A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Potentiation of Anticancer Antibody Efficacy by Antineoplastic Drugs: Detection of Antibody-drug Synergism Using the Combination Index Equation
* These authors contributed equally
In This Article
Summary
This protocol describes how to assess synergism between an anticancer antibody and antineoplastic drugs in preclinical models by using the combination index equation of Chou and Talalay.
Abstract
Potentiation of hostile monoclonal antibodies (mAb) by chemotherapeutic agents constitutes a valuable strategy for designing effective and safer therapy against cancer. Here we provide a protocol to identify a rational combination at the preclinical step. First, we describe a cell-based assay to assess the synergism between anticancer mAb and cytotoxic drugs, that uses the combination index equation of Chou and Talalay1. This includes the measurement of tumor cell drug- and antibody-sensitivity using an MTT assay, followed by an automated computer analysis to calculate the combination index (CI) values. CI values of <1 indicate synergism between tested mAbs and cytotoxic agents1. To corroborate the in vitro findings in vivo, we further describe a method to assess the combination regimen efficacy in a xenograft tumor model. In this model, the combined regimen significantly delays tumor growth, which results in a significant extended survival in comparison to single-agent controls. Importantly, the in vivo experimentation reveals that the combination regimen is well tolerated. This protocol allows the effective evaluation of anticancer drug combinations in preclinical models and the identification of rational combination to evaluate in clinical trials.
Introduction
The conventional approach in the treatment of a large number of different types of cancer was based on monotherapy. Even if it is still used in many cases, this method met several obstacles leading to opting for combined therapies2. Particularly, cancer cells are more susceptible to develop resistance when treated with a single drug by inducing alternative survival mechanisms3, resulting in therapeutic failure in patients4. Moreover, in monotherapy, drugs are usually administrated at a high dose. This situation often results in the occurrence of strong dose-dependent side effects that can be intolerable and force physicians to stop the treatment2. For these reasons, the association of anticancer molecules is now preferred to monotherapy.
Ideal drug combinations would be those that act in synergy against tumor cells, without increased toxicity against normal cells. Synergism refers to the interaction of two or more drugs that produces a therapeutic effect greater than the sum of each individual drug acting separately. Such interactions may result in enhanced clinical therapeutic efficacy2. It limits treatment resistance, increases efficacy, and can also reduce toxicity2. In fact, the dosage of each drug can be reduced to lower their side effects by targeting different pathways. In addition, one of the molecules can also serve as a sensitizing agent against cancer cells. The effect of the second drug may be enhanced on sensitized cells and fewer dosages can be used5.
Combined therapy can include two or more chemotherapeutic drugs and/or biologics, such as monoclonal antibodies6. These mAbs specifically target cells expressing a cell surface antigen of interest and are able to kill tumor cells through immunological pathways including antibody-dependent cell-mediated cytotoxicity (ADCC), with the involvement of immune effector cells7, and complement-dependent cytotoxicity (CDC)6. They can also act via a non-immunological mechanism mediated by apoptosis8,9,10,11. In this case, the induction of the process of programmed cell death may sensitize cancer cells, weaken their function, and make the associated chemotherapeutic drug more effective at a lower dosage. As such, proapoptotic mAb are good candidates for designing combination regimens with antineoplastic drugs.
Different mathematical models have been described to assess drug synergism; one of them is based on the combination index method1. This method is based on the median-effect principle developed by Chou1. The median-effect equation correlates the drug dose and drug effect as follows.
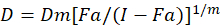
Here, D is the drug dose; Dm is the median-effect dose; Fa is the fraction affected by the dose; m is an exponent that signifies the shape of the dose-effect plot1. The median-effect dose is used to calculate the dose Dx of a drug that inhibits or kills "x" percent of cells. The CI value is then calculated to assess the additive effect of the drug combination, as follows1.
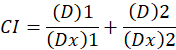
A CI value of 1 indicates an additive effect and a CI value of <1 indicates a synergistic effect, while a CI value of >1 indicates antagonism1. The application of this method is further facilitated by the availability of a computer program, CompuSyn, that determines synergism and antagonism at all doses or effect levels simulated automatically12.
Our group has developed the mAb 8B6 specific for O-acetyl-GD2 ganglioside (OAcGD2) neuroblastoma antigen13 and further demonstrated that this mAb is able to induce cell death with attributes of apoptosis11. To test whether mAb 8B6 can sensitize neuroblastoma cells to the antineoplastic agent topotecan, we adapted the above-mentioned method developed by Chou1. First, we determine the effective dose 50 (ED50) values of mAb 8B6 and topotecan. Next, the neuroblastoma cells with equipotent ratios of the two compounds based on ED50 values are exposed to determine the CI values using the above-mentioned simulation software. This method allows us to demonstrate synergism between mAb 8B6 and topotecan in vitro. Next, we describe a protocol to further assess the potency and the safety of this combination regimen in vivo. This protocol can be easily applied to select potent and safe anticancer mAb and chemotherapeutic agent combinations in preclinical studies. A schematic representation of this study is provided in Figure 1.
Protocol
Animal housing and experimental procedure were approved by the French Government (agreements #C44-278 and #APAFIS 03479.01). Animal care and procedures were conducted under directive EU 2010/63/EU and French Law #2013-118 on the protection of animals used for scientific purposes.
1. Evaluation of the Drug Interaction Between mAb 8B6 and Topotecan In Vitro
- 96-well sample preparation
CAUTION: Consult the institution's Health and Safety committee and follow local regulation rules related to laboratory safety. Review the Material and Safety Data Sheet information before working with any media, cell lines, or reagents. Use proper sterile technique and work in a laminar flow hood. All solutions/equipment that are used to manipulate cells must be sterile.
NOTE: The following protocol was designed for use with adherent cells. Modifications are required to apply the method to nonadherent cells growing in suspension; this protocol uses quadruplicate for each experimental condition.- Grow IMR5 cells in a T75 flask.
- On the first day (day 0), observe the cell culture under a microscope to check the cell confluency. Aspirate the cell medium from the flask, wash it with 5 mL of phosphate-buffered saline (PBS), and add 3 mL of 0.05% ethylenediaminetetraacetic acid (EDTA)/PBS solution. Return the flask to the incubator for 3 min (37 °C, 5% CO2).
- Examine the cell culture under a microscope for cell detachment.
NOTE: If necessary, return the flask to the incubator for an additional 3 to 5 min, depending on tumor cell type. - Add 10 mL of complete cell medium to the flask and transfer the cell suspension to a sterile 15 mL conical tube. Centrifuge the cells for 5 min at 300 x g. Count the cells using a hemocytometer.
- Remove and discard the supernatant. Resuspend the cell pellet in complete growth medium. Adjust the medium volume to obtain a final concentration of 1 x 105 cells/mL.
- Seed 84 wells of a 96-well culture plate with 104 cells each, which is 100 µL of cell suspension. Follow the experimental layout shown in Figure 2.
- Incubate the cells for 18 h in the cell incubator (37 °C, 5% CO2).
- Drug solution preparation
NOTE: For drug/mAb sensitization studies, modify the timing, the length, and the concentration treatment to suit the particular drug/mAb in question. Note that the initial concentration is 3x the final concentration.- The next morning (day 1), prepare the following drug solutions using complete growth medium.
- mAb solution preparation
- Dilute mAb in 500 µL of complete growth medium to obtain an antibody working solution with an mAb concentration of 240 µg/mL.
- Perform five two-fold serial dilutions as indicated in Figure 2.
- Topotecan solution preparation
- Dilute, as above, the drug in 500 µL of complete growth medium to obtain a drug working solution with a final concentration of 120 nM.
- Perform five two-fold serial dilutions as indicated in Figure 2.
- Antibody and drug solution preparation
- Dilute the drug and mAb solutions in 500 µL of complete growth medium to obtain a solution at 120 nM drug and 240 µg/mL mAb (working solution).
- Perform five two-fold serial dilutions as indicated in Figure 2.
- mAb solution preparation
- To arrive at the final concentration, transfer 50 µL of each drug solution into the corresponding wells, as indicated in the experimental layout ( Figure 2).
NOTE: Transfer 50 µL of complete growth medium into the untreated cell wells, as indicated in Figure 2. - Incubate the cells for 72 h in the incubator (37 °C, 5% CO2).
- The next morning (day 1), prepare the following drug solutions using complete growth medium.
- MTT assay
- Add 10 µL of MTT reagent solution into each well.
- Incubate at 37 °C for 4 h.
- Add 100 µL of lysis solution (10% SDS in 0.01 M HCl) into each well, using a multichannel pipette, and mix thoroughly by pipetting.
- Incubate at 37 °C for 4 h in a humidified chamber (95% humidity).
- Read the absorbance at 570 nm (A570) and 620 nm (A620) using a spectrophotometer.
NOTE: Mix each sample again by pipetting before reading the absorbance; absorbance at 620 nm allows the correction of nonspecific background values. - Calculate the corrected absorbance: corrected absorbance = A570- A620.
- Calculate the cell viability as follows: cell viability = 100 x (sample mean corrected absorbance / control mean corrected absorbance).
- Calculate the fraction-affected values (Fa) using the following equation: 1 - (sample mean corrected absorbance / control mean corrected absorbance).
- Drug interaction analytical simulation software for single and drug combination studies
- Run the simulation software to open the start window.
- Click on the New Experiment button to open the Main window.
- Type the name of the experiment in the Name window.
NOTE: A date can be added in the Date window. - Click on the New Single Drug button.
- Type the name in the Full Name window.
- Type the abbreviation in the Abbrev window.
- Type the drug concentration unit in the Units window.
- Enter Data Point 1 Dose and Fa value, press Enter.
- Repeat this step until all Data Points are entered.
- Click on the Finished button.
- Follow the same steps to enter mAb Data Points.
NOTE: Use the same concentration unit as is used by Drug. - Click on the New Drug Combo button.
- Select Drug and mAb.
- Select Constant Ratio and click on OK.
- Type the name in the Full Name window.
- Type the abbreviation in the Abbrev window.
- Type the drug/mAb ratio in the Ratio of window.
- Enter Data Point 1 Dose and press Enter.
NOTE: The program will automatically calculate the doses of mAb and Combo. - Enter the Data Point 1 Fa value and press Enter.
- Repeat this step until all Data Points are entered.
- Click on the Finished button and, then, click on the Generate Report button.
- Select drug and mAb and, then, click OK.
- Select Combo and, then, click OK.
- Select Header, CI table, and Summary table. Then, click OK.
- Type the file name of the analysis file and click SAVE to generate the report.
NOTE: After clicking OK, the report will automatically open in the computer's default web browser. - To print the report, choose Print from the web browser's file menu. The report contains a Summary Table Section that includes title, date, file name, description note, parameters (m, Dm, and r), ED50 for either agent used in monotherapy or in combination, and the CI table for each combination at ED50, ED75, ED90, and ED95.
NOTE: A CI value of <1 indicates synergism, a CI value of =1 indicates additivity, and a CI value of >1 indicates antagonism.
2. Generation of Human Neuroblastoma Xenografts in Nonobese Diabetic NOD Scid Gamma Mice (NSG Mice)
NOTE: Exclude any contamination of the cell culture. Since the basement membrane matrix forms a gel above 5 °C, all cultureware or media coming in contact with the basement membrane matrix reagent should be prechilled/ice-cold. Keep the basement membrane matrix on ice during the entire process.
- Preparation of the IMR5 cell suspension
- Thaw the basement membrane matrix reagent overnight by submerging the vial in ice in a 4 °C refrigerator before use.
- On day 0, harvest the cultured IMR5 cells as detailed above.
- Transfer the cells to a 15 mL conical tube and centrifuge at 300 x g for 5 min.
- Discard the supernatant. Wash the cells 2x with 15 mL of ice-cold PBS, and prepare a cell suspension of 5 x 107 cells/mL in ice-cold PBS.
NOTE: If necessary, transfer the cell suspension to a 1.5 mL microcentrifuge tube. - Swirl the basement membrane matrix vial.
NOTE: The basement membrane matrix reagent should be thawed and dispersed. - Add one volume of basement membrane matrix reagent and mix it by pipetting to obtain a cell suspension of 2.5 x 107 cells /mL.
- Keep the cell suspension on ice.
- Preparation of the mice
NOTE: The mice should be six to seven weeks old.- Maintain mice under a specific pathogen-free condition.
- Allow a three- to five-day acclimatization period after the mice have arrived.
- On the day of inoculation, shave the flank where the injection will be done (see step 2.3.6).
- Preparation of the tumor cell injection
NOTE: Keep the ice-cold basement membrane matrix cell suspension aseptic throughout the procedure.- Mix the cells and carefully draw the cell suspension into a 1 mL syringe mounted with a 21 G needle.
- Check to be sure that there are no air bubbles in the syringe.
- Disinfect the inoculation area of the mouse with an antiseptic solution.
- Gently squeeze the mouse's skin on the flank between fingers, at the injection site.
- Insert the needle exactly into the skin fold. Do not place the needle deep into the tissue to ensure a subcutaneous injection.
- Inject 100 µL of IMR5 cell suspension (i.e., 2.5 x 106 cells) subcutaneously into the lower right flank of the mice.
- Rotate the syringe to prevent leakage and withdraw the needle.
- Monitoring of body weight changes and tumor growth
- Measure the length (A) and the width (B) of the tumor with a caliper.
- Calculate the tumor volume using the formula (A x B2) x 0.5.
- Start therapy when the tumors have reached an average volume of ~50 - 60 mm3.
3. Drug and Antibody Administration in Mice
- Intravenous administration of mAb 8B6
- Carefully fill a 1 mL syringe mounted with a 25 G needle with mAb solution.
- Place the mouse under a heat lamp for 10 min to dilate the tail vein.
- Restrain the mouse in a rodent restrainer.
- Disinfect the inoculation area of the mouse with an antiseptic solution.
- Insert the needle parallel to the tail vein, penetrating 2 - 4 mm into the lumen while keeping the bevel of the needle face upward (Figure 3A).
- Inject 100 µL of antibody solution intravenously (i.v.).
- When the injection is finished, gently pressure the injection site to prevent bleeding.
- Intraperitoneal administration of topotecan
- Draw the drug solution in a 1 mL syringe mounted with a 25 G needle.
- Hold the mouse in a supine position, with its posterior end slightly elevated.
- Disinfect the inoculation area of the mouse with an antiseptic solution.
- Locate the mouse's abdomen midline and mentally divide the abdomen into quadrants. Locate the injection site in the right or left lower quadrant (Figure 3B).
- Insert the needle into the abdomen (5 mm deep) at ~10° angle, in the right or left lower quadrant.
- Inject 100 µL of drug solution intraperitoneally (i.p.).
- Disinfect the inoculation site.
Results
The representative results and figures are adapted with permission from earlier published work14.
Anti-OAcGD2 mAb 8B6 Synergistically Enhances the Inhibitory Effects of Topotecan on Neuroblastoma Cell Line Growth:
To establish the drug and the antibody concentrations to be used for assessing synergism between topotecan and mAb 8...
Discussion
To predict the effect of drug interactions, three methods can be used: the isobologram methodology17, the nonlinear mixture model18, and the combination index1. Combination index analysis is the most commonly used because its application is simplified by the availability of a user-friendly computer program. For this purpose, we first characterized the dose-effect response of each agent used alone or in combination, by performing an MTT assay
Disclosures
S.Fa., J.F., and S.B. are designated as inventors of pending patents covering the clinical application of anti-O-acetyl-GD2 therapeutic antibodies.
Acknowledgements
Grant support: Fondation de Projet de L'Université de Nantes, les Bagouz' à Manon, La Ligue contre le Cancer comité de Loire-Atlantique, comité du Morbihan, and comité de Vendée, une rose pour S.A.R.A.H, L'Etoile de Martin and la Société Française de Lutte contre les Cancers et les leucémies de L'Enfant et de L'adolescent (SFCE). M.B. and J.F. are supported by La Ligue Contre Le Cancer. The authors thank the UTE-facility of the Structure Fédérative de Recherche François Bonamy. The authors also thank Dr. S. Suzin (Inserm, Paris) for providing the IMR5 cells and Ms. H. Estéphan for her technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Cell Proliferation kit (MTT) | Roche | 11-465-007-001 | |
| CompuSyn software | ComboSyn | Combosyn can be downloaded for free at http://www.combosyn.com | |
| Electric shaver | Bioseb | BIO-1556 | |
| Fetal calf serum | Eurobio | CVFSVFF00-01 | 10% heat-inactivated fetal calf serum in RPMI 1640 |
| Firefox | Mozilla Corporation | Firefox can be downloaded for free at http://www.mozilla.org/en-US/firefox/ | |
| Heat lamp | Verre&Quartz | 4003/1R | |
| Human neuroblastoma IMR-5 cell line | Accegen Biotechnology | ABC-TC0450 | IMR-5 is a clone of the human neuroblastoma cell line IMR32 5459762. IMR-5 cells were generously provided by Dr. Santos Susin (U.872, Paris, France) |
| L-glutamine | Gibco | 25030-024 | 2 mM in RPMI 1640 |
| Lysis solution | Roche | 11-465-007-001 | |
| mAb 8B6 | University of Nantes | N/A | |
| Matrigel | Corning | 354248 | |
| Multiskan FC | Thermofischer Scientific | N08625 | |
| Needle 21G 1 ½ | BD Microlance | 304432 | |
| Needle 25G 1 | Terumo | NN-2525R | |
| NSG mice | Charles River Laboratories | 5557 | |
| Nunc MicroWell 96-well microplates | Thermofisher | 167008 | |
| PBS | VWR | L182-10 | |
| PBS, 0,05% EDTA | Sigma-Aldrich | E9884 | |
| PC that runs windows 7 | Microsoft | Windows 7 can be purchased at http://www.microsoft.com/en-gb/software-download/windows7 | |
| Penicillin-Streptomycin | Gibco | 15140-122 | 100 units/mL penicillin and 100 mg/mL streptomycin in RPMI 1640 |
| Reagent reservoir | Thermofischer Scientific | 8094 | |
| Rodent restrainer | Bioseb | TV-150-SM | |
| RPMI 1640 | Gibco | 31870-025 | |
| Syringe 1 mL | Henke Sass Wolf | 5010.200V0 | |
| Topotecan | Sigma-Aldrich | T2705 |
References
- Chou, T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Reviews. 58 (3), 621-681 (2006).
- Bayat Mokhtari, R., et al. Combination therapy in combating cancer. Oncotarget. 8 (23), 38022-38043 (2017).
- Zahreddine, H., Borden, K. L. Mechanisms and insights into drug resistance in cancer. Frontiers in Pharmacology. 4, 28 (2013).
- Martin, T. P., Baguley, D., et al. Re: "Postoperative validation of bone-anchored implants in the single-sided deafness population." Snapp et al. Otol Neurotol 2012: 33;291-6. Otol Neurotol. 34 (4), 777-778 (2013).
- Choi, B., et al. Sensitization of lung cancer cells by altered dimerization of HSP27. Oncotarget. 8 (62), 105372-105382 (2017).
- Weiner, L. M., Surana, R., Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology. 10 (5), 317-327 (2010).
- Mellor, J. D., Brown, M. P., Irving, H. R., Zalcberg, J. R., Dobrovic, A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. Journal of Hematology & Oncology. 6, 1 (2013).
- Kowalczyk, A., et al. The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Letters. 281 (2), 171-182 (2009).
- Retter, M. W., et al. Characterization of a proapoptotic antiganglioside GM2 monoclonal antibody and evaluation of its therapeutic effect on melanoma and small cell lung carcinoma xenografts. Cancer Research. 65 (14), 6425-6434 (2005).
- Nakamura, K., et al. Apoptosis induction of human lung cancer cell line in multicellular heterospheroids with humanized antiganglioside GM2 monoclonal antibody. Cancer Research. 59 (20), 5323-5330 (1999).
- Cochonneau, D., et al. Cell cycle arrest and apoptosis induced by O-acetyl-GD2-specific monoclonal antibody 8B6 inhibits tumor growth in vitro and in vivo. Cancer Letters. 333 (2), 194-204 (2013).
- Chou, T. C., Martin, N. . CompuSyn for drug combinations: PC software and user’s guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. , (2005).
- Alvarez-Rueda, N., et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS One. 6 (9), e25220 (2011).
- Faraj, S., et al. Neuroblastoma chemotherapy can be augmented by immunotargeting O-acetyl-GD2 tumor-associated ganglioside. Oncoimmunology. 7 (1), e1373232 (2017).
- Ishikawa, F., et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 106 (5), 1565-1573 (2005).
- Ullman-Cullere, M. H., Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Laboratory Animal Science. 49 (3), 319-323 (1999).
- Teicher, B. A. Assays for in vitro and in vivo synergy. Methods in Molecular Medicine. 85, 297-321 (2003).
- White, D. B., Slocum, H. K., Brun, Y., Wrzosek, C., Greco, W. R. A new nonlinear mixture response surface paradigm for the study of synergism: a three drug example. Current Drug Metabolism. 4 (5), 399-409 (2003).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65 (1-2), 55-63 (1983).
- Huyck, L., Ampe, C., Van Troys, M. The XTT cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay and Drug Development Technologies. 10 (4), 382-392 (2012).
- Thompson, J., et al. Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clinical Cancer Research. 5 (11), 3617-3631 (1999).
- Tan, M., Fang, H. B., Tian, G. L., Houghton, P. J. Experimental design and sample size determination for testing synergism in drug combination studies based on uniform measures. Statistic in Medicine. 22 (13), 2091-2100 (2003).
- Tang, X. X., et al. Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proceding of the National Academy of Sciences of the United States of America. 97 (20), 10936-10941 (2000).
- Mehta, R. R., Graves, J. M., Hart, G. D., Shilkaitis, A., Das Gupta, T. K. Growth and metastasis of human breast carcinomas with Matrigel in athymic mice. Breast Cancer Research and Treatment. 25 (1), 65-71 (1993).
- Mullen, P., Ritchie, A., Langdon, S. P., Miller, W. R. Effect of Matrigel on the tumorigenicity of human breast and ovarian carcinoma cell lines. International Journal of Cancer. 67 (6), 816-820 (1996).
- Feng, C., Tang, S., Wang, J., Liu, Y., Yang, G. Topotecan plus cyclophosphamide as maintenance chemotherapy for children with high-risk neuroblastoma in complete remission: short-term curative effects and toxicity. Nan Fang Yi Ke Da Xue Xue Bao. 33 (8), 1107-1110 (2013).
- Cheung, N. K., et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. Journal of Clininical Oncology. 5 (9), 1430-1440 (1987).
- Nair, A. B., Jacob, S. A simple practice guide for dose conversion between animals and human. Journal of Basic Clinical Pharmacy. 7 (2), 27-31 (2016).
- Dayde, D., et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 113 (16), 3765-3772 (2009).
- Racki, W. J., et al. NOD-scid IL2rgamma(null) mouse model of human skin transplantation and allograft rejection. Transplantation. 89 (5), 527-536 (2010).
- Sherif, A., Winerdal, M., Winqvist, O. Immune Responses to Neoadjuvant Chemotherapy in Muscle Invasive Bladder Cancer. Bladder Cancer. 4 (1), 1-7 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved