A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Production of Membrane-Filtered Phase-Shift Decafluorobutane Nanodroplets from Preformed Microbubbles
In This Article
Summary
This protocol describes a method of generating large volumes of lipid encapsulated decafluorobutane microbubbles using probe-tip sonication and subsequently condensing them into phase-shift nanodroplets using high-pressure extrusion and mechanical filtration.
Abstract
There are many methods that can be used for the production of vaporizable phase-shift droplets for imaging and therapy. Each method utilizes different techniques and varies in price, materials, and purpose. Many of these fabrication methods result in polydisperse populations with non-uniform activation thresholds. Additionally, controlling the droplet sizes typically requires stable perfluorocarbon liquids with high activation thresholds that are not practical in vivo. Producing uniform droplet sizes using low-boiling point gases would be beneficial for in vivo imaging and therapy experiments. This article describes a simple and economical method for the formation of size-filtered lipid-stabilized phase-shift nanodroplets with low-boiling point decafluorobutane (DFB). A common method of generating lipid microbubbles is described, in addition to a novel method of condensing them with high-pressure extrusion in a single step. This method is designed to save time, maximize efficiency, and generate larger volumes of microbubble and nanodroplet solutions for a wide variety of applications using common laboratory equipment found in many biological laboratories.
Introduction
Ultrasound contrast agents (UCAs) are rapidly growing in popularity for imaging and therapy applications. Microbubbles, the original UCAs, are currently the mainstream agents used in clinical diagnostic applications. Microbubbles are gas-filled spheres, typically 1-10 µm in diameter, surrounded by lipid, protein, or polymer shells1. However, their size and in vivo stability can limit their functionality in many applications. Phase-shift nanodroplets, which contain a superheated liquid core, can overcome some of these limitations due to their smaller size and improved circulation-life2. When exposed to heat or acoustic energy, the superheated liquid core vaporizes to form a gas microbubble2,3,4,5. Since the vaporization threshold is directly related to droplet size5,6, formulating droplet suspensions with uniform size would be highly desirable for achieving consistent activation thresholds. Formulation methods that produce uniform droplet sizes are often complex and costly, whereas more cost-effective approaches result in polydisperse solutions7. Another limitation is the ability to generate stable phase-shift droplets with low-boiling point perfluorocarbon (PFC) gases, which is critical for efficient activation in vivo8. In this manuscript, a protocol is described for generating stable filtered low-boiling point vaporizable phase-shift droplets for in vivo imaging and therapy applications.
There are many methods of producing monodispersed submicron phase-shift droplets7. One of the most robust methods of controlling size is the use of microfluidic devices. These devices can be costly, have slow rates of droplet production (~104-106 droplets/s)7, and require extensive training. Microfluidic devices also generally require high-boiling point gases to avoid spontaneous vaporization and clogging of the system7. However, a recent study by de Gracia Lux et al.9 demonstrates how cooling a microfluidizer can be used to generate high concentrations of sub-micron phase-shift (1010-1012/mL) using low-boiling point decafluorobutane (DFB) or octafluoropropane (OFP).
In general, low-boiling point gases such as DFB or OFP are easier to handle using preformed gas bubbles. Vaporizable droplets can be produced from precursor lipid-stabilized bubbles by condensing the gas using low temperatures and elevated pressure5,10. The concentration of droplets produced using this method depends on precursor microbubble concentration and efficiency of conversion of bubbles to droplets. Concentrated microbubbles have been reported from tip sonication approaching > 1010 MB/mL11, while a separate study has reported droplet concentrations ranging from ~1-3 x1011 droplets/mL from condensed OFP and DFP bubbles12. When monodispersed droplets are not a concern, condensation methods are the most straightforward and lowest-cost methods of generating lipid-stabilized phase-shift droplets using low-boiling point PFCs. Methods of generating uniform size bubbles before condensing can help create more monodisperse populations of droplets. However, generating monodisperse precursor bubbles is also difficult, requiring more costly approaches such as microfluidics or repeated differential centrifugation techniques11. An alternative approach to producing DFB and OFB nanodroplets has recently been published using spontaneous nucleation of droplets in liposomes13. This method, utilizing an "Ouzo" effect, is a simple way to generate low-boiling point PFC droplets without needing to condense bubbles. The size-distribution of the PFC droplets can be controlled by delicate titration and mixing PFC, lipid, and ethanol components used to initiate nucleation of the droplets. It is also worth noting that mixing of perfluorocarbons can be used to control stability and activation thresholds of nanodroplets14,15. More recent work by Shakya et al. demonstrates how nanodroplet activation can be tuned by emulsifying high boiling-point PFCs within a hydrocarbon endoskeleton to facilitate heterogenous nucleation within the droplet core16, which is an approach that can be considered along with other forms of droplet size filtration.
Once formed, phase-shift droplets can be extruded after formation to create more monodisperse populations. In fact, a similar protocol to the method described here has been published previously by Kopechek et al.17 using high boiling-point dodecofluorpentane (DDFP) as the droplet core. Readers seeking to use phase-shift droplets with high-boiling point perfluorocarbons (stable at room temperature) should reference the article above instead. Generating and extruding droplets with low boiling point gasses, such as DFB and OFP, is more complicated and is best approached by condensing preformed gas bubbles.
In this protocol, a common method of generating preformed lipid microbubbles with a DFB gas core using probe tip sonication is described. Next, a commercial extruder is used to condense preformed microbubbles into submicron phase-shift nanodroplets (Figure 1). The resulting droplets are then activatable by heat and ultrasound. This method can produce larger volumes of nanodroplet solution than conventional condensation methods with narrower size-distributions without the need for expensive microfluidic devices. The production of nanodroplet solutions with narrow size distributions can likely generate more uniform vaporization thresholds. This will maximize their potential for numerous applications such as imaging, ablation, drug delivery, and embolization1,3,4,6.
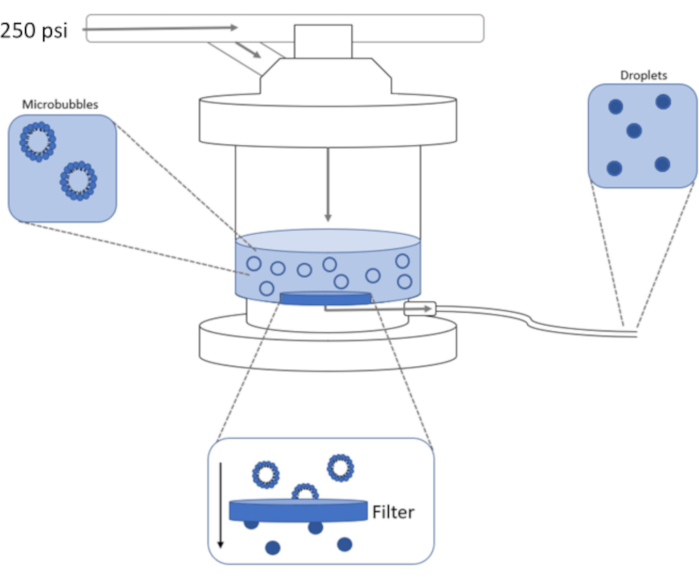
Figure 1: Schematic of high-pressure extrusion setup for condensing preformed microbubbles into phase-shift nanodroplets. Microbubble solution is added to and contained in the extruder chamber, and 250 psi, from the nitrogen tank, is applied through the chamber inlet valve. The nitrogen gas will push the microbubble solution through the filter at the base of the chamber, condensing the sample to nanodroplets. Solution is finally pushed out of extruder through the sample outlet tube and collected. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Making lipid films
- Prepare lipid films for microbubble generation using 90% DSPC and 10% DSPE-PEG2K by mixing the lipids at the correct ratio using the following directions:
- Make stock lipids of DSPC and DSPE-PEG2K in chloroform. Weigh 50 mg of each lipid powder in separate vials. Add 1 mL of chloroform to each vial using a 1 mL glass syringe.
- Add 287 µL of DSPC stock and 113 µL of DSPE-PEG2K stock (both 50 mg/mL) into a 20 mL scintillation vial using a glass syringe.
- Dry the mixed lipids to remove chloroform using nitrogen. Using an appropriate length of tubing connected to house nitrogen, lightly flow nitrogen gas over the headspace of the vial while mixing. Continue until no chloroform is observed, and the remaining lipid film starts to turn white. Use polypropylene screw caps, cover the sample while introducing nitrogen in the headspace.
- Place vials under vacuum overnight using a vacuum desiccator to remove any residual chloroform. A thin translucent film will remain that coats the bottom of the vial.
- Store vials at -20 °C until needed.
2. Generating microbubbles from lipid films
- To make the microbubbles, add 10 mL of 1x phosphate buffer saline (PBS) containing 20% v/v Propylene Glycol and 20% v/v Glycerol (final pH 7.2-7.4) to a dry lipid film.
- Re-cap the sample and warm sample to 65 °C for 30 min on a heating block (or heated water bath).
- While the sample is warming, prepare the bath sonicator by increasing the bath temperature to 65 °C.
NOTE: This process is faster if the water is preheated in a microwave or hotplate before placing in the bath sonicator. - Place the scintillation vial containing the warmed sample in the bath sonicator so that only the portion of the vial containing the lipid solution is submerged in the water bath.
- Sonicate the warm lipid solution for a minimum of 15 min. Ensure that the water temperature remains at 65 °C. Continue to sonicate in intervals of 10-15 min until the solution is completely clear (Figure 2).
NOTE: If a bath sonicator is not available, the solution can be tip sonicated at 10% power until clear. However, the microtip will wear out quicker and is more expensive to replace.
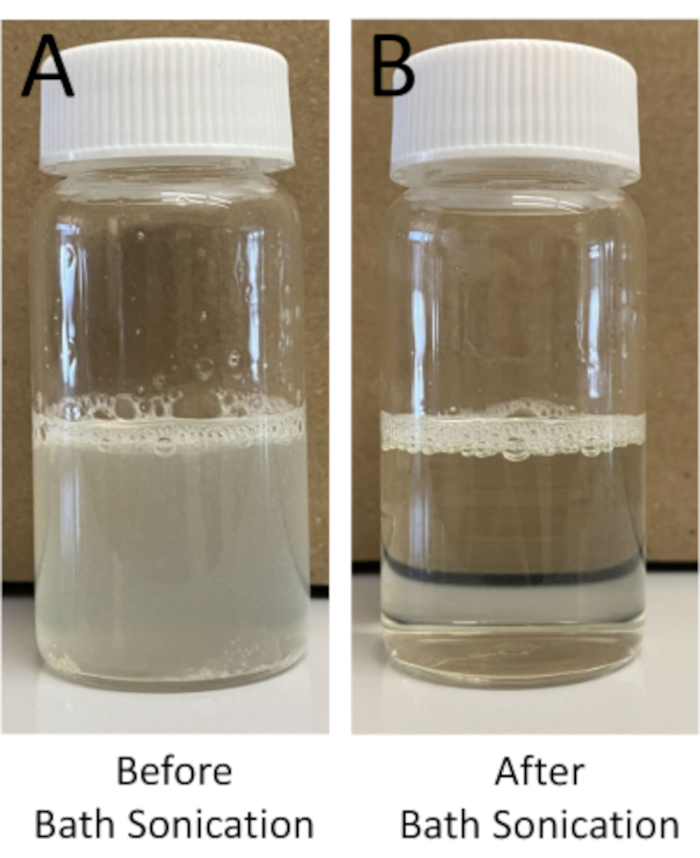
Figure 2: Example of hydrated lipid films. Example of hydrated lipid film (A) before and (B) after bath sonication to form uni-lamellar vesicles. Following bath sonication, the lipid solution should shift from a more opaque to translucent solution. Please click here to view a larger version of this figure.
- While still warm, remove the cap and clamp the vial into the sonicator's soundproof enclosure so that the microtip attachment of the sonicator is submerged just beneath the air/liquid interface (Figure 3).
- Place the tank of decafluorobutane next to the soundproof enclosure of the sonicator.
- Prepare an ice-bath and place it next to the soundproof enclosure. This will be used later in Step 2.14.
- Turn on the power switch for the sonicator.
- After the system starts, set the power level to 70%. Do not exceed 70% amplitude with the microtip attachment. Do not start the sonicator at this time.
- Attach an appropriate length of tubing to guide the gas from the DFB tank outlet into the warm lipid solution held in the enclosure. The tube should be placed just into the neck of the vial to allow gas to flow into the headspace during sonication (Figure 3).
- Open the tank valve slowly until the gas can be seen flowing over the lipid solution. This will cause slight ripples on the surface of the liquid. If the gas flow is too high, the solution will overflow during microbubble formulation.
- Start the sonicator and run for 10 s continuously to generate microbubbles. If the bubble solution starts to overflow during sonication, immediately stop the sonicator.
- Turn off the sonicator and immediately close the DFB tank valve.
- Quickly cap the microbubble solution and submerge the vial in the ice bath to cool the sample below 55 °C (glass transition temperature of DSPC)
- Leave the microbubble samples in the ice bath until needed.
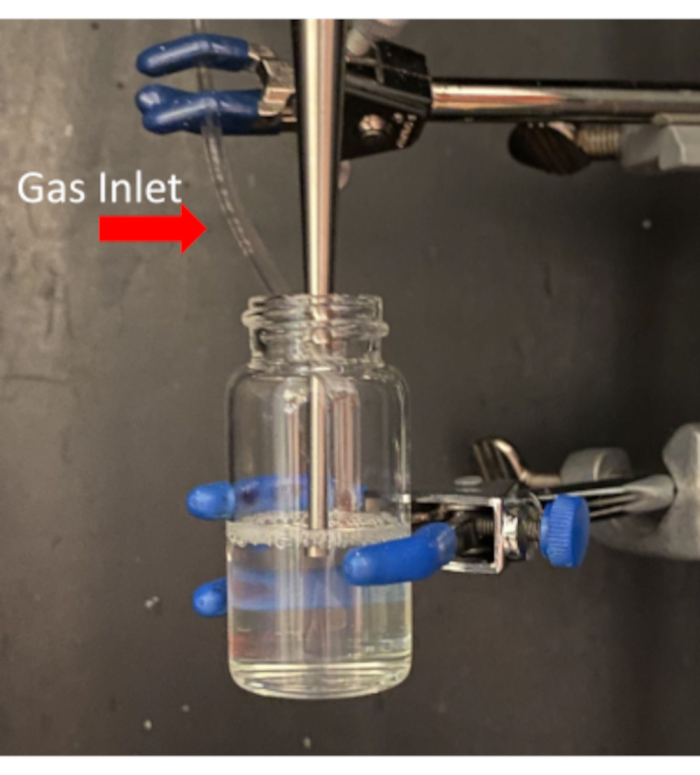
Figure 3: Placement of probe tip into lipid solution to optimize microbubble formation. Take care to not allow the tip of the probe to touch the glass. Please click here to view a larger version of this figure.
3. Preparing extruder for microbubble condensation
- Assemble high-pressure extruder as detailed in the user's manual using a 200 nm ceramic filter (supplied from manufacturer).
- Place the extruder in the center of a watertight container so that the sample outlet tube is not pressed against the side or crimped.
- Couple the extruder to the nitrogen gas tank using the adaptor supplied by the manufacturer.
- Make a -2 °C salted ice bath in the watertight container around the extruder using 400 mL of water and 10 g of sodium chloride.
- Place the end of the outlet tube in a scintillation vial to collect the extruded sample.
NOTE: Secure the tube to the container with tape if it does not lay flat or stay within the vial.
4. Priming the extruder for microbubble condensation
- Open and close the release valve to make sure there is no pressure within the extruder.
- Remove the chamber lid and add 5 mL of 1x PBS to the extruder chamber.
- Replace the lid making sure that it clicks securely back into place.
- Open the nitrogen gas tank so that the pressure gauge reads 250 psi. Make sure the pressure control valve is in the closed position.
- Close the gas tank and open the extruder chamber inlet valve. The PBS solution will be pushed through the system and out the sample outlet tube into the scintillation vial.
- When only gas is exiting the tubing, open the release valve and allow the pressure to fall to 0 psi.
- Remove the scintillation vial.
5. Pre-cooling microbubbles for extrusion
- Open and close the release valve to make sure there is no pressure within the extruder. Place a new scintillation vial at the end of the outlet tube.
- Fill a steel container with 2-methyl butane and add dry ice to bring the temperature down to -18 °C.
- Insert the microbubble solution into the chilled 2-methyl butane so the sample is submerged for 2 min. Move the scintillation vial throughout the 2 min to gently mix the bubbles. Add dry ice as needed to maintain the temperature between -15 and -18 °C. Be careful not to exceed -20 ˚C or the excipient solution will freeze and destroy the bubble sample.
NOTE: Steps 5.2 and 5.3 can also be done by cooling the bubble sample in a laboratory freezer over a more extended time period. However, caution should be used to carefully monitor the temperature of the freezer and avoid freezing the sample. - After 2 min, remove the microbubbles from the chilled 2-methyl butane, gently shake the vial to mix the microbubbles and use a chilled 10 mL syringe to transfer the solution to the extruder.
- Remove the extruder chamber lid and add the microbubble solution to the chamber by slowly pushing the plunger on the syringe. Replace the extruder cap making sure it clicks securely back in place.
- Verify that the pressure control valve and the release valve of the extruder are in the closed position.
- Open the nitrogen gas tank until the pressure gauge reads 250 psi, close the gas tank, and turn the pressure control valve to the open position.
- When the solution has filled the scintillation vial at the exit tubing, and only gas is exiting the tube, slowly open the pressure release valve and allow the pressure to fall to 0 psi.
- Place the scintillation vial in an ice bath or fridge for storage.
- For long-term storage and minimizing spontaneous vaporization, store the sample in a standard freezer. Ensure the temperature is -20 °C or higher to avoid freezing the sample (the excipient solution of 20% PPG and 20% Glycerol will keep the sample from freezing in most laboratory freezers).
6. Separating droplets from liposomes by centrifugation
- Transfer 10 mL of the extruded droplet solution to a 15 mL centrifuge tube.
- Centrifuge the extruded sample at 1,500 x g for 10 min at 4 °C. A pellet comprised of DFB nanodroplets will be apparent at the bottom of the tube (Figure 4). Spontaneously vaporized droplets will appear at the top of the solution and should be discarded.

Figure 4: Example of phase-shift DFB droplets pelleting after centrifugation. DFB nanodroplets are more dense than liposomes and will collect at the bottom of the centrifuge tube in a pellet, (red box). Please click here to view a larger version of this figure.
- Remove the supernatant and resuspend the pellet in 2 mL of 1x PBS with 20% glycerol and 20% propylene glycol.
- Mix the tube gently to obtain a homogeneous solution and transfer the droplets to a smaller 2 mL centrifuge tube.
- Wash sample two more times in 2 mL centrifuge tube.
- After the last wash, resuspend the pellet in 100 µL of 1x PBS with 20% glycerol and 20% propylene glycol and store on ice or in the freezer until needed.
7. Microscopy verification of droplet vaporization
- Make a diluted droplet solution by adding 2.5 µl of concentrated droplets to 7.5 µl of 1x PBS.
- Prepare a microscope slide with 10 µl of the diluted sample. Using a 40x objective, observe the sample and save images.
- Remove the slide from the microscope and place it on a 65 ˚C heat plate for 1 min to vaporize nanodroplets into microbubbles.
- Use the same 40x objective to observe the sample after heating to verify droplet vaporization.
Access restricted. Please log in or start a trial to view this content.
Results
Representative results of the size distribution are included using dynamic light scattering (DLS) and tunable resistive pulse sensing (TRSP) analysis. Figure 5 shows the size distribution of condensed bubble solutions with and without extrusion. Without extrusion, the protocol ends at step 5.3. The chilled bubbles are condensed by venting the sample to atmospheric pressure while cold. The condensed only sample has a much wider distribution centered near 400 nm. The extruded sample has a narr...
Access restricted. Please log in or start a trial to view this content.
Discussion
A comprehensive body of literature is available that discusses the formulation, physics, and potential applications of microbubbles and phase-shift droplets for in vivo imaging and therapy. This discussion pertains explicitly to generating lipid microbubbles and converting them into sub-micron phase-shift droplets using a low boiling point DFB gas and high-pressure extrusion. The method outlined here is meant to provide a relatively simple method of producing large amounts of lipid microbubbles and DFB phase-shift drople...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dominique James in Dr. Ken Hoyt's lab for providing TRSP analysis of vaporizable phase-shift nanodroplets
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL Centrifuge Tubes | Falcon | 352095 | Collecting and centrifuging droplets |
| 200 nm polycarbonate filter | Whatman | 110606 | Extruder filters |
| 2-methylbutane | Fisher Chemical | 03551-4 | Rapid precooling of microbubble solution prior to extrusion |
| 3-prong clamps X2 | Fisher | 02-217-002 | Holding scintilation vials in place for probe tip sonication |
| 400W Analog Probe Tip Sonicator with Horn | Branson | 101-063-198R | Used to generate lipid microbubbles from lipid solution |
| Bath Sonicator | Fisher Scientific | 15337402 | Used to help breakdown liposomes into unilamellar vesicles |
| Chloroform | Fisher Bioreagents | C298-4 | Used to make lipid film for microbubble preperation |
| Decafluorobutane (Perfluorobutane) Gas | FluoroMed L.P. | 1 kg | generating microbubbles via probe tip sonication |
| Dry Ice | - | - | Rapid precooling of microbubble solution prior to extrusion |
| DSPC Lipid Powder | NOF America | COATSOME MC-8080 | Component of lipid film |
| DSPE-PEG-2K Lipid Powder | NOF America | SUNBRIGHT DSPE-020CN | Component of lipid film |
| General Thermometer | - | - | Used to measure ice bath temperature and 2-methylbutane temperature ( needs to accommodate -20C temperatures) |
| Glass Syringes | Hamilton | 81139 | Used to mix lipids in chloroform |
| Glycerol | Fisher Bioreagents | BP229-1 | Reduces freezing temperature of PBS solution |
| Heating Block | VWR Scientific Products | Heating lipid films and vaporizing droplets | |
| Lipex 10 mL Extruder | Evonik | Commercial high-pressure extrusion system | |
| Mini Vortex Mixer | Fisher brand | 14-955-151 | Used to remove excess chloroform from lipid films |
| Nitrogen Tank | - | - | Used to operate extruder |
| Phosphate Buffer Saline | Fisher Scientific | Hydrate lipid films and washing droplets | |
| Polyester Drain Disk | Whatman | 230600 | Provides support for polycarbonate filter |
| Polypropylene Caps | Fisher Scientific | 298417 | Used for solution storage |
| Propylene Glycol | Fisher Chemical | P355-1 | Reduces freezing temperature of PBS solution |
| Scintiliation Vials | DWK Life Sciences Wheaton | 986532 | Used for lipid films and microbubble generation |
| Small hammer | - | - | Used to break apart dry ice for cooling methylbutane |
| Sonicator Microtip Attachment | Branson | 101148070 | Used to generate microbubbles from lipid solution |
| Steel Container | Medegen | 79310 | Rapid precooling of microbubble solution prior to extrusion ( any container rated to -20C will work) |
| Vacuume Dessicator | Bel-Art SP Scienceware | 08-648-100 | Removes excess chloroform from lipid films |
| 2mL Centrifuge Tube | Fisher | 02682004 | Used for concentrating nanodroplets |
References
- Sirsi, S., Borden, M. Microbubble compositions, properties and biomedical applications. Bubble Science Engineering and Technology. 1 (1-2), 3-17 (2009).
- Sheeran, P. S., Dayton, P. A. Phase-change contrast agents for imaging and therapy. Current Pharmaceutical Design. 18 (15), 2152-2165 (2012).
- Mountford, P. A., Smith, W. S., Borden, M. A. Fluorocarbon nanodrops as acoustic temperature probes. Langmuir: The ACS Journal of Surfaces and Colloids. 31 (39), 10656-10663 (2015).
- Mountford, P. A., Thomas, A. N., Borden, M. A. Thermal activation of superheated lipid-coated perfluorocarbon drops. Langmuir: The ACS Journal of Surfaces and Colloids. 31 (16), 4627-4634 (2015).
- Sheeran, P. S., Luois, S., Dayton, P. A., Matsunaga, T. O. Formulation and acoustic studies of a new phase-shift agent for diagnostic and therapeutic ultrasound. Langmuir: The ACS Journal of Surfaces and Colloids. 27 (17), 10412-10420 (2011).
- Sheeran, P. S., Dayton, P. A. Improving the performance of phase-change perfluorocarbon droplets for medical ultrasonography: current progress, challenges, and prospects. Scientifica. 2014, 579684(2014).
- Sheeran, P. S., et al. Methods of generating submicrometer phase-shift perfluorocarbon droplets for applications in medical ultrasonography. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 64 (1), 252-263 (2017).
- Sheeran, P. S., et al. Decafluorobutane as a phase-change contrast agent for low-energy extravascular ultrasonic imaging. Ultrasound in Medicine & Biology. 37 (9), 1518-1530 (2011).
- de Gracia Lux, C., et al. Novel method for the formation of monodisperse superheated perfluorocarbon nanodroplets as activatable ultrasound contrast agents. RSC Advances. 7 (77), 48561-48568 (2017).
- Mountford, P. A., Sirsi, S. R., Borden, M. A. Condensation phase diagrams for lipid-coated perfluorobutane microbubbles. Langmuir: The ACS Journal of Surfaces and Colloids. 30 (21), 6209-6218 (2014).
- Feshitan, J. A., Chen, C. C., Kwan, J. J., Borden, M. A. Microbubble size isolation by differential centrifugation. Journal of Colloid and Interface Science. 329 (2), 316-324 (2009).
- Wu, S. -Y., et al. Focused ultrasound-facilitated brain drug delivery using optimized nanodroplets: vaporization efficiency dictates large molecular delivery. Physics in Medicine and Biology. 63 (3), 035002(2018).
- Li, D. S., et al. Spontaneous Nucleation of stable perfluorocarbon emulsions for ultrasound contrast agents. Nano Letters. 19 (1), 173-181 (2019).
- Sheeran, P. S., Luois, S. H., Mullin, L. B., Matsunaga, T. O., Dayton, P. A. Design of ultrasonically-activatable nanoparticles using low boiling point perfluorocarbons. Biomaterials. 33 (11), 3262-3269 (2012).
- Kawabata, K., Sugita, N., Yoshikawa, H., Azuma, T., Umemura, S. Nanoparticles with multiple perfluorocarbons for controllable ultrasonically induced phase shifting. Japanese Journal of Applied Physics. 44 (6), 4548-4552 (2005).
- Shakya, G., et al. Vaporizable endoskeletal droplets via tunable interfacial melting transitions. Science Advances. 6 (14), 7188(2020).
- Kopechek, J. A., Zhang, P., Burgess, M. T., Porter, T. M. Synthesis of phase-shift nanoemulsions with narrow size distributions for acoustic droplet vaporization and bubble-enhanced ultrasound-mediated ablation. Journal of Visualized Experiments: JoVE. (67), e4308(2012).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved