A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
SA-β-Galactosidase-Based Screening Assay for the Identification of Senotherapeutic Drugs
* These authors contributed equally
In This Article
Summary
Cellular senescence is the key factor in the development of chronic age-related pathologies. Identification of therapeutics that target senescent cells show promise for extending healthy aging. Here, we present a novel assay to screen for the identification of senotherapeutics based on measurement of senescence associated β-Galactosidase activity in single cells.
Abstract
Cell senescence is one of the hallmarks of aging known to negatively influence a healthy lifespan. Drugs able to kill senescent cells specifically in cell culture, termed senolytics, can reduce the senescent cell burden in vivo and extend healthspan. Multiple classes of senolytics have been identified to date including HSP90 inhibitors, Bcl-2 family inhibitors, piperlongumine, a FOXO4 inhibitory peptide and the combination of Dasatinib/Quercetin. Detection of SA-β-Gal at an increased lysosomal pH is one of the best characterized markers for the detection of senescent cells. Live cell measurements of senescence-associated β-galactosidase (SA-β-Gal) activity using the fluorescent substrate C12FDG in combination with the determination of the total cell number using a DNA intercalating Hoechst dye opens the possibility to screen for senotherapeutic drugs that either reduce overall SA-β-Gal activity by killing of senescent cells (senolytics) or by suppressing SA-β-Gal and other phenotypes of senescent cells (senomorphics). Use of a high content fluorescent image acquisition and analysis platform allows for the rapid, high throughput screening of drug libraries for effects on SA-β-Gal, cell morphology and cell number.
Introduction
Cellular senescence was described for the first time by Leonard Hayflick and Paul Moorhead, who showed that normal cells had a limited ability to proliferate in culture1. Senescent cells fail to proliferate despite the presence of nutrients, growth factors and lack of contact inhibition, but remain metabolically active2. This phenomenon is known as replicative senescence and was mainly attributed to the telomere shortening, at least in human cells3. Further studies have shown that cells can also be induced to undergo senescence in response to other stimuli, such as oncogenic stress (oncogene induced senescence, OIS), DNA damage, cytotoxic drugs, or irradiation (stress induced senescence, SIS)4,5,6. In response to DNA damage, including telomere erosion, cells either senesce, start uncontrolled cell growth, or undergo apoptosis if the damage cannot be repaired. In this case, cell senescence seems to be beneficial as it acts in a tumor suppressive manner2. In contrast, senescence is increased with aging due to the accumulation of cellular damage including DNA damage. Since senescent cells can secrete cytokines, metalloproteinases and growth factors, termed the senescence-associated secretory phenotype (SASP), this age-dependent increase in cellular senescence and SASP contributes to decreased tissue homeostasis and subsequently aging. Also, this age-dependent increase in the senescence burden is known to induce metabolic diseases, stress sensitivity, progeria syndromes, and impaired healing7,8 and is, in part, responsible for the numerous age-related diseases, such as atherosclerosis, osteoarthritis, muscular degeneration, ulcer formation, and Alzheimer's disease9,10,11,12,13. Eliminating senescent cells can help to prevent or delay tissue dysfunction and extend healthspan14. This has been shown in transgenic mouse models14,15,16 as well as by using senolytic drugs and drug combinations that were discovered through both drug screening efforts and bioinformatic analysis of pathways induced specifically in senescent cells17,18,19,20,21,22. Identifying more optimal senotherapeutic drugs, able to more effectively reduce the senescent cell burden, is an important next step in the development of therapeutic approaches for healthy aging.
Senescent cells show characteristic phenotypic and molecular features, both in culture and in vivo. These senescence markers could be either the cause or the result of senescence induction or a byproduct of molecular changes in these cells. However, no single marker is found specifically in senescent cells. Currently, senescence-associated β-galactosidase (SA-β-Gal) detection is one of the best-characterized and established single-cell based methods to measure senescence in vitro and in vivo. SA-β-Gal is a lysosomal hydrolase with an optimal enzymatic activity at pH 4. Measuring its activity at pH 6 is possible because senescent cells show increased lysosomal activity23,24. For living cells, increased lysosomal pH is obtained by lysosomal alkalinization with the vacuolar H+-ATPase inhibitor Bafilomycin A1 or the endosomal acidification inhibitor chloroquine25,26. 5-Dodecanoylaminofluorescein Di-β-D-galactopyranoside (C12FDG) is used as substrate in living cells as it retains the cleaved product in the cells due to its 12 carbon lipophilic moiety25. Importantly, SA-β-Gal activity itself is not directly connected with any pathway identified in senescent cells and is not necessary to induce senescence. With this assay, senescent cells can be identified even in the heterogeneous cell populations and aging tissues, such as skin biopsies from older individuals. It also has been used to show a correlation between cell senescence and aging23 as it is a reliable marker for senescent cell detection in several organisms and conditions27,28,29,30. Here, a high throughput SA-β-Gal screening assay based on the fluorescent substrate C12FDG using primary mouse embryonic fibroblasts (MEFs) with robustly oxidative stress induced cell senescence is described and its advantages and disadvantages are discussed. Although this assay can be performed with different cell types, the use of Ercc1-deficient, DNA repair impaired MEFs allows for more rapid induction of senescence under conditions of oxidative stress. In mice, reduced expression of the DNA repair endonuclease ERCC1-XPF causes impaired DNA repair, accelerated accumulation of endogenous DNA damage, elevated ROS, mitochondrial dysfunction, increased senescent cell burden, loss of stem cell function and premature aging, similar to natural aging31,32. Similarly, Ercc1-deficient MEFs undergo senescence more rapidly in culture17. An important feature of the senescent MEF assay is that each well has a mixture of senescent and non-senescent cells, allowing for the clear demonstration of senescent cell-specific effects. However, although we believe that the use of oxidative stress in primary cells to induce senescence is more physiologic, this assay also can be used with cell lines where senescence is induced with DNA damaging agents like etoposide or irradiation.
Protocol
Animal use was approved by the Scripps Florida Institutional Animal Care and Use Committee.
1. Generation of senescent murine embryonic fibroblast (MEF) – 12-15 days
- Isolate wild type and Ercc1-/- MEFs from pregnant female mice at embryonic day 13 (E13) as described previously33.
NOTE: All following steps are carried out in a tissue culture hood under aseptic conditions and using sterile instruments. - Resect the embryo head above the eyes.
- Remove the red tissue (heart and liver) and use them for genotyping if necessary.
- Prepare 500 mL of a 1:1 mixture of Dulbecco’s Modified Eagle’s Medium (DMEM) and Ham’s F10 with 10% fetal bovine serum, 1x nonessential amino acids, penicillin, and streptomycin as growth medium and warm it up to 37 °C for around 15 min before each use. Store growth medium at 4 °C.
- Incubate the rest of the embryo with 0.25% trypsin/EDTA for 10 min.
- Mince the embryo into 1 mm pieces and pipette the tissue up and down several times.
- Add 10 mL of growth medium and plate tissues of one embryo per 10 cm diameter cell culture plate (passage 0).
- Cultivate cells at 37 °C, 3% O2, 5% CO2.
NOTE: Only MEF cells attach to non-coated tissue culture plates under these conditions. - Change medium every day in passage 0 to remove non-attached tissue and cell fragments.
NOTE: Depending on the size of the embryo and the quality of the isolation, cell usually reach confluency after 2 to 3 days. - Trypsinization
- Carefully remove the growth medium and wash cells with 10 mL of 1x PBS two times.
- Add 2 mL of a 0.025% trypsin/EDTA solution to cells in 10 cm diameter plates and incubate at 37 °C for 2-3 min.
- Make sure that cells are detached from the surface by inspecting the cells under the microscope.
- Terminate trypsin digestion by adding the same amount of growth medium.
- Transfer the cells to a conical tube and centrifuge cells at 200 x g for 3 min and discard the supernatant.
- Resuspend cells carefully in fresh growth medium, count cells and seed them in new plates at the projected cell density.
- For non-senescent sub cultivation split confluent cells 1:4 and extend for another passage at 3% O2, 37 °C to yield more cells (passage 1).
NOTE: At this point the cells can either be maintained in culture or stored for later use in liquid nitrogen, in cryovials containing approximately 1 million cells each. This step also offers the possibility to generate a mixed batch of cells from different animals to reduce variability coming from single animal analysis. - To induce cell senescence, seed split confluent cells from passage 1 at a ratio of 1:4 and incubate them at 20% O2, 37 °C, 5% CO2 for 3 days; these culture conditions are atmospheric for oxygen.
NOTE: Cultivation of cells and blastocysts under ambient oxygen concentrations can elevate markers of cellular senescence specifically when DNA damage repair is impaired34,35,36. - Repeat this procedure for 2 more passages.
- To monitor cellular senescence, measure the gradual increase in cell diameter and cell volume during each trypsinization step using an advanced Coulter cell counter system.
- Assess the reduction in cell proliferation by determination of population doubling (PD) using the equation
PDT = (t2-t1)/3.32 x (log n2 – log n1)
NOTE: Population doubling time was only used for non-senescent cells. - Use early passage wild-type or Ercc1-/- MEF cells that were kept at 3% O2, 37 °C, 5% CO2 as non-senescent control cells.
2. Senescent associated β-Gal screening assay – 2-3 days
- Prepare 10 mM stock solutions in DMSO of all drugs to be tested and store aliquots at -80 °C. Do not freeze-thaw stock solutions as this may decrease the activity of drugs.
NOTE: Here, the HSP90 inhibitor 17DMAG was used as a senolytic drug capable of specifically killing senescent cells17. - On the day of the experiment thaw the aliquot, dilute the drugs in fresh culture medium and add to the cells containing conditioned medium at a 1:1 ratio to yield the final concentration in growth medium.
- Use 96-well pre-dilution plates for serial dilutions and drug combinations.
NOTE: For MEF cells, it was empirically determined that DMSO concentrations should not exceed 2% and control cells treated with highest DMSO concentrations used should be included in each run. - Seed 5 x 103 senescent cells or 3 x 103 non-senescent cells per well in 96 well plates at least 6 h prior to treatment in 100 µL of growth medium and incubate at 20% O2, 37 °C, 5% CO2.
NOTE: Cells should be about 80% confluent before treatment. - Use black wall/clear bottom tissue culture, treated 96 well plates to minimize fluorescent signal crosstalk and background.
NOTE: However, clear plates have also been tested successfully. - Add drug dilutions to MEF cells and incubate for 24 h to 48 h under 20% O2, 37 °C, 5% CO2 conditions.
- Keep non-senescent cells under 3% O2, 37 °C, 5% CO2 conditions.
- For lysosomal alkalinization, prepare a 10 mM bafilomycin A1 solution, aliquot and keep frozen at -20 °C.
- For fluorescence analysis of SA-β-Gal activity, prepare a 2 mM C12FDG stock solution, store at -20 °C, and protect from light.
- For the working solution, prepare 100 µM C12FDG in growth medium on the day of experiment.
NOTE: All (incubation) steps involving C12FDG should be performed in the dark. - Remove the drug solution and wash cells 1 time with 100 µL of 1x PBS.
- Induce lysosomal alkalinization by pretreating cells with 90 µL of a 100 nM bafilomycin A1 solution prepared in fresh cell culture medium for 1 hour at 20% O2, 37 °C, 5% CO2.
- Add 10 µL of 100 µM C12FDG working solution to the culture medium (final concentration 10 µM).
- Incubate cells for 2 h.
- Add 2 µL of a 100 µg/mL Hoechst 33342 dye (final concentration 2 µg/mL) to the culture and incubate for 20 min.
- Remove media and add 100 µL of fresh growth medium.
3. Quantitative high content fluorescent image analysis
- Use a high content fluorescent image acquisition and analysis platform to acquire fluorescent images of the cells in the two channels appropriate for the capture of Hoechst and C12FDG fluorescence (e.g., DAPI and FITC channel presets, respectively).
NOTE: Acquisition protocols require the definition of several variables that are specific to the assay. The purpose of an acquisition protocol is to capture an adequate number of in-focus fluorescent images of sufficient numbers of cells for downstream quantitative analysis. - Develop an appropriate analysis protocol by selecting each channel and defining, by adjusting one or more selective criteria, what qualifies as a feature of interest in each channel.
- For the nucleus, use so segmentation pre-sets (e.g., nuclear segmentation, vesicle segmentation, cytoplasm segmentation) that will allow the identification of cellular organelles on the basis of multiple criteria including morphology, size, and signal intensity. Adjust these criteria to include nuclei while excluding nuclear fragments and debris which may have a signal, but which are, for example, too large or too small to be nuclei.
- Check the signal in the FITC channel which is fluorescence from cleaved C12FDG and represents the amount of senescence-associated β-galactosidase activity in the cells.
NOTE: Senescent cells have a higher senescence-associated β-galactosidase activity than non-senescent cells; however, C12FDG fluorescence will be non-discrete and continuous, necessitating the establishment of a threshold between what is considered a C12FDG-positive and a C12FDG-negative cell. - Using commercially available analytical software, generate a count of instances in which a defined region surrounding the nucleus (a presumptive cell) has overlapped at least once with an above-threshold C12FDG.
NOTE: The analytical software automatically generates a count using target linking. This is the assay’s practical definition of a senescent, C12FDG-positive cell.
- Analyze all samples in triplicate with 3-5 fields per well and mean values and standard deviations being calculated accordingly.
- Calculate the percentage of senescent cells using the following formula:
Senescent cells (%) =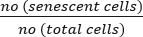 x 100
x 100
4. Assay validation parameters
- For all samples intra- and inter-assay coefficient of variations (%CVs) were calculated using the following formula:
Intra-assay CV (n = 10 repeats measured in one experiment) = x 100 (%)
x 100 (%)
Inter-assay CV (n = 5 independent experiments) = x 100 (%)
x 100 (%) - For screening purposes, determine the Z’ value, a statistical parameter to evaluate the quality of an assay, from cells treated with 200 nM rapamycin for 24 h at 20% O2, 37 °C, 5% CO2 (a positive control for senotherapeutic drugs) and untreated senescent cells (negative control).
NOTE: The Z’ value was calculated according to Zhang et al.37. Z’ values between 0.5 and 1 indicate that an assay can be used for drug screening.
Results
SA-β-Gal activity can be detected in cells that are induced to senesce by various ways from replicative exhaustion, genotoxic and oxidative stress, to oncogene activation23,25,38. In the current model using Ercc1-deficient mouse embryonic fibroblast cells, normoxic growth conditions (20% O2) were sufficient to induce cell senescence after cultivating them for a few passages. ...
Discussion
SA-β-Gal is a well-defined biomarker for cellular senescence originally discovered by Dimri et al. (1995) showing that senescent human fibroblasts have increased activity of SA-β-Gal when assayed at pH 623 compared to proliferating cells. Meanwhile, in vitro and in vivo assay for SA-β-Gal have been established for different cell types and tissues25,39,40. The fluorescence base...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH Grants AG043376 (Project 2 and Core A, PDR; Project 1 and Core B, LJN) and AG056278 (Project 3 and Core A, PDR; and Project 2, LJN) and a grant from the Glenn Foundation (LJN).
Materials
| Name | Company | Catalog Number | Comments |
| DMEM | Corning | 10-013-CV | medium |
| Ham's F10 | Gibco | 12390-035 | medium |
| fetal bovine serum | Tissue Culture Biologics | 101 | serum |
| 1x non-essential amino acids | Corning | 25-025-Cl | amino-acids |
| bafilomycin A1 | Sigma | B1793 | lysosomal inhibitor |
| C12FDG | Setareh Biotech | 7188 | b-Gal substrate |
| Hoechst 33342 | Life Technologies | H1399 | DNA intercalation agent |
| 17DMAG | Selleck Chemical LLC | 50843 | HSP90 inhibitor |
| InCell6000 Cell Imaging System | GE Healthcare | High Content Imaging System |
References
- Hayflick, L., Moorhead, P. S. The serial cultivation of human diploid cell strains. Experimental Cell Research. 25, 585-621 (1961).
- Campisi, J., di Fagagna, F. D. Cellular senescence: when bad things happen to good cells. Nature Reviews Molecular Cell Biology. 8 (9), 729-740 (2007).
- Harley, C. B., Futcher, A. B., Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 345 (6274), 458-460 (1990).
- Zhu, J., Woods, D., McMahon, M., Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes and Development. 12 (19), 2997-3007 (1998).
- Dimri, G. P., Itahana, K., Acosta, M., Campisi, J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Molecular and Cellular Biology. 20 (1), 273-285 (2000).
- Michaloglou, C., et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 436 (7051), 720-724 (2005).
- Niedernhofer, L. J. Tissue-specific accelerated aging in nucleotide excision repair deficiency. Mechanisms of Ageing and Development. 129 (78), 408-415 (2008).
- Gitenay, D., et al. Glucose metabolism and hexosamine pathway regulate oncogene-induced senescence. Cell Death & Disease. 5, e1089 (2014).
- Erusalimsky, J. D., Kurz, D. J. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Experimental Gerontology. 40 (8-9), 634-642 (2005).
- Kassem, M., Marie, P. J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 10 (2), 191-197 (2011).
- Campisi, J., Andersen, J. K., Kapahi, P., Melov, S. Cellular senescence: A link between cancer and age-related degenerative disease. Seminars in Cancer Biology. 21 (6), 354-359 (2011).
- Golde, T. E., Miller, V. M. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer's and other neurodegenerative diseases. Alzheimers Research & Therapy. 1 (2), 5 (2009).
- Telgenhoff, D., Shroot, B. Cellular senescence mechanisms in chronic wound healing. Cell Death & Differentiation. 12 (7), 695-698 (2005).
- Baker, D. J., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 479 (7372), 232-236 (2011).
- Baker, D. J., et al. Naturally occurring p16-positive cells shorten healthy lifespan. Nature. , (2016).
- Childs, B. G., et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 354 (6311), 472-477 (2016).
- Fuhrmann-Stroissnigg, H., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nature Communications. 8 (1), 422 (2017).
- Zhu, Y., et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 9 (3), 955-963 (2017).
- Zhu, Y., et al. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell. , (2015).
- Zhu, Y., et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. , (2015).
- Baar, M. P., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 169 (1), 132-147 (2017).
- Jeon, O. H., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature. , (2017).
- Dimri, G. P., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences USA. 92 (20), 9363-9367 (1995).
- Itahana, K., Campisi, J., Dimri, G. P. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods in Molecular Biology. 371, 21-31 (2007).
- Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J., Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-[beta]gal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols. 4 (12), 1798-1806 (2009).
- Cahu, J., Sola, B. A sensitive method to quantify senescent cancer cells. Journal of Visualized Experiments. 78 (78), (2013).
- Collado, M., et al. Tumour biology: Senescence in premalignant tumours. Nature. 436 (7051), 642 (2005).
- Krishnamurthy, J., et al. Ink4a/Arf expression is a biomarker of aging. The Journal of Clinical Investigation. 114 (9), 1299-1307 (2004).
- Mishima, K., et al. Senescence-associated beta-galactosidase histochemistry for the primate eye. Investigative Ophthalmology, Visual Science. 40 (7), 1590-1593 (1999).
- Melk, A., et al. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney International. 65 (2), 510-520 (2004).
- Niedernhofer, L. J., et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 444 (7122), 1038-1043 (2006).
- Wang, J., Clauson, C. L., Robbins, P. D., Niedernhofer, L. J., Wang, Y. The oxidative DNA lesions 8,5′-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell. 11 (4), 714-716 (2012).
- Jozefczuk, J., Drews, K., Adjaye, J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. Journal of Visualized Experiments. (64), (2012).
- Jagannathan, L., Cuddapah, S., Costa, M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Current Pharmacology Reports. 2 (2), 64-72 (2016).
- Meuter, A., et al. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J Assist Reprod Genet. 31 (10), 1259-1267 (2014).
- Robinson, A. R., et al. Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biology. 17, 259-273 (2018).
- Zhang, J. H., Chung, T. D., Oldenburg, K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal Of Biomolecular Screening. 4 (2), 67-73 (1999).
- Zhao, H., Darzynkiewicz, Z. Biomarkers of cell senescence assessed by imaging cytometry. Methods in Molecular Biology. 965, 83-92 (2013).
- Yang, N. -. C., Hu, M. -. L. A fluorimetric method using fluorescein di-β-d-galactopyranoside for quantifying the senescence-associated β-galactosidase activity in human foreskin fibroblast Hs68 cells. Analytical Biochemistry. 325 (2), 337-343 (2004).
- Zhao, J., et al. Quantitative Analysis of Cellular Senescence in Culture and In Vivo. Current Protocols in Cytometry. 79, (2017).
- Capparelli, C., et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, "fueling" tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 11 (19), 3599-3610 (2012).
- Coppe, J. P., Desprez, P. Y., Krtolica, A., Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Pathology. 5, 99-118 (2010).
- Mah, L. J., El-Osta, A., Karagiannis, T. C. GammaH2AX as a molecular marker of aging and disease. Epigenetics. 5 (2), 129-136 (2010).
- Hewitt, G., et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nature Communications. 3, 708 (2012).
- Narita, M., et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 113 (6), 703-716 (2003).
- Georgakopoulou, E. A., et al. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging-Us. 5 (1), 37-50 (2013).
- Severino, J., Allen, R. G., Balin, S., Balin, A., Cristofalo, V. J. Is β-Galactosidase Staining a Marker of Senescence in Vitro and in Vivo?. Experimental Cell Research. 257 (1), 162-171 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved