A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Accurate Follicle Enumeration in Adult Mouse Ovaries
In This Article
Summary
Here, we describe, compare, and contrast two different techniques for accurate follicle counting of fixed mouse ovarian tissues.
Abstract
Sexually reproducing female mammals are born with their entire lifetime supply of oocytes. Immature, quiescent oocytes are found within primordial follicles, the storage unit of the female germline. They are non-renewable, thus their number at birth and subsequent rate of loss largely dictates the female fertile lifespan. Accurate quantification of primordial follicle numbers in women and animals is essential for determining the impact of medicines and toxicants on the ovarian reserve. It is also necessary for evaluating the need for, and success of, existing and emerging fertility preservation techniques. Currently, no methods exist to accurately measure the number of primordial follicles comprising the ovarian reserve in women. Furthermore, obtaining ovarian tissue from large animals or endangered species for experimentation is often not feasible. Thus, mice have become an essential model for such studies, and the ability to evaluate primordial follicle numbers in whole mouse ovaries is a critical tool. However, reports of absolute follicle numbers in mouse ovaries in the literature are highly variable, making it difficult to compare and/or replicate data. This is due to a number of factors including strain, age, treatment groups, as well as technical differences in the methods of counting employed. In this article, we provide a step-by-step instructional guide for preparing histological sections and counting primordial follicles in mouse ovaries using two different methods: [1] stereology, which relies on the fractionator/optical dissector technique; and [2] the direct count technique. Some of the key advantages and drawbacks of each method will be highlighted so that reproducibility can be improved in the field and to enable researchers to select the most appropriate method for their studies.
Introduction
The immature, meiotically-arrested oocytes stored within primordial follicles in the ovary are the storage unit for the female germline and comprise an individual’s lifetime ovarian reserve. Primordial follicle numbers decline naturally with age1, or alternatively, can be prematurely depleted following exposure to exogenous chemicals, including some pharmaceuticals and environmental toxicants in air, food and water2. Given that the primordial follicle number is finite, the quantity and quality of follicles present within the ovary largely determine female fertility and offspring health. Thus, accurate quantification of primordial follicle number in women is essential for evaluating the off-target impacts of exogenous insults on the ovarian reserve.
In women, analysis of the whole ovary is generally not possible, thus non-invasive surrogate measures of the ovarian reserve must be utilized in a clinical setting. Anti-Mϋllerian hormone (AMH) is the most widely used surrogate biomarker clinically3. Serum AMH levels are often measured in women of advanced maternal age, or before and after cancer treatment, such as chemotherapy. However, AMH is produced by growing follicles and not by primordial follicles, and thus, serum levels do not inform on absolute primordial follicle number.
With the absence of methods to accurately determine primordial follicle number in women in situ, counting ovarian follicles in small animal models, such as rodents, remains an essential research tool to assess the degree by which exogenous insults impact on primordial follicles and thus, fertility. Unfortunately though, reports throughout the literature of primordial follicle numbers in rodent models are highly variable4. A major reason for this is widely reported technical differences in the counting method employed. Predominately, there are two different techniques described in the literature for enumerating primordial follicles in mice. These include stereology, which employs the fractionator optical dissector method, and direct follicle counts.
Stereology is widely regarded the gold standard as it uses systematic uniform random sampling5, making it the most accurate method of quantifying primordial follicle number in whole mouse, or rat ovaries4,6,7. Stereology is unbiased, as it accounts for the three-dimensional structure of the object of interest8. Using an optical dissector/fractionator method, three levels of sampling are applied to quantify primordial follicles using thick tissue sections (e.g., 20 µm) within a known fraction of the total mouse ovary. Firstly, the sampling interval is chosen (e.g., every 3rd section) at a random start (sampling fraction 1, f1)4. Sections are then sampled in a systematic, uniform manner from this point through the whole ovary. Then, an unbiased counting frame is superimposed over the ovarian section and progressively moved along a defined, randomized counting grid (sampling fraction 2, f2)8. Lastly, a known fraction of the section thickness is optically sampled (e.g., 10 µm) and follicles within this area are counted (sampling fraction 3, f3)4. The raw follicle number is multiplied by the inverse of these sampling fractions to obtain the final value. This method requires expert training and equipment, including a microscope with a motorized stage driven by stereological software. Tissues should be preserved in a specialized Bouin’s fixative, and embedded in glycolmethacrylate resin to allow for thick tissue sections to be cut using a glycolmethacrylate resin microtome with a glass knife. This method is designed to account for tissue shrinkage and deformation, to best preserve the three-dimensional morphological structure of the ovary and follicles9.
Direct follicle counting is the most frequently used method for counting follicles10. More common fixatives (i.e., formalin) can be used, followed by paraffin wax embedding and exhaustive serial sectioning using a standard microtome at a thickness of between 4-6 µm. Follicles are systematically counted in the entire tissue section at a defined interval, and then multiplied by the inverse of the sampling interval to obtain the total follicle estimate. This method is quick, easy, can be performed using archived tissues, and prepared using standard histological techniques. It requires only a light microscope with standard imaging capabilities. However, despite these advantages, direct follicle counting lacks the accuracy and strict counting parameters of stereology, making it more prone to investigator bias. Additionally, tissues may undergo shrinkage and deformation during processing, disrupting the integrity and morphology of the ovary and thus making follicle classification and quantification difficult.
The aim of this article is to describe two commonly-used methods to quantitatively assess primordial follicle number in mouse ovaries: stereology and direct follicle counting. We will provide detailed protocols for these two methods and highlight some of their strengths and weaknesses, in order to enhance reproducibility in our field and allow researchers to make an informed decision of the most appropriate counting method for their studies.
Protocol
Ovaries were collected from female C57BL6J mice. All animal procedures and experiments were performed in accordance with the NHMRC Australian Code of Practice for the Care and Use of Animals and approved by the Monash Animal Research Platform Animal Ethics Committee.
NOTE: A chemotherapy agent shown to deplete primordial follicle oocytes, as determined using stereology11 and direct counts12,13 was used in this report to compare the two counting methods in the same animal. Female, 8-week-old (young adult) mice were weighed prior to a single intraperitoneal injection of 75 mg/kg/bodyweight of cyclophosphamide, or saline vehicle control (n=5/group). This dose has been shown to cause an approximate 50% depletion of primordial follicles, but not reported to cause morbidity or mortality in mice14. Ovaries were harvested 48 hours after treatment. One ovary from each animal was fixed in 10% (v/v) neutral buffered formalin solution for 24 hours, and the other fixed in Bouin’s solution for 24 hours. Tissue was then embedded in either glycolmethacrylate resin and serially sectioned at 20 µm, or in paraffin and serially sectioned at 5 µm. All tissues were stained with periodic acid Schiff and haematoxylin.
1. Histological preparation: fixation, processing, embedding and sectioning mouse ovaries
- Dissect mouse ovaries by trimming the oviduct and all surrounding fat, without damaging or cutting the ovary itself. If necessary, use a dissecting microscope and fine blade for this step (Figure 1A).
- Fix tissues immediately by placing into a small labelled tube containing either Bouin’s fixative (stereology), or formalin fixative (direct counts) for 24 hours (Figure 1B), before transferring tissues into 70% ethanol.
NOTE: Follicle morphology is conserved best within Bouin’s fixed ovarian tissue, embedded into glycolmethacrylate resin (Figure 2). - Process whole fixed ovaries and then embed in glycolmethacrylate resin for stereology (Supplementary File 1), or paraffin wax for direct counts using a standard histological protocol.
CAUTION: Resin is toxic, so ensure all tissue processing steps are performed in a fume hood and gloves are worn at all times. - Use a specialized resin methacrylate microtome (Figure 1C) fitted with a glass knife (Figure 1D) to exhaustively cut thick glycolmethacrylate resin sections (e.g., 20 µm). Collect the sections at a regular interval (e.g., every 3rd section) onto glass microscope slides for stereology.
- Use a standard microtome to cut thin paraffin sections (e.g., 4-6 µm). Collect tissue sections at a regular interval (e.g., every 9th section) onto a glass microscope slide for direct follicle counts.
- Stain the slides with periodic acid Schiff and haematoxylin (Supplementary File 2).
- Coverslip with standard DPX for paraffin sections, or thick DPX for glycolmethacrylate resin sections (Figure 1E).
CAUTION: Glycolmethacrylate resin DPX is hazardous, so perform this step in to fume hood.
NOTE: Glycolmethacrylate resin DPX is extremely viscous. The glass coverslip must be adhered firmly by pressing it down with a spatula to ensure the DPX is evenly and thinly dispersed, and there are no air bubbles present under the coverslip (Figure 1F).
2. Stereological estimation of primordial follicle number using the optical fractionator
- Turn on the computer, the multi-control unit, the camera and the light source within the stereology setup and set the microscope objective to a low magnification (e.g., 10x).
- Open the stereology software.
- Put the first slide securely on the microscope stage.
- Adjust the light exposure by checking Automatic under Exposure in the Camera Settings panel (Supplementary Figure 1A). Alternatively, manually adjust the light exposure.
- Use the joystick to locate the first tissue sample and bring the sample into focus.
- Adjust the white balance, either by clicking on More Settings (located bottom right of the Camera Settings panel), and then click White Balance and click on Automatic (Supplementary Figure 1B). Alternatively, click the White Balance button adjacent to the More Settings button (or Select Area in More Settings), to set the white balance manually by selecting a white area on the section.
- Go to the Probes drop down menu and click on Optical Fractionator Workflow. Then click Start New Project and click OK.
- If an existing sampling configuration has been saved, under Sampling Parameters click Yes | … and select the desired sampling configuration.
- If not, click No and manually enter the serial section information (Supplementary Figure 1C) and define the probe configuration at step 2.13.
- Click on Next, set the microscope to Low Magnification and choose 10x magnification from the dropdown menu.
- Click on Next, and then trace around the entire ovarian section – start by left clicking around the section and at the end, right click and choose Close Contour.
- Click on Next, set the microscope to High Magnification and choose 100x Oil magnification from the dropdown menu.
- Place a drop of oil on the tissue section on the slide and move the microscope objective to 100x magnification.
- Adjust the light exposure (as in step 2.4) and click Next.
- Set up the Sampling Parameters to define the probe configuration. Here, the counting frame was set to 47.5 µm x 47.5 µm (2,256.25 µm2) and the step length was set to 100 µm x 100 µm (10,000 µm2) (Supplementary Figure 2). Once the sampling parameters are established, save the template and re-open during subsequent analysis sessions at step 2.7.
- Click on Start Counting (Supplementary Figure 1D). Focus to the top of the sample, click OK and begin counting.
- Use the focusing knob to move through the 10 µm sampling depth and count any primordial follicles whose oocyte nucleus comes into focus. Click Next to move to the next area.
- Classify follicles as a primordial if the oocyte is surrounded by squamous (flattened) granulosa cells, but no cuboidal granulosa cells (Figure 2A).
NOTE: Primordial follicles are distinct from intermediate/transitional follicles, which comprise a combination of cuboidal and squamous granulosa cells (Figure 2B), and primary follicles, which are surrounded predominantly by cuboidal granulosa cells (Figure 2C). These follicle classes should be quantified separately. - Only count follicles in which the oocyte nucleus is visible. The oocyte nucleus must appear within the counting frame or be touching the green inclusion lines of the counting frame (Supplementary Figure 1E,F).
- If the oocyte nucleus falls outside the counting frame (Supplementary Figure 1G) or touches the red exclusion lines of the counting frame, do not count this follicle.
- When assessing primordial follicle depletion in response to an exogenous chemical (e.g., chemotherapy), ensure all follicles counted are healthy and thus have normal morphology (Figure 2). Count any abnormal or atretic follicles separately. Often, follicle death is induced by insults such as chemotherapy, and quantification of the atretic follicles should be obtained separately in order to distinguish between healthy and atretic follicles, as only healthy follicles comprise the ovarian reserve15.
- Classify follicles as a primordial if the oocyte is surrounded by squamous (flattened) granulosa cells, but no cuboidal granulosa cells (Figure 2A).
- Once counting is complete on that section, do one of the following:
- Click Add New Section, and then return to step 2.3 to set up the next section for counting.
- Click I’ve Finished Counting to end the session. Return the objective to 10x, exit the stereology software and turn off the light source, camera, multi-control unit and computer.
- Obtain the sum raw follicle number (Q) from each tissue section sampled from the entire ovary, then using a spreadsheet, use the equation below to obtain the final value from each replicate animal (N)4.
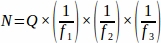 , where:
, where:
N = Total estimated number of follicles within the ovary.
Q = Raw primordial follicle count.
f1 = Sampling interval. Every 3rd section was sampled thus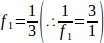 .
.
f2 = Relationship between the counting frame (sample area) and stepper, calculated as . Since the sample area was 2256 µm2 (47.5 µm x 47.5 µm) and the stepper area was 10000 µm2 (100 µm x 100 µm),
. Since the sample area was 2256 µm2 (47.5 µm x 47.5 µm) and the stepper area was 10000 µm2 (100 µm x 100 µm),  .
.
f3 = Fraction of ovarian section sampled. Since 10 µm of the 20 µm section was sampled,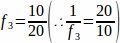 .
.
Therefore,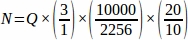 .
.
NOTE: This protocol describes how to apply these principles of stereological analyses using a widely cited stereology software (Table of Materials); however, other stereological software is available. The principles applied during stereological analyses of ovarian follicles are the same, regardless of the software used to set up the parameters. Stereology is most accurate when 100 or more objects are counted in an adult mouse ovary4, as this gives a coefficient of error for the estimate below 10%16. The sampling parameters outlined here have been optimized to ensure a minimum of approximately 100 objects (i.e., primordial, transitional and primary follicles) can be counted in control adult wild-type C57BL6J ovaries. A pilot study can be conducted including a small sample size to establish the optimal sampling parameters, such as the interval and number of sections to be analyzed and the number of optical dissectors within the sampled sections17.
3. Estimation of primordial follicle number by direct ovarian follicle counts
- Place the microscope slide securely under a standard light microscope and perform direct counts to obtain raw primordial follicle number.
- Classify follicles as a primordial if the oocyte is clearly visible and is surrounded by squamous (flattened) granulosa cells, but no cuboidal granulosa cells (Figure 2D).
NOTE: Primordial follicles are distinct from intermediate/transitional follicles, which comprise a combination of cuboidal and squamous granulosa cells (Figure 2E), and primary follicles, which are surrounded predominantly by cuboidal granulosa cells (Figure 2F). These follicle classes should be quantified separately. - Ensure all follicles counted are healthy and thus have normal morphology (Figure 2). Count any abnormal or atretic follicles separately, as only healthy follicles comprise the ovarian reserve.
- Classify follicles as a primordial if the oocyte is clearly visible and is surrounded by squamous (flattened) granulosa cells, but no cuboidal granulosa cells (Figure 2D).
- Alternatively, take multiple, or stitched high-power (e.g., 20x) photomicrographs of the entire ovarian tissue section to perform counts by opening the image file(s). This can be done manually, or using an automated slide scanner.
- Obtain the sum raw follicle number (Q) from each tissue section sampled from the entire ovary at the predetermined interval. Multiply this number by the inverse of the sampling fraction to obtain the final value for each replicate animal (N), using the equation below.
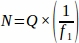 , where:
, where:
N = Total estimated number of follicles within the ovary.
Q = Raw follicle count (of each individual type, calculated separately).
f1 = Sampling interval. Every 9th section was sampled thus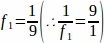 .
.
Therefore,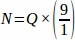 .
.
Results
A well-characterized model of follicle depletion was used, whereby young adult female mice were administered a single dose of cyclophosphamide chemotherapy, or saline vehicle control (n=5/group) and both ovaries were harvested from each animal after 48 hours. One ovary per animal was prepared as described in Step 1 for each of the two methods: stereology or direct counts. The left and right ovary from each animal was randomly assigned to each group. These data show that when using stereology, a significant depletion of m...
Discussion
This article provides a step-by-step protocol for the gold standard technique for enumerating mouse primordial follicles, stereology, and the more commonly employed method of direct follicle counting. Chemotherapy treatment was used to compare and contrast the results obtained from these two different methods within the left and right ovary from the same animal. Both methods revealed high inter-animal variability in primordial follicle numbers. A significant depletion of the ovarian reserve was recorded using stereology,...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS and supported by funding from National Health and Medical Research Council (ALW #1120300) and Australian Research Council (KJH #FT190100265). The authors would like to acknowledge the technical support of the Monash Animal Research Platform, Monash Histology Platform and Monash Micro Imaging facility.
Materials
| Name | Company | Catalog Number | Comments |
| 1-Butanol (HPLC) | Fisher Chemical | #A383-1 | |
| Acid alcohol | Amber Scientific | #ACDL | |
| Bouin’s fixative | Sigma-Aldrich | #HT10132 | Picric acid 0.9% (w/v), formaldehyde 9% (v/v), acetic acid 5% (w/v) |
| Cyclophosphamide | Sigma-Aldrich | #C0768-5G | |
| Dibutylphthalate Polystyrene Xylene (DPX) | Sigma-Aldrich | #06522 | |
| Ethanol | Amber Scientific | #ETH | Ethanol 100% |
| Micro Feather opthalmic scalpel with aluminium handle | Designs for Vision | #FEA-745-SR | Feather blade for dissections (seen in Figure 1) |
| Formalin fixative | Australian Biostain | #ANBFC | |
| Glass coverslip | Thermo Scientific | #MENCS22501GP | 22 mm x 50 mm |
| Glycomethacrylate resin RM2165 microtome | Leica Microsystems | #RM2165 | |
| Glycolmethacrylate DPX | *made in house | *Mix 1.5 L Xylene; 800 g polystyrene pellets; 100mL Dibutyl phthalate for 3 weeks | |
| Histolene | Trajan | #11031 | |
| Mayer’s haematoxylin | Amber Scientific | #MH | |
| Olympus BX50 microscope | Olympus | #BX50 | Brightfield microscope fitted with 10x dry & 100x oil immersion objective (numerical aperture 1.3) |
| Olympus immersion oil type-F | Olympus | #IMMOIL-F30CC | |
| Olympus TH4-200 light source | Olympus | #TH4-200 | |
| Paraffin wax | Sigma-Aldrich | #03987 | |
| Periodic acid | Trajan | #PERI1% | Periodic acid 1% |
| Rotary Microtome CUT 4060 | MicroTec | #4060R/F | Used to cut paraffin sections |
| Schiff’s reagent | Trajan | #SCHF | |
| Scott's tap water | Amber Scientific | #SCOT | Potassium carbonate, magnesium sulphate, water |
| StereoInvestigator Stereological System | MBF Bioscience | Includes StereoInvestigator software, multi-control unit, automatic stage and joystick | |
| Superfrost microscope slides | Thermo Scientific | #MENSF41296SP | 1 mm, 72 pcs |
| Technovit 7100 Plastic embedding system | Emgrid Australia | #64709003 | 500 mL/5 x 1 g/40 mL |
| Technovit 3040 yellow | Emgrid Australia | #64708805 | 100 g/80 mL |
References
- Stringer, J. M., Winship, A., Liew, S. H., Hutt, K. The capacity of oocytes for DNA repair. Cellular and Molecular Life Sciences. 75 (15), 2777-2792 (2018).
- Winship, A. L., Stringer, J. M., Liew, S. H., Hutt, K. J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Human Reproduction Update. 24 (2), 119-134 (2018).
- Broer, S. L., Broekmans, F. J., Laven, J. S., Fauser, B. C. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Human Reproduction Update. 20 (5), 688-701 (2014).
- Myers, M., Britt, K. L., Wreford, N. G., Ebling, F. J., Kerr, J. B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 127 (5), 569-580 (2004).
- Boyce, R. W., Dorph-Petersen, K. A., Lyck, L., Gundersen, H. J. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicologic Pathology. 38 (7), 1011-1025 (2010).
- Ristic, N., et al. Maternal dexamethasone treatment reduces ovarian follicle number in neonatal rat offspring. Journal of Microscopy. 232 (3), 549-557 (2008).
- Soleimani Mehranjani, M., Mansoori, T. Stereological study on the effect of vitamin C in preventing the adverse effects of bisphenol A on rat ovary. International Journal of Reproductive Biomedicine (Yazd). 14 (6), 403-410 (2016).
- Brown, D. L. Bias in image analysis and its solution: unbiased stereology. Journal of Toxicologic Pathology. 30 (3), 183-191 (2017).
- Hasselholt, S., Lykkesfeldt, J., Overgaard Larsen, J. Thick methacrylate sections devoid of lost caps simplify stereological quantifications based on the optical fractionator design. Anatomical Record (Hoboken). 298 (12), 2141-2150 (2015).
- Tilly, J. L. Ovarian follicle counts--not as simple as 1, 2, 3. Reproductive Biology and Endocrinology. 1, 11 (2003).
- Nguyen, Q. N., et al. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death and Disease. 9 (6), 618 (2018).
- Meirow, D., Assad, G., Dor, J., Rabinovici, J. The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Human Reproduction. 19 (6), 1294-1299 (2004).
- Winship, A. L., et al. The PARP inhibitor, olaparib, depletes the ovarian reserve in mice: implications for fertility preservation. Human Reproduction. , (2020).
- Meirow, D., Lewis, H., Nugent, D., Epstein, M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Human Reproduction. 14 (7), 1903-1907 (1999).
- Nguyen, Q. N., et al. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Molecular Human Reproduction. , (2019).
- Gundersen, H. J., Jensen, E. B. The efficiency of systematic sampling in stereology and its prediction. Journal of Microscopy. 147, 229-263 (1987).
- Olesen, M. V., Needham, E. K., Pakkenberg, B. The Optical Fractionator Technique to Estimate Cell Numbers in a Rat Model of Electroconvulsive Therapy. Journal of Visualized Experiments. (125), e55737 (2017).
- Findlay, J. K., Hutt, K. J., Hickey, M., Anderson, R. A. How Is the Number of Primordial Follicles in the Ovarian Reserve Established. Biology of Reproduction. 93 (5), 111 (2015).
- Omari, S., et al. Mcl-1 is a key regulator of the ovarian reserve. Cell Death and Disease. 6, 1755 (2015).
- Winship, A. L., Bakai, M., Sarma, U., Liew, S. H., Hutt, K. J. Dacarbazine depletes the ovarian reserve in mice and depletion is enhanced with age. Scientific Reports. 8 (1), 6516 (2018).
- Miller, P. B., Charleston, J. S., Battaglia, D. E., Klein, N. A., Soules, M. R. An accurate, simple method for unbiased determination of primordial follicle number in the primate ovary. Biology of Reproduction. 56 (4), 909-915 (1997).
- Miller, P. B., Charleston, J. S., Battaglia, D. E., Klein, N. A., Soules, M. R. Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biology of Reproduction. 61 (2), 553-556 (1999).
- Charleston, J. S., et al. Estimating human ovarian non-growing follicle number: the application of modern stereology techniques to an old problem. Human Reproduction. 22 (8), 2103-2110 (2007).
- Hansen, K. R., Craig, L. B., Zavy, M. T., Klein, N. A., Soules, M. R. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause. 19 (2), 164-171 (2012).
- Hansen, K. R., et al. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Human Reproduction. 23 (3), 699-708 (2008).
- Sonigo, C., et al. High-throughput ovarian follicle counting by an innovative deep learning approach. Scientific Reports. 8 (1), 13499 (2018).
- McKey, J., Cameron, L. A., Lewis, D., Batchvarov, I. S., Capel, B. Combined iDISCO and CUBIC tissue clearing and lightsheet microscopy for in toto analysis of the adult mouse ovarydagger. Biology of Reproduction. 102 (5), 1080-1089 (2020).
- Kagami, K., Shinmyo, Y., Ono, M., Kawasaki, H., Fujiwara, H. Three-dimensional evaluation of murine ovarian follicles using a modified CUBIC tissue clearing method. Reproductive Biology and Endocrinology. 16 (1), 72 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved