A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation of High Quality Murine Atrial and Ventricular Myocytes for Simultaneous Measurements of Ca2+ Transients and L-Type Calcium Current
* These authors contributed equally
In This Article
Summary
Mouse models allow studying key mechanisms of arrhythmogenesis. For this purpose, high quality cardiomyocytes are necessary to perform patch-clamp measurements. Here, a method to isolate murine atrial and ventricular myocytes via retrograde enzyme-based Langendorff perfusion, which allows simultaneous measurements of calcium-transients and L-type calcium current, is described.
Abstract
Mouse models play a crucial role in arrhythmia research and allow studying key mechanisms of arrhythmogenesis including altered ion channel function and calcium handling. For this purpose, atrial or ventricular cardiomyocytes of high quality are necessary to perform patch-clamp measurements or to explore calcium handling abnormalities. However, the limited yield of high-quality cardiomyocytes obtained by current isolation protocols does not allow both measurements in the same mouse. This article describes a method to isolate high-quality murine atrial and ventricular myocytes via retrograde enzyme-based Langendorff perfusion, for subsequent simultaneous measurements of calcium transients and L-type calcium current from one animal. Mouse hearts are obtained, and the aorta is rapidly cannulated to remove blood. Hearts are then initially perfused with a calcium-free solution (37 °C) to dissociate the tissue at the level of intercalated discs and afterwards with an enzyme solution containing little calcium to disrupt extracellular matrix (37 °C). The digested heart is subsequently dissected into atria and ventricles. Tissue samples are chopped into small pieces and dissolved by carefully pipetting up and down. The enzymatic digestion is stopped, and cells are stepwise reintroduced to physiologic calcium concentrations. After loading with a fluorescent Ca2+-indicator, isolated cardiomyocytes are prepared for simultaneous measurement of calcium currents and transients. Additionally, isolation pitfalls are discussed and patch-clamp protocols and representative traces of L-type calcium currents with simultaneous calcium transient measurements in atrial and ventricular murine myocytes isolated as described above are provided.
Introduction
Cardiac arrhythmias are common and one of the current major healthcare challenges since they affect millions of people worldwide. Arrhythmias are associated with high morbidity and mortality1,2 and represent the underlying cause for the majority of sudden cardiac deaths3. Up to date treatment options have improved patient survival but are still mainly symptomatic treatments rather than targeting the underlying mechanisms. Thus, these treatments have limited efficacy and may frequently cause severe side effects4,5,6. An improvement of current treatment options requires insight into the underlying pathophysiology, creating the need for suitable models to study. Small animal models - and specifically mouse models - play a crucial role in arrhythmia research as they allow to study key mechanisms of arrhythmogenesis, for example the genetic impact on cellular electrophysiology, ion channel function or calcium handling7,8.
For this purpose, isolated atrial and ventricular cardiomyocytes of sufficient quantity and viability are required. A broad spectrum of different isolation approaches to obtain atrial and ventricular myocytes has been previously described9,10,11,12,13 and some groups have presented data from simultaneous measurements of L-type current and calcium current induced calcium transients from either atrial14 or ventricular15 murine cardiomyocytes. However, to our best knowledge there is no data available of atrial as well as ventricular measurements from one animal. Researchers focus on a broad variety of topics ranging from electrophysiology to proteomics, functional studies as cell contractility or protein interactions, mitochondrial function, or genetics – all in need of isolated cardiomyocytes. Many of the published protocols thus have not been specifically developed for patch clamp studies, leading to limited yields and insufficient cell quality for patch clamp studies. Thus, simultaneous patch clamp and calcium transient measurements of atrial and ventricular cells isolated from one animal cannot be performed with established protocols.
Isolation of murine – especially atrial – myocytes for patch clamp experiments remains challenging. This article provides a simple and fast method for the isolation of high-quality murine atrial and ventricular myocytes via retrograde enzyme based Langendorff perfusion, which subsequently allows simultaneous measurements of both net membrane current and current induced calcium transients from one animal. This article elaborates a protocol for the isolation of atrial and ventricular myocytes derived from wild type mice and mice carrying genetic mutations. This protocol can be used for male and female mice alike. The myocyte isolation, images, and representative results described below were obtained from wild type C57Bl/6 mice at the age of 6 (± 1) months. Nevertheless, this protocol has successfully been used for mice at various ages ranging from 2 to 24 months with different genotypes. Figure 1 shows the isolation setup and a close-up of a cannulated heart during enzyme perfusion.
Protocol
All animal procedures were approved by the Lower Saxony Animal Review Board (LAVES, AZ-18/2900) and were conducted in accordance with all institutional, national, and international guidelines for animal welfare.
1. Prearrangements
- Prepare 1 L of 10x perfusion buffer (Table 1), 500 mL of 1x perfusion buffer (Table 2), 50 mL of digestion buffer (Table 3), 10 mL of stop buffer (Table 4), 1 L of Tyrode solution (Table 5), 10 mL of each calcium step solution (Tyrode solution with glucose and respective amount of calcium as indicated), 1 L of 4-AP solution (Table 5), 100 mL of pipette solution (Table 6) and 5 mL of pluronic acid according to the provided recipes.
NOTE: Bath solutions (Tyrode and 4-AP solution) can be prepared (without glucose) in advance and stored at +4 °C, glucose is added on the experimental day. Pipette solution can be stored at -20 °C, calcium indicator is added on the experimental day and solution then stored on ice until further use. 10x perfusion buffer can be stored at room temperature, 1x perfusion buffer, digestion buffer and stopping buffer should be freshly prepared on experimental day. - Turn on the water bath and roller pump.
- Prefill Langendorff apparatus with perfusion buffer; make sure it is air-free.
- Prepare aortic cannula by fixing it under the dissection microscope, connect with a 1 mL syringe filled with perfusion buffer and clear air by rinsing the cannula.
NOTE: It is crucial to avoid any air inside the perfusion system, as this will directly affect coronary perfusion and thus digestion effectiveness. A bubble trap might be added to the setup if necessary, to safely avoid any air trapping. - Prepare Petri dishes with enough perfusion buffer for organ collection and microscopy (buffer should securely cover the entire organ, a few millilitres – depending on the used Petri dish size – should be enough).
- Prepare 3 Petri dishes with digestion buffer for tissue dissociation and microscopy, buffer should cover the organ for dissection under the microscope within the respective Petri dish, amount depends on the used Petri dish size. For the dissociation use 3 mL for ventricular tissue and 1.5 mL for atrial tissue.
2. Organ harvest
- Inject mouse with 0.1 mL of heparin (1,000 U/mL) i.p. using a 1 mL syringe with a 27 G cannula and wait for 5-10 min.
- Place the mouse into an induction chamber along with a small tissue soaked in approximately 500 µL of isoflurane. The animal should not be in contact with the tissue. To avoid that, one can use a plastic biopsy-embedding cassette to cover the tissue. Once the animal is fully anesthetized, check for toe pinch reflex and as soon as it is not present anymore, quickly euthanize the mouse by cervical dislocation.
- Place the mouse on a platform on its back (e.g., on Styrofoam covered with a paper towel) and fix the paws down with cannulas to hold it in place.
- Remove fur and skin covering the chest and part of the abdomen with a clear cut from jugulum towards symphysis and open the abdomen right under the xiphoid without injuring any organ structure using scissors. Lift the sternum with surgical forceps and cut the diaphragm with scissors along the edge of the ribs, then cut the ribs in medial axillary line and remove the rib cage to expose the heart.
- Carefully remove the pericardium using blunt forceps and quickly remove the heart by lifting it with blunt forceps from below and by cutting the large vessels with one single cut using scissors.
- Put the heart into room temperature perfusion buffer and cannulate the aorta with a blunt end needle under the microscope as quickly as possible.
NOTE: Remove any lung tissue and fatty tissue attached to the organ without losing too much time on it. While cannulating, make sure that the end of the needle does not extend through the aortic valve, as this will impair results by preventing buffers from entering the coronary arteries. - Tie the heart with a piece of suturing silk firmly to the needle and disconnect from syringe.
NOTE: The entire procedure from obtaining the heart (the moment when the large vessels are cut) until suturing the aorta to the needle should take as little time as possible. It is recommended not taking longer than 90-180 s from removing the heart until start of perfusion.
3. Enzymatic digestion
- After aortic cannulation, immediately connect the cannulated heart to the Langendorff apparatus avoiding any air entering the system.
NOTE: It can help to have a hanging drop of perfusion buffer at the bottom of the Langendorff apparatus as well as a drop of perfusion buffer sitting on the top of the needle in order to avoid any air entering the system. - Perfuse the heart with perfusion buffer for 1 min at a temperature of exactly 37 °C and a perfusion rate of exactly 4 mL/min.
NOTE: In order to have a temperature of 37 °C at the tip of the perfusion needle, water bath temperature has to be set slightly above at approximately 40 °C. This should be tested regularly by measuring the temperature at the perfusion tip. - Switch perfusion to digestion buffer and perfuse for exactly 9 min at a temperature of exactly 37 °C and a perfusion rate of exactly 4 mL/min.
- Transfer the digested heart to a Petri dish with enough digestion buffer to keep it fully covered. Then carefully dissect the atria and ventricles under the microscope.
- Transfer the atria into a Petri dish with 1.5 mL of digestion buffer and the ventricles into another Petri-dish with 3 mL of digestion buffer.
- Atrial dissection
- Carefully, but without loss of time, pull the atria apart into tiny pieces using blunt forceps.
- Dissolve the tissue by carefully pipetting up and down using a 1,000 µL pipette tip, which has previously been cut to widen the tip opening.
- Transfer the solution to a 15 mL centrifuge tube and add an equivalent amount of stop buffer (1.5 mL) by carefully pipetting down at the side of the tube to end the reaction.
- Carefully pass all 3 mL of cell/tissue-solutions through a 200 µm nylon mesh to remove remaining larger tissue pieces that have not been fully digested.
NOTE: A successful digestion will leave almost no solid chunks.
- Ventricular dissection
- Quickly chop the ventricular tissue into tiny pieces using dissection scissors and pipette up and down to dissolve. Use another 1,000 µL pipette tip for pipetting up and down, it may be shortened to widen the opening.
- Transfer cell/tissue-solution into a 15 mL centrifuge tube and add an equivalent amount of stop buffer (3 mL) by carefully pipetting down at the side of the tube to end the reaction.
- Carefully pass all 6 mL of cell/tissue-solution through a 200 µm nylon mesh to remove larger tissue pieces that have not been fully digested.
NOTE: A successful digestion will leave almost no solid chunks.
- Leave both tubes (atrial and ventricular cell suspension) on the bench at room temperature for 6 min to settle.
- Centrifuge both 15 mL tubes at 5 x g for 2 min.
4. Calcium reintroduction
NOTE: The following steps are identical for both atrial and ventricular cells (unless otherwise mentioned) and are performed at room temperature.
- Discard the supernatant using a plastic Pasteur pipette and carefully resuspend pellet in 10 mL of calcium free Tyrode solution.
- Leave cells for 8 min for sedimentation.
- Centrifuge at 5 x g for 1 min (only atrial cells, sedimentation is enough for ventricular cells).
- Discard supernatant and carefully resuspend pellet in 10 mL of Tyrode solution with 100 µM calcium concentration.
- Leave cells for 8 min for sedimentation.
- Centrifuge at 5 x g for 1 min (only atrial cells, sedimentation is enough for ventricular cells).
- Discard the supernatant and carefully resuspend pellet in 10 mL of Tyrode solution with 400 µM calcium concentration.
- Leave cells for 8 min for sedimentation.
- Centrifuge at 5 x g for 1 min (only atrial cells, sedimentation is enough for ventricular cells).
- Discard the supernatant and carefully resuspend the pellet in 1 mL (atrial)/ 5 mL (ventricular) of Tyrode solution with 1 mM calcium concentration.
5. Loading of myocytes with fluorescent calcium-indicator Fluo-3 AM
NOTE: Due to the light sensitivity of the fluorescent calcium indicator, the following steps should be executed protected from light (e.g., by covering tubes with aluminium foil).
- Prepare Fluo-3 AM stock solution by adding 44 µL of 20% pluronic F-127 in anhydrous DMSO to 50 µg of Fluo-3AM (can be stored at -20 °C protected from light).
- Add 10 µL of Fluo-3 AM stock solution to 1 mL of cell suspension and incubate for 10 min at room temperature protected from light.
- Centrifuge at 5 x g for 1 min.
- Discard the supernatant using a plastic Pasteur pipette and resuspend the pellet in a reasonable amount of bath solution to obtain a good working concentration (1-5 mL of bath solution depending on the cell density).
- Leave for 30 min for de-esterification before starting with experiments.
6. Simultaneous patch-clamp and epifluorescent Ca2+-transient measurements as previously described16
NOTE: Patch clamp measurements are not the topic of this article, the interested reader may be referred to major publications providing in depth descriptions of this method17,18,19,20,21,22. Nevertheless, for a better overall understanding, a summary on a protocol to measure L-type calcium currents along with current induced calcium transients is provided.
- Transfer myocytes into a cell chamber and superfuse with bath solution at 37 °C.
- Block potassium currents by adding 4-aminopyridine and barium chloride to the bath solution as indicated in Table 5.
- Ensure that borosilicate microelectrodes have a tip resistance of 2-5 MΩ filled with pipette solution (Table 6).
- Setup measurements to allow for simultaneous recording of both electrical signals and epifluorescence at the same time. Voltage clamp mode is used to measure L-type Ca2+-current with a protocol holding the cell at -80 mV and a 600 ms ramp-pulse to -40 mV to inactivate the fast Na+-current, followed by a 100 ms test-pulse to +10 mV at 0.5 Hz (Figure 2).
- Use excitation at 488 nm, emitted light at <520 nm to detected and convert to [Ca2+]I assuming
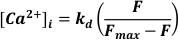
Where kd = dissociation constant of Fluo-3 (864 nM), F = Fluo-3 fluorescence; Fmax = Ca2+-saturated fluorescence obtained at the end of each experiment19.
Results
Isolation yield is determined after calcium reintroduction by pipetting 10 µL of cell suspension onto a microscope slide. More than 100 viable, rod-shaped, non-contracting cells/10 µL for atrial cell isolation and more than 1,000 viable, rod-shaped, non-contracting cells/10 µL for ventricular cell isolation are considered as sufficient yield and are commonly obtained using this protocol. Atrial cells obtained with this protocol showed a variety of different cell types containing cells of the cardiac conduc...
Discussion
This article provides an easy and functional way to obtain high quality atrial and ventricular myocytes from the same mouse for patch-clamp studies with simultaneous calcium transient recordings. The quality of the obtained data highly depends on the quality of the cell isolation. As mentioned above, many methods to isolate murine cardiomyocytes have been described previously9,10,11,12. The iso...
Disclosures
None
Acknowledgements
This work was supported by German Research Foundation (DFG; Clinician Scientist Program In Vascular Medicine (PRIME), MA 2186/14-1 to P. Tomsits and D. Schüttler; VO1568/3-1, IRTG1816, and SFB1002 project A13 to N. Voigt), German Research Foundation under Germany’s Excellence Strategy (EXC 2067/1- 390729940 to N. Voigt), German Centre for Cardiovascular Research (DZHK; 81X2600255 to S. Clauss and N. Voigt; 81Z0600206 to S. Kääb), the Corona Foundation (S199/10079/2019 to S. Clauss), the ERA-NET on Cardiovascular Diseases (ERA-CVD; 01KL1910 to S. Clauss), the Heinrich-and-Lotte-Mühlfenzl Stiftung (to S. Clauss) and the Else-Kröner-Fresenius Foundation (EKFS 2016_A20 to N. Voigt). The funders had no role in manuscript preparation.
Materials
| Name | Company | Catalog Number | Comments |
| 2,3-Butanedione monoxime | Sigma-Aldrich | 31550 | |
| 27G cannula | Servoprax | L10220 | |
| 4-Aminopyridine | Sigma-Aldrich | A78403 | |
| Anhydrous DMSO | Sigma-Aldrich | D12345 | |
| Aortic cannula | Radnoti | 130163-20 | |
| BaCl2 | Sigma-Aldrich | 342920 | |
| blunt surgical forceps | Kent Scientific | INS650915-4 | |
| Bovine Calf Serum | Sigma-Aldrich | 12133C | |
| CaCl2 | Sigma-Aldrich | C5080 | |
| Circulating heated water bath | Julabo | ME | |
| Collagenase Type II | Worthington | LS994177 | |
| disscetion scissors | Kent Scientific | INS600124 | |
| DL-aspartat K+-salt | Sigma-Aldrich | A2025 | |
| EGTA | Sigma-Aldrich | E4378 | |
| Fluo-3 | Invitrogen | F3715 | |
| Fluo-3 AM | Invitrogen | F1242 | |
| Glucose | Sigma-Aldrich | G8270 | |
| Guanosine 5′-triphosphate tris salt | Sigma-Aldrich | G9002 | |
| Heating coil | Radnoti | 158821 | |
| Heparin | Ratiopharm | 25.000 IE/5ml | |
| HEPES | Sigma-Aldrich | H9136 | |
| induction chamber | CWE incorporated | 13-40020 | |
| Isoflurane | Cp-pharma | 1214 | |
| Jacketed heart chamber | Radnoti | 130160 | |
| KCl | Merck | 1049360250 | |
| KH2PO4 | Sigma-Aldrich | P5655 | |
| MgCl x 6H2O | Sigma-Aldrich | M0250 | |
| MgSO4 x 7H2O | Sigma-Aldrich | M9397 | |
| Na2ATP | Sigma-Aldrich | A2383 | |
| Na2HPO4 x 2H2O | Sigma-Aldrich | S5136 | |
| NaCl | Sigma-Aldrich | S3014 | |
| NaHCO3 | Sigma-Aldrich | S5761 | |
| Nylon mesh (200 µm) | VWR-Germany | 510-9527 | |
| pasteur pipette | Sigma Aldrich | Z331759 | |
| petri-dishes | Thermo Fisher | 150318 | |
| Pluronic Acid F-127 | Sigma-Aldrich | P2443 | |
| Probenecid | Sigma-Aldrich | P8761 | |
| Roller Pump | Ismatec | ISM597D | |
| surgical forceps | Kent Scientific | INS650908-4 | |
| surgical scissors | Kent Scientific | INS700540 | |
| suturing silk | Fine Science Tools | NC9416241 | |
| syringe | Merck | Z683531-100EA | |
| Taurin | Sigma-Aldrich | 86330 |
References
- Camm, A. J., et al. Guidelines for the management of atrial fibrillation: the Task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace. 12 (10), 1360-1420 (2010).
- Chugh, S. S., et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 129 (8), 837-847 (2014).
- Tonchev, I., Luria, D., Orenstein, D., Lotan, C., Biton, Y. For whom the bell tolls : Refining risk assessment for sudden cardiac death. Current Cardiology Reports. 21 (9), 106 (2019).
- Kirchhof, P., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal. 37 (38), 2893-2962 (2016).
- Dobrev, D., Nattel, S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 375 (9721), 1212-1223 (2010).
- Heijman, J., Voigt, N., Carlsson, L. G., Dobrev, D. Cardiac safety assays. Current opinion in pharmacology. 15, 16-21 (2014).
- Schüttler, D., et al. Animal models of atrial fibrillation. Circulation Research. 127 (1), 91-110 (2020).
- Clauss, S., et al. Animal models of arrhythmia: classic electrophysiology to genetically modified large animals. Nature Reviews. Cardiology. 16 (8), 457-475 (2019).
- Voigt, N., Pearman, C. M., Dobrev, D., Dibb, K. M. Methods for isolating atrial cells from large mammals and humans. Journal of Molecular and Cellular Cardiology. 86, 187-198 (2015).
- Jansen, H. J., Rose, R. A. Isolation of atrial myocytes from adult mice. Journal of Visualized Experiments. (149), e59588 (2019).
- Blackwood, E. A., Bilal, A. S., Azizi, K., Sarakki, A., Glembotski, C. C. Simultaneous isolation and culture of atrial myocytes, ventricular myocytes, and non-myocytes from an adult mouse heart. Journal of Visualized Experiments. (160), e61224 (2020).
- Omatsu-Kanbe, M., Yoshioka, K., Fukunaga, R., Sagawa, H., Matsuura, H. A simple antegrade perfusion method for isolating viable single cardiomyocytes from neonatal to aged mice. Physiological Report. 6 (9), 13688 (2018).
- Köhncke, C., et al. Isolation and Kv channel recordings in murine atrial and ventricular cardiomyocytes. Journal of Visualized Experiments. (73), e50145 (2013).
- Brandenburg, S., et al. Axial tubule junctions activate atrial Ca(2+) release across species. Frontiers in Physiology. 9, 1227 (2018).
- Hofhuis, J., et al. Dysferlin links excitation-contraction coupling to structure and maintenance of the cardiac transverse-axial tubule system. Europace. 22 (7), 1119-1131 (2020).
- Voigt, N., Zhou, X. B., Dobrev, D. Isolation of human atrial myocytes for simultaneous measurements of Ca2+ transients and membrane currents. Journal of Visualized Experiment. (77), e50235 (2013).
- Voigt, N., Makary, S., Nattel, S., Dobrev, D. Voltage-clamp-based methods for the detection of constitutively active acetylcholine-gated I(K,ACh) channels in the diseased heart. Methods in Enzymology. 484, 653-675 (2010).
- Voigt, N., Nattel, S., Dobrev, D. Proarrhythmic atrial calcium cycling in the diseased heart. Advances in Experimental Medicine and Biology. 740, 1175-1191 (2012).
- Trafford, A. W., Díaz, M. E., Eisner, D. A. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Archiv: European Journal of Physiology. 437 (3), 501-503 (1999).
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. European Journal of Physiology. 391 (2), 85-100 (1981).
- Voigt, N., et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 125 (17), 2059-2070 (2012).
- Fakuade, F. E., et al. Altered atrial cytosolic calcium handling contributes to the development of postoperative atrial fibrillation. Cardiovascular Research. , (2020).
- Chen, W., Frangogiannis, N. G. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Failure Reviews. 15 (5), 415-422 (2010).
- Plačkić, J., Kockskämper, J. Isolation of atrial and ventricular cardiomyocytes for in vitro studies. Methods in Molecular Biology. 1816, 39-54 (2018).
- Díaz, M. E., Trafford, A. W., Eisner, D. A. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophysical Journal. 80 (4), 1915-1925 (2001).
- Zimmerman, A. N., Hülsmann, W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 211 (5049), 646-647 (1966).
- Chen, X., O'Connell, T. D., Xiang, Y. K. With or without Langendorff: A new method for adult myocyte isolation to be tested with time. Circulation Research. 119 (8), 888-890 (2016).
- Kappadan, V., et al. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. blebbistatin-uncoupled isolated rabbit hearts. Frontiers in Physiology. 11, 464 (2020).
- Brack, K. E., Narang, R., Winter, J., Ng, G. A. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Experiment in Physiology. 98 (5), 1009-1027 (2013).
- Seibertz, F., Reynolds, M., Voigt, N. Single-cell optical action potential measurement in human induced pluripotent stem cell-derived cardiomyocytes. Journal of Visual Experiment. , e61890 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved