker donor plasmid (pGolden-Neo or pGolden-Hygro), and homology arm PCR product overhangs.
2. Generation of Cas9-sgRNA Expression Plasmids
- Define the targeting site(s) for mutagenesis. For the single-cut, selection-free method, target 5' proximal coding exons to increase the probability of a non-functional mutation. For the two-cut method, select two sites that span as much of the gene as necessary.
NOTE: In our experience, deletions up to 53 kb are efficiently created. - Input 200 to 300 bp of the targeted region sequence into the crispr.mit.edu design tool. For selection-based cloning, use 200-300 bp of the identified homology regions that are proximal to the deletion locus (Figure 1A). Select 2-3 of the highest-ranked sgRNAs per targeted region to account for differences in their activity, and to isolate independent clones that do not share the same potential off-target effects.
- To design duplexed oligonucleotides with appropriate overhangs for insertion into the expression plasmid, omit the ending PAM sequence (NGG) and append a 5'-CACC overhang followed by a G to the top strand oligo. For the bottom strand oligo, append a 5'-AAAC overhang to the reverse-complemented target sequence, followed by a 3'-C, as illustrated below:
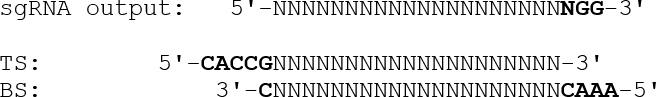
- Insert synthetic oligonucleotides corresponding to the sgRNAs into the pSpCas9(BB) plasmid using Golden Gate cloning28 as described in the Zhang lab protocol29.
3. Generation of Homology-directed Repair Template Plasmids
NOTE: Only necessary if using selection-based editing. The homology-directed repair template plasmid consists of a drug resistance cassette flanked by two regions that are complementary to the genome just outside the two sgRNA target sites (Figure 1B).
- From the broader homology regions identified in step 1.1, select 800-1,000 bp26 no more than 5-10 bp away from the designed Cas9 cut sites, to serve as the homology arms (Figure 1A). Be sure not to include the sgRNA target site and its PAM in the homology arms, as this will cause CRISPR/Cas9 cleavage of the template plasmid itself. If the homology arms do contain internal BsaI sites, use alternate sgRNA sites.
- PCR amplify homology arms from genomic DNA using a high-fidelity DNA polymerase, per manufacturer's instructions, using forward and reverse primers with additional 5' sequence that introduces BsaI sites (GGTCTC) and unique overhangs on either end of the homology region. The overhangs must contain proper sequences to generate correct assembly order and orientation, as described below (Figure 1B):
Left homology arm FP overhang: 5' GGGTCTCAGGCC
Left homology arm RP overhang: 5' GGGTCTCTCACG
Right homology arm FP overhang: 5' GGGTCTCAGTCC
Right homology arm RP overhang: 5' GGGTCTCTCCAC - Check homology arms for appropriate amplification via gel electrophoresis before proceeding forward.
- Assemble the right and left homology arm regions with the resistance cassette into an HDR template plasmid by Golden Gate cloning28. Use pGolden-Neo or pGolden-Hygro plasmids30 as the source of loxP-flanked resistance cassettes (loxP-PGK-Neo-pA-loxP or loxP-PGK-Hyg-pA-loxP). Use pUC19-BsaI, a modified pUC19 with BsaI sites in the multiple cloning region and eliminated BsaI sites elsewhere, as the recipient vector (available upon request). Use a ratio of 1:1:1:1 for the three inserts and vector.
- Prepare the following reaction mixture30:
Right homology arm PCR product 0.06 pmol (30-40ng) Left homology arm PCR product 0.06 pmol (30-40ng) pGolden-Neo plasmid (100 ng/µL) 1 µL pUC19-BsaI plasmid (100 ng/µL) 1 µL 2x T7 DNA ligase buffer 5 µL BsaI (10 U/µL) 0.75 µL T7 DNA ligase (3000 U/µL) 0.25 µL Water up to 10 µL - Use the following thermocycler parameters: 37 °C for 5 min, 20 °C for 5 min. Repeat for 30 cycles.
- Treat the cloning product with an exonuclease per manufacturer's instructions to digest away any remaining linearized DNA.
- Transform the reaction mixture into a competent E. coli strain according to the protocol supplied with the cells, and plate on ampicillin-containing dishes.
- Prepare the following reaction mixture30:
- To identify clones with the correct template assembly, perform bacterial colony PCR to amplify the homology arm subregions of the assembled insert (as the whole insert may be too large to amplify reliably). Use one primer annealing to the flanking plasmid sequence and a second primer complementary to the edge of the resistance cassette (Figure 1B, the sequence is common to both Neo and Hygro inserts), generating an assembly-dependent product that is approximately 200 bp longer than the contained homology arm:
pUC19-Bsal-Left: 5' GGCTCGTATGTTGTGTGGAATTGTGAG
Resistance-Left: 5' AAAAGCGCCTCCCCTACCC
pUC19-BsaI-Right: 5' GCTATTACGCCAGCTGGCGAAA
Resistance-Right: 5' AAGACAATAGCAGGCATGCTGGG- Pick bacterial colonies with sterile toothpicks and suspend the bacteria in 20 µL water. Heat at 95 °C for 15 min, and spin down in a tabletop centrifuge at maximal speed for 5 min. Place immediately on ice.
- Prepare the following PCR mixture31:
Dilute colony template 1.25 µL 10x Taq reaction buffer 1.25 µL 20mM dNTPs 0.25 µL 10 µM Forward primer 0.25 µL 10 µM Reverse primer 0.25 µL Taq Polymerase 0.25 µL Water 9 µL - Use the following thermocycler parameters: 95 °C for 30 s, (95 °C 30 s, 61 °C for 30 s, 72 °C for 1 min/kb), repeat for 25 cycles, 72 °C for 2 min.
- Check PCR product for correct amplicon size by agarose gel electrophoresis.
- Optionally, miniprep plasmid DNA from individual colonies with correct insert size and sequence the homology arm regions by Sanger sequencing with the amplification primers mentioned above.
4. Transfection of CRISPR Components into Cultured Cells
- Prior to transfection: culture T-REx293 cells in DMEM media supplemented with 10% FBS at 37 °C and 5% CO2.
- Plate cells onto 6-well plates and grow to approximately 70% confluency. Include a well for an untransfected control.
- Transfect cells with 2.5 µg total plasmid using a commercial transfection protocol. In parallel, maintain the untransfected control.
NOTE: Transfection method and efficiency vary depending on cell type. Determine the appropriate transfection method for the system prior to the experiment.- For transfection of cells with selection, use 0.75 µg Cas9-sgRNA 1 (left cut), 0.75 µg Cas9-sgRNA 2 (right cut), and 1.0 µg homologous recombination template.
- For transfection of cells without selection, use 2.5 µg Cas9-sgRNA plasmid.
5. Drug Selection
- 48 h after transfection, treat the cells with the appropriate drug (Neomycin/Hygromycin). Carry out selection until all cells in the untransfected control die (typically 3-5 days for Neo, 7-14 days for Hygro).
NOTE: Use concentrations of 500 µg/mL and 10 µg/mL for Neomycin and Hygromycin, respectively for T-REx293 cells. For other cell types, it may be useful to carry out a prior titration of drug on untransfected cells to determine the effective concentration.
6. Isolation of Clonal Populations
- Grow cells to 100% confluency in the original well after selection. Seed cells into 96 well plates at a density of 0.33 cells per well.
NOTE: Seeding three 96 well plates is a good starting point to ensure isolation of more than one correct clone, but more or less may be needed depending on sgRNA and HDR efficiencies. - Observe colonies over a 2-4 week period, until colonies are visible to the eye. Pick visible colonies with a sterile pipette tip and reseed in new wells to encourage monolayer growth. When using suspension cells, even lower seeding densities can be used to ensure a greater proportion of single-colony wells.
7. Screening Candidates
- Screening candidates without the use of selection: dot blot
- Grow individual clones to 50-100% confluency. Dislodge the monolayer of cells by pipetting within the well.
- Aliquot 90 µL of the 100 µL total volume to a clean microcentrifuge tube. Spin down at 6000 rpm for 5 min, remove media, and lyse cell pellet in 10 µL of 1x Lysis Buffer or 1x SDS loading buffer (5x: 250 mM Tris-Cl pH 6.8, 8% SDS, 0.1% bromophenol blue, 40% glycerol, 100 mM DTT). Add 90 µL of new media to the remainder of the cells in the well to continue propagating.
- Pipette 1 µL of cell lysate onto a dry nitrocellulose membrane to form a dot. Blot each sample twice on two separate membranes, creating two identical patterns of samples.
- Block the membranes in 5% milk in TBST (Tris Buffered Saline + 0.01% Tween 20: 8 g NaCl, 0.2g KCl, 3 g Tris base, up to 1 L distilled water, pH 7.4)31 for 1 h at room temperature (with rocking, here and throughout the procedure).
- Blot using the primary antibody against the target protein on one membrane, and the primary antibody for a control protein (tubulin, GAPDH, or any other protein that is not expected to change) on the other. Use the recommended primary antibody dilution (0.2 µg/mL for ELAVL1 and 0.1 µg/mL for Pum2 in this case) in TBST + 5% milk. Incubate 1 h at room temperature.
- Wash 3 times with TBST for 5 min.
- Incubate each membrane with appropriate HRP-conjugated secondary antibody in TBST + 5% milk for 1 h at room temperature.
- Wash 3 times with TBST for 5 min.
- Apply chemiluminescence substrate solution (see Materials Table) following the manufacturer's instructions and image the blotted membrane on a digital chemiluminescence imager.
- Quantify dot intensities for the target and control proteins using the appropriate software.
- Eliminate candidates with low control protein signal, and calculate background-subtracted ratios of target to control protein intensities for the remaining candidates. Select the candidates with the lowest ratios for passage and further validation by western blot.
- Screening candidates with the use of selection: colony PCR
- Collect cell lysate using a DNA prep kit (See Materials Table).
- Duplicate individual colonies in a new 96 well and grow until 100% confluency.
- Remove media from one set of the clones, and resuspend cells in 30 µL of extraction buffer from the kit. Transfer to a clean 1.5 mL microcentrifuge tube.
- Heat the solution to 96 °C for 15 min and let cool to room temperature.
- Add 30 µL of Stabilization buffer from the kit. Mix well.
- Identify wildtype and monoallelic/biallelic mutant lines by colony PCR.
- Design two separate sets of PCR primers (for the left and right side of the targeted locus) to amplify regions around the homology arms based on either successful or unsuccessful integration of the resistance cassette (Figure 2C). On each side, use a common primer (red) that anneals outside of the homology arm. Use it with a corresponding paired primer that is complementary to the endogenous sequence, spanning the homology region (blue), to test for the wild-type allele. Use another paired primer, complementary to the inserted resistance cassette (see primers in section 2.3), to test for the desired mutation.
- (Recommended) Validate the primer sets using cell lysate from the original selected bulk cell population, since it will contain a mixture of both WT and mutant alleles (Figure 2D).
- Prepare the following reaction mixture:
Cell lysate 0.5 µL 10x KOD buffer 1.25 µL 25mM MgSO4 0.75 µL 2 mM dNTPs 1.25 µL 10 µM Forward primer 0.375 µL 10 µM Reverse primer 0.375 µL KOD polymerase 0.25 µL Water 7.75 µL - Use the following thermocycler conditions: 95 °C for 2 min, (95 °C for 20 s, Primer Tm for 10 s, 70 °C for 20 s/kb) repeat for 25 cycles.
- Visualize the PCR product by agarose gel electrophoresis.
- Repeat the colony PCR testing for each side of the predicted integration. Select and expand candidates that show presence of integration and no endogenous sequence product for the deleted region.
- Validate positive candidates by western blot and/or RT-qPCR.
- Collect cell lysate using a DNA prep kit (See Materials Table).
8. Verify the Genomic Mutation by Sequencing
- Isolate genomic DNA from mutant cell lines by phenol chloroform extraction31.
- PCR amplify the sgRNA target region, using primers with 5' overhangs that add BsaI restriction sites to each end.
NOTE: For clones edited with HDR, where both alleles may be identical, the PCR product can be sequenced directly. - Clone the amplified region into pUC19-BsaI by Golden Gate cloning.
- Transform the reaction mixture into a competent E. coli strain.
- Miniprep plasmid DNA from 6-10 individual colonies and sequence the cloned region by Sanger sequencing.
Results
For the generation of ELAVL1 knockout lines, a robust antibody was available, so editing using single sgRNAs (Figure 1A, left) was performed, followed by dot immunoblot. Three sgRNAs were transfected independently to compare efficiencies and to rule out off target effects in the resulting clones. After collecting and blotting cell lysates from clonal populations onto two nitrocellulose membranes, the blots were probed for both ELAVL1 and PUM2 (as a normalizat...
Discussion
The CRISPR/Cas9 system has allowed for efficient generation of stable genomic modifications, which provide a more consistent alternative to other transient manipulation methods. Here, we have presented two methods for rapid identification of CRISPR/Cas9 gene knockouts in mammalian cell lines. Both methods require little cellular material, so testing can be done in early stages of clonal culture, saving time and reagents. To increase the efficiency of both methods, we recommend testing multiple sgRNAs, as efficiencies var...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
The authors would like to acknowledge Gissell Sanchez, Megan Lee, and Jason Estep for experimental assistance, and Weifeng Gu and Xuemei Chen for sharing reagents.
Materials
| Name | Company | Catalog Number | Comments |
| Competent E. coli cells | |||
| plasmid prep kit | |||
| pSpCas9(BB) plasmid | Addgene | 42230 | Ran et al. 2013, cloning sgRNAs |
| BbsI enzyme | ThermoFisher | FD1014 | Ran et al. 2013, cloning sgRNAs |
| T7 DNA ligase | NEB | M0318L | Ran et al. 2013, cloning sgRNAs |
| Tango Buffer | ThermoFisher | BY5 | Ran et al. 2013, cloning sgRNAs |
| PlasmidSafe exonuclease | Epicentre | E3105K | Ran et al. 2013, cloning sgRNAs |
| Q5 hot start high fidelity polymerase | NEB | M0494A | HA amplification |
| pUC19-BsaI | Modified pUC19 plasmid, mutated existing BsaI site and inserted two outward facing BsaI sites after BamHI/EcoRI digestion | ||
| pGolden-Neo | Addgene | 51422 | Resistance cassette |
| pGolden-Hygro | Addgene | 51423 | Resistance cassette |
| BsaI enzyme | NEB | R3535S | Homology arm contruction |
| T7 DNA ligase | NEB | M0318L | |
| PlasmidSafe exonuclease | Epicentre | E3105K | |
| Toothpicks | bacterial PCR | ||
| Taq polymerase | bacterial colony PCR | ||
| HEK293 human cells | |||
| DMEM | Corning | 10-013-CV | |
| FBS | Corning | MT35010CV | |
| Penicillin-Strep (opt.) | Gibco | 15140-122 | |
| 6 well plates | BioLite | 12556004 | |
| TransIT-LTI Transfection Reagent | Mirus | MIR2300 | for lipofection only |
| Opti-MEM | ThermoFisher | 31985062 | for lipofection only |
| G418 (Neomycin) | Sigma Aldrich | A1720-5G | |
| Hygromycin | Sigma Aldrich | H3274-250MG | |
| 96 well plates | ThermoFisher | 12556008 | |
| Passive Lysis Buffer, 5x | Promega | ||
| 1x SDS loading buffer | recipe decribed in protocol | ||
| Nitrocellulose Membrane | Bio-Rad | 162-0115 | |
| TBST | recipe decribed in protocol | ||
| Dehydrated milk | |||
| SuperSignal West Dura Extended Duration Substrate | ThermoFisher | 34075 | for HRP-conjugated secondary antibodies |
| Extracta DNA prep for PCR | Quantabio | 95091-025 | |
| KOD polymerase | Novagen | 71316 |
References
- Carroll, D. Genome engineering with zinc-finger nucleases. Genetics. 188 (4), 773-782 (2011).
- Kim, Y. G., Cha, J., Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 93 (3), 1156-1160 (1996).
- Bibikova, M., et al. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 21 (1), 289-297 (2001).
- Bibikova, M., Golic, M., Golic, K. G., Carroll, D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 161 (3), 1169-1175 (2002).
- Porteus, M. H., Cathomen, T., Weitzman, M. D., Baltimore, D. Efficient gene targeting mediated by adeno-associated virus and DNA double-strand breaks. Mol Cell Biol. 23 (10), 3558-3565 (2003).
- Bogdanove, A. J., Voytas, D. F. TAL effectors: customizable proteins for DNA targeting. Science. 333 (6051), 1843-1846 (2011).
- Miller, J. C., et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 29 (2), 143-148 (2011).
- Moscou, M. J., Bogdanove, A. J. A simple cipher governs DNA recognition by TAL effectors. Science. 326 (5959), 1501 (2009).
- Christian, M., et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 186 (2), 757-761 (2010).
- Jiang, W., Marraffini, L. A. CRISPR-Cas: New Tools for Genetic Manipulations from Bacterial Immunity Systems. Annu Rev Microbiol. 69, 209-228 (2015).
- Barrangou, R., et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 315 (5819), 1709-1712 (2007).
- Karginov, F. V., Hannon, G. J. The CRISPR system: small RNA-guided defense in bacteria and archaea. Mol Cell. 37 (1), 7-19 (2010).
- Wiedenheft, B., Sternberg, S. H., Doudna, J. A. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 482 (7385), 331-338 (2012).
- Mojica, F. J., Diez-Villasenor, C., Garcia-Martinez, J., Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 60 (2), 174-182 (2005).
- Pourcel, C., Salvignol, G., Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 151 (Pt 3), 653-663 (2005).
- Brouns, S. J., et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 321 (5891), 960-964 (2008).
- Deltcheva, E., et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 471 (7340), 602-607 (2011).
- Garneau, J. E., et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 468 (7320), 67-71 (2010).
- Marraffini, L. A., Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 322 (5909), 1843-1845 (2008).
- Gasiunas, G., Barrangou, R., Horvath, P., Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 109 (39), E2579-E2586 (2012).
- Jinek, M., et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337 (6096), 816-821 (2012).
- Davis, A. J., Chen, D. J. DNA double strand break repair via non-homologous end-joining. Transl Cancer Res. 2 (3), 130-143 (2013).
- Guirouilh-Barbat, J., et al. Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol Cell. 14 (5), 611-623 (2004).
- Moore, J. K., Haber, J. E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 16 (5), 2164-2173 (1996).
- Liang, F., Han, M., Romanienko, P. J., Jasin, M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci U S A. 95 (9), 5172-5177 (1998).
- Gratz, S. J., et al. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics. 196 (4), 961-971 (2014).
- Cong, L., et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 339 (6121), 819-823 (2013).
- Engler, C., Kandzia, R., Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 3 (11), e3647 (2008).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 8 (11), 2281-2308 (2013).
- Luo, Y., Lin, L., Bolund, L., Sorensen, C. B. Efficient construction of rAAV-based gene targeting vectors by Golden Gate cloning. Biotechniques. 56 (5), 263-268 (2014).
- Green, M. R., Sambrook, J., Sambrook, J. . Molecular cloning: a laboratory manual. , (2012).
- Lin, S., Staahl, B. T., Alla, R. K., Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 3, e04766 (2014).
- Kim, S., Kim, D., Cho, S. W., Kim, J., Kim, J. S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24 (6), 1012-1019 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved