A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Utilizing Percutaneous Ventricular Assist Devices in Acute Myocardial Infarction Complicated by Cardiogenic Shock
In This Article
Summary
Percutaneous ventricular assist devices are increasingly being utilized in patients with acute myocardial infarction and cardiogenic shock. Herein, we discuss the mechanism of action and hemodynamic effects of such devices. We also review algorithms and best practices for the implantation, management and weaning of these complex devices.
Abstract
Cardiogenic shock is defined as persistent hypotension, accompanied by evidence of end organ hypo-perfusion. Percutaneous ventricular assist devices (PVADs) are used for the treatment of cardiogenic shock in an effort to improve hemodynamics. Impella is currently the most common PVAD and actively pumps blood from the left ventricle into the aorta. PVADs unload the left ventricle, increase cardiac output and improve coronary perfusion. PVADs are typically placed in the cardiac catheterization laboratory under fluoroscopic guidance via the femoral artery when feasible. In cases of severe peripheral arterial disease, PVADs can be implanted through an alternative access. In this article, we summarize the mechanism of action of PVAD and the data supporting their use in the treatment of cardiogenic shock.
Introduction
Cardiogenic shock (CS) is defined as persistent hypotension (systolic blood pressure <90 mmHg for >30 minutes, or the need for vasopressors or inotropes), end-organ hypo-perfusion (urine output <30 mL/h, cool extremities or lactate > 2 mmol/L), pulmonary congestion (pulmonary capillary wedge pressure (PCWP) ≥ 15 mmHg) and decrease cardiac performance (cardiac index <2.2 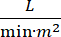 )1,2 due to a primary cardiac disorder. Acute myocardial infarction (AMI) is the most common cause of CS3. CS occurs in 5-10% of AMI and historically has been associated with significant mortality3,4. Mechanical circulatory support (MCS) devices such as intra-aortic balloon pump (IABP), percutaneous ventricular assist devices (PVAD), extracorporeal membrane oxygenation (ECMO) and percutaneous left atrial to aortic devices are frequently used in patients with CS5. Routine use of IABP has demonstrated no improvement in clinical outcomes or survival in AMI-CS1. Given the poor outcomes associated with AMI-CS, the difficulties in conducting trials in AMI-CS, and the negative results of IABP use in AMI-CS, clinicians are increasingly looking to other forms of MCS.
)1,2 due to a primary cardiac disorder. Acute myocardial infarction (AMI) is the most common cause of CS3. CS occurs in 5-10% of AMI and historically has been associated with significant mortality3,4. Mechanical circulatory support (MCS) devices such as intra-aortic balloon pump (IABP), percutaneous ventricular assist devices (PVAD), extracorporeal membrane oxygenation (ECMO) and percutaneous left atrial to aortic devices are frequently used in patients with CS5. Routine use of IABP has demonstrated no improvement in clinical outcomes or survival in AMI-CS1. Given the poor outcomes associated with AMI-CS, the difficulties in conducting trials in AMI-CS, and the negative results of IABP use in AMI-CS, clinicians are increasingly looking to other forms of MCS.
PVADs are increasingly utilized in patients with AMI-CS6. In this article, we will focus our discussion primarily on the Impella CP, which is the most common PVAD used currently6. This device utilizes an axial flow Archimedes-screw pump which actively and continuously propels blood from the left ventricle (LV) into the ascending aorta (Figure 1). The device is most frequently placed in the cardiac catheterization laboratory under fluoroscopic guidance via the femoral artery. Alternatively, it can be implanted through an axillary or transcaval access when necessary7,8.
Protocol
This protocol is the standard of care in our institution.
1. Insertion of the PVAD (e.g., Impella CP)
- Obtain common femoral access over the lower half of the femoral head under fluoroscopic and ultrasound guidance using a micro-puncture needle9,10. Position the micro-puncture sheath and obtain an angiogram of the femoral artery to confirm appropriate arteriotomy location11.
- Insert a 6 Fr sheath in the femoral artery.
- If there is concern for ilio-femoral disease, insert a pigtail catheter in the inferior portion of the abdominal aorta and perform an angiogram of the iliofemoral system to ensure there is no significant peripheral artery disease (PAD) that may preclude PVAD insertion. If there is moderate disease or calcification of the iliac arteries consider using a longer 25 cm 14 French sheath so that the tip of the sheath is in a relatively healthy segment of the abdominal aorta.
- Serially dilate the arteriotomy site over a stiff .035" wire using 8, 10 and 12 Fr dilators sequentially. Then, insert the 14 Fr peel away sheath under fluoroscopic guidance, ensuring the tip advances without resistance.
- Administer heparin bolus (~100 U/kg body weight) for an ACT goal of 250 to 300 s. Alternative anticoagulation include bivalirudin and argatroban.
- Use a pigtail catheter to cross into the LV using a .035" J tipped wire. Remove the J wire and check an LVEDP.
- Shape the tip of the exchange length 0.018" wire included in the kit and insert it into the LV so that it forms a stable curve at the LV apex.
- Make sure ACT is at goal (250 to 300 s) before insertion12,13.
- Remove the pigtail catheter and insert the pump by loading the wire on the pre-assembled loading red lumen (e.g., EasyGuide) until it exits near the label.
- Remove the loading red lumen by gently pulling on the label while holding the catheter.
- Advance the device in small increments under fluoroscopic guidance into the LV over the 0.018" wire.
- Position the pump in the LV with its inlet 4 cm below the aortic valve and make sure it is free from the mitral chordae. Being too close to the apex can cause PVCs and trigger "suction alarms". Remove the .018" wire and once removed, start the pump. Remove excess slack so the pump rests against the lesser curvature of the aorta.
- Monitor the console to make sure the motor current is pulsatile and aortic waveform is displayed. If a ventricular waveform is displayed, the pump may need to be pulled back.
- If the device needs to be left in situ, remove the peal-away sheath and insert the repositioning sheath pre-loaded on the device.
- Check the device position on fluoroscopy and the waveforms on the console again.
- Palpate (or sense with Doppler) the distal lower extremity arterial pulses including dorsalis pedis and posterior tibial prior to and after insertion of the device. Document this appropriately in the patient's medical record.
- If pulses or dopplers cannot be obtained, consider taking a lower extremity angiogram using the wire re-introducing port located on the side of the device or using another access to ensure non-obstructive flow to the lower limb.
- If flow is obstructed, place a reperfusion sheath prior to transferring the patient to the CCU. In patients with PAD who are at high risk for obstructive flow, strongly consider inserting the reperfusion sheath prior to placement of the 14 Fr sheath (i.e., after step 1.4 listed above).
- Monitor patients treated with a PVAD in the critical care unit (CCU) by personnel trained in its use.
2. Post-procedural care
- Apply sterile dressing.
- Position the device at a 45° angle when entering the skin (gauze underneath the repositioning sheath can be helpful to maintain this angle). Failure to do so may result in the arteriotomy oozing, leading to formation of a hematoma. It is also helpful to place sutures with forward pressure to avoid device migration and to prevent bleeding.
NOTE: Securing the lower extremity with a knee immobilizer can also limit device migration as a reminder to patient not to bend/move the effected limb. This should not be fastened too tightly so as not to compromise circulation. - Continue to perform routine pulse checks (palpable or Doppler).
3. Positioning
- Use bedside transthoracic echocardiogram to confirm appropriate device position either prior to transfer or immediately on arrival to the cardiac ICU, depending on availability of a point of care ultrasound.
- Use a parasternal long axis view to assess device position. A subxyphoid view may also be used if parasternal long axis view is not obtainable. A measurement from aortic valve to the device inlet should ideally be 3-4 cm for proper positioning of the device.
- Use echocardiograms to note the position of the device as it relates to the mitral valve.
- When a device needs to be repositioned, turn down the device to P2, unscrew the locking mechanism on the sterile cover to advance or retract the device. One can torque as advancing or retracting if the pigtail or inlet is too close to the mitral valve.
- Lock the device in the new position and document the new position.
- Following this, increase the device to the desired level of support.
- After increasing the level of support, reevaluate the device position as the device can jump forward when speed increases.
NOTE: If the device has been pulled back across the aortic valve, repositioning is better done in the cath lab under fluoroscopy guidance.
4. Weaning
- Consider weaning when vasopressors/inotropes are at low doses or completely weaned off. Hemodynamics should be continuously monitored to maintain a CPO > 0.6 W. Carefully monitor right ventricular (RV) hemodynamics with a goal to maintain right atrial pressure (RAP) <12 mmHg and pulmonary artery pulsatility index (PAPI) >1.014. Also consider obtaining pH, mixed venous saturations and lactate every 2-6 hours to monitor cardiac work and end-organ perfusion.
- Decrease power by 1-2 levels over 2 hours, noting CPO, PAPI, RAP, MAP and urine output. If CPO drops <0.6 W, RAP begins to increase, urine output drops > 20 mL/h or MAP <60 mmHg, increase power to previous level.
5. Removal12
- Use vascular closure devices to close the arteriotomy access site with complete deployment of the device performed when the large bore sheath is removed14. Temporary endovascular balloon tamponade or "dry field closure technique" is an effective and safe way to ensure hemostasis of the large bore access site15.
- Dial down to P1 and pull back the device into the aorta followed by change to P0 and disconnect the device from the console as the catheter is pulled out of the body.
- Note that the device should not be left across the aortic valve at P0 due to the risk of aortic regurgitation.
- If considering manual hemostasis, wait until ACT <150 and hold 3 minutes of pressure per French size.
Results
Table 1 shows the safety and efficacy of PVAD implantation35,36,37,38,39,40.
Optimizing PVAD Outcomes
PVADs are a resource-heavy intervention that requires significant experience and expertise to optimize outcomes. The following best practices should be consi...
Discussion
Minimizing the Risks and Complications of PVAD (Table 2)
The hemodynamic benefits of PVAD can be significantly neutralized if complications from large-bore access occur, such as major bleeding and acute limb ischemia28,29. It is thus essential to minimize the risk and complications of the device.
In order to decrease access site complications and reduce the number of access attempts, ultrasound and fluoroscopic g...
Disclosures
Dr. Aditya Bharadwaj is a consultant, proctor, and member of the Speakers Bureau for Abiomed.
Dr. Mir Basir is a consultant for Abbott Vascular, Abiomed, Cardiovascular System, Chiesi, Procyrion and Zoll.
Acknowledgements
None
Materials
| Name | Company | Catalog Number | Comments |
| 4 Fr-018-10 cm Silhouette Stiffened Micropuncture Set | Cook | G48002 | Microvascular access |
| 5 Fr Infiniti Pigtail Catheter | Cordis | 524-550S | pigtail catheter |
| Impella CP Intra-cardiac Assist Catheter | ABIOMED | 0048-0003 | Impella catheter kit |
References
- Holger, T., et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction. Circulation. 139 (3), 395-403 (2019).
- Hochman, J. S., et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. New England Journal of Medicine. 341 (9), 625-634 (1999).
- van Diepen, S., et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 136 (16), 232-268 (2017).
- Kolte, D. h. a. v. a. l., et al. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. Journal of the American Heart Association. 3 (1), 000590 (2014).
- Aditya, M., Sunil, R. V. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock. Circulation: Cardiovascular Interventions. 10 (5), 004337 (2017).
- Amit, A. P., et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation. 141 (4), 273-284 (2020).
- Kajy, M., et al. Deploying Mechanical Circulatory Support Via the Axillary Artery in Cardiogenic Shock and High-Risk Percutaneous Coronary Intervention. The American Journal of Cardiology. 128, 127-133 (2020).
- Afana, M., et al. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheterization and Cardiovascular Interventions. , 29235 (2020).
- Sandoval, Y., et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC: Cardiovascular Interventions. 10 (22), 2233-2241 (2017).
- Seto, A. H., et al. Real-Time Ultrasound Guidance Facilitates Femoral Arterial Access and Reduces Vascular Complications. JACC: Cardiovascular Interventions. 3 (7), 751-758 (2010).
- Mignatti, A., Friedmann, P., Slovut, D. P. Targeting the safe zone: A quality improvement project to reduce vascular access complications: Vascular Access Complications Postcardiac Catheterization. Catheterization and Cardiovascular Interventions. 91 (1), 27-32 (2018).
- Rihal, C. S., et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. Journal of the American College of Cardiology. 65 (19), 7-26 (2015).
- Burzotta, F., et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. International Journal of Cardiology. 201, 684-691 (2015).
- Basir, M. B., et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheterization and Cardiovascular Interventions. 93 (7), 1173-1183 (2019).
- Kaki, A., et al. Access and closure management of large bore femoral arterial access. Journal of Interventional Cardiology. 31 (6), 969-977 (2018).
- Basir, M. B., et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. The American Journal of Cardiology. 119 (6), 845-851 (2017).
- Tehrani, B. N., et al. Standardized Team-Based Care for Cardiogenic Shock. Journal of the American College of Cardiology. 73 (13), 1659-1669 (2019).
- Ouweneel, D. M., et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. Journal of the American College of Cardiology. 69 (3), 278-287 (2017).
- Alushi, B., et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. 6 (1), 000987 (2019).
- Ginwalla, M., Tofovic, D. S. Current Status of Inotropes in Heart Failure. Heart Failure Clinics. 14 (4), 601-616 (2018).
- O'Neill, W. W., et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. American Heart Journal. 202, 33-38 (2018).
- O'neill, W. W., et al. The Current Use of Impella 2.5 in Acute Myocardial Infarction Complicated by Cardiogenic Shock: Results from the USpella Registry. Journal of Interventional Cardiology. 27 (1), 1-11 (2014).
- Hernandez, G. A., et al. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. Journal of Cardiac Failure. 25 (5), 364-371 (2019).
- Thayer, K., et al. Pulmonary Artery Catheter Usage and Mortality in Cardiogenic Shock. The Journal of Heart and Lung Transplantation. 39 (4), 54-55 (2020).
- Fincke, R., et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. Journal of the American College of Cardiology. 44 (2), 340-348 (2004).
- Lim, H. S., Gustafsson, F. Pulmonary artery pulsatility index: physiological basis and clinical application. European Journal of Heart Failure. 22 (1), 32-38 (2020).
- Korabathina, R., et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheterization and Cardiovascular Interventions. 80 (4), 593-600 (2012).
- Lauten, A., et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circulation. Heart Failure. 6 (1), 23-30 (2013).
- Dixon, S. R., et al. A Prospective Feasibility Trial Investigating the Use of the Impella 2.5 System in Patients Undergoing High-Risk Percutaneous Coronary Intervention (The PROTECT I Trial): Initial U.S. Experience. JACC: Cardiovascular Interventions. 2 (2), 91-96 (2009).
- Abu-Fadel, M. S., et al. Fluoroscopy vs. Traditional guided femoral arterial access and the use of closure devices: A randomized controlled trial. Catheterization and Cardiovascular Interventions. 74 (4), 533-539 (2009).
- Lata, K., Kaki, A., Grines, C., Blank, N., Elder, M., Schreiber, T. Pre-close technique of percutaneous closure for delayed hemostasis of large-bore femoral sheaths. Journal of Interventional Cardiology. 31 (4), 504-510 (2018).
- Basir, M. B., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheterization and Cardiovascular Interventions. 91 (3), 454-461 (2018).
- Udesen, N. J., et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. American Heart Journal. 214, 60-68 (2019).
- Clinical Research. Protected PCI Community Available from: https://www.protectedpci.com/clinical-research/ (2020)
- Seyfarth, M., et al. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. Journal of the American College of Cardiology. 52 (19), 1584-1588 (2008).
- Schrage, B., et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation. 139 (10), 1249-1258 (2019).
- Casassus, F., et al. The use of Impella 2.5 in severe refractory cardiogenic shock complicating an acute myocardial infarction. Journal of Interventional Cardiology. 28 (1), 41-50 (2015).
- Joseph, S. M., Brisco, M. A., Colvin, M., Grady, K. L., Walsh, M. N., Cook, J. L. Women With Cardiogenic Shock Derive Greater Benefit From Early Mechanical Circulatory Support: An Update From the cVAD Registry. Journal of Interventional Cardiology. 29 (3), 248-256 (2016).
- Lauten, A., et al. Percutaneous Left-Ventricular Support With the Impella-2.5-Assist Device in Acute Cardiogenic Shock. Circulation: Heart Failure. 6 (1), 23-30 (2013).
- Ouweneel, D. M., et al. Impella CP Versus Intra-Aortic Balloon Pump in Acute Myocardial Infarction Complicated by Cardiogenic Shock: The IMPRESS trial. Journal of the American College of Cardiology. , 23127 (2016).
- Badiye, A. P., Hernandez, G. A., Novoa, I., Chaparro, S. V. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO Journal. 62 (1), 11-14 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved