A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Quail Chorioallantoic Membrane - A Tool for Photodynamic Diagnosis and Therapy
In This Article
Summary
The chorioallantoic membrane (CAM) of the avian embryo is a very useful and applicable tool for various areas of research. A special ex ovo model of Japanese quail CAM is suitable for photodynamic treatment investigation.
Abstract
The chorioallantoic membrane (CAM) of an avian embryo is a thin, extraembryonic membrane that functions as a primary respiratory organ. Its properties make it an excellent in vivo experimental model to study angiogenesis, tumor growth, drug delivery systems, or photodynamic diagnosis (PDD) and photodynamic therapy (PDT). At the same time, this model addresses the requirement for the replacement of experimental animals with a suitable alternative. Ex ovo cultivated embryo allows easy substance application, access, monitoring, and documentation. The most frequently used is chick CAM; however, this article describes the advantages of the Japanese quail CAM as a low-cost and high-throughput model. Another advantage is the shorter embryonic development, which allows higher experimental turnover. The suitability of quail CAM for PDD and PDT of cancer and microbial infections is explored here. As an example, the use of the photosensitizer hypericin in combination with lipoproteins or nanoparticles as a delivery system is described. The damage score from images in white light and changes in fluorescence intensity of the CAM tissue under violet light (405 nm) was determined, together with analysis of histological sections. The quail CAM clearly showed the effect of PDT on the vasculature and tissue. Moreover, changes like capillary hemorrhage, thrombosis, lysis of small vessels, and bleeding of larger vessels could be observed. Japanese quail CAM is a promising in vivo model for photodynamic diagnosis and therapy research, with applications in studies of tumor angiogenesis, as well as antivascular and antimicrobial therapy.
Introduction
The chicken chorioallantoic membrane (CAM) model is well known and widely used in various areas of research. It is a richly vascularized extraembryonic organ that provides gas exchange and mineral transport1. Due to the transparency and accessibility of this membrane, individual blood vessels and their structural changes can be observed in real time2. Despite the advantages, chick CAM also has some limitations (e.g., larger breeding facilities, egg production, and feed consumption) that could be avoided by using other avian species. In this protocol, an alternative ex ovo CAM model using Japanese quail (Coturnix japonica) embryo is described. Due to its small size, it allows the use of a much larger number of experimental individuals than chicken CAM. Moreover, the shorter 16-day embryonic development of quail embryos is another advantage. The first larger vessels on quail CAM appear on embryonic day (ED) 7. This can be directly compared with chick embryo development (stages 4-35); however, the later stages of development are no longer comparable and require less time for the quail embryo3. Of interest is the regular occurrence of microvascular branching similar to that of chicken CAMs4,5,6. Rapid sexual maturation, high egg production, and low-cost breeding are other examples that favor the use of this experimental model7.
An avian CAM model is often used in photodynamic therapy (PDT) studies8. PDT is used to treat several forms of cancer (small localized tumors) and other non-oncological diseases. Its principle is in the delivery of a fluorescent drug, a photosensitizer (PS), to the damaged tissue and its activation with light of the appropriate wavelength. One prospective PS used in research is hypericin, originally isolated from the medicinal plant St. John's wort (Hypericum perforatum)9. The strong photosensitizing effects of this compound are based on its photochemical and photophysical properties. These are characterized by multiple fluorescence excitation peaks in the 400-600 nm range, which induce the emission of fluorescence at about 600 nm. The absorption maxima of hypericin within the spectral band are in the 540-590 nm range, and the fluorescence maxima are in the 590-640 nm range9. To achieve these photosensitizing effects, hypericin is excited by laser light at a wavelength of 405 nm after local administration10. In the presence of light, hypericin can exhibit virucidal, antiproliferative, and cytotoxic effects11, while there is no systemic toxicity, and it is rapidly released from the organism. Hypericin is a lipophilic substance that forms water-insoluble, non-fluorescent aggregates, which is why several types of nanocarriers, such as polymeric nanoparticles12,13 or high- and low-density lipoproteins (HDL, LDL)14,15, are used to help its delivery and penetration into the cells. Since CAM is a naturally immunodeficient system, tumor cells can be implanted directly on the membrane surface. The model is also well suited for recording the extent of PDT-induced vascular damage according to a defined score16,17. Light of lower intensity compared to PDT can be used for photodynamic diagnosis (PDD). Monitoring the tissue under violet excitation LED light also leads to photoactivation of photosensitizers18,19,20 that results in an emission of fluorescent light, yet it does not provide enough energy to start a PDT reaction and damage the cells. It makes it a good tool for tumor visualization and diagnosis or monitoring the pharmacokinetics of used PSs14,15.
This article describes the preparation of the quail ex ovo CAM assay with survival rates over 80%. This ex ovo culture was successfully applied in a large number of experiments.
Protocol
The research was performed in compliance with institutional guidelines. All equipment and reagents must be autoclaved or sterilized with 70% ethanol or UV light.
1. Egg incubation
- Store fertilized quail eggs at 10-15 °C for a maximum of 4-5 days before starting incubation. Use only clean and undamaged eggs.
- Incubate the eggs in a forced draught incubator for ~ 53-54 h. Lay the eggs horizontally with the egg rotation switched off, at 50%-60% humidity and 37.5 °C incubation temperature.
2. Ex ovo culture preparation
NOTE: After initial incubation, the eggs are suitable for starting the ex ovo cultivation.
- Disinfect the egg surface with 70% ethanol, without rotating the egg.
- In a sterile laminar-flow cabinet, open the eggshell using small sterile surgical scissors and transfer the contents into a 6-well culture plate. If properly done, the embryo will lie on top of an undamaged yolk. After each egg, disinfect the scissors with 70% ethanol.
- Add approximately 5 mL of sterile water to the gaps in the 6-well plate, as humidity is essential to prevent the CAM from drying out.
- Place the embryos in an incubator until further experiments, while maintaining a temperature of 37 °C and 80%-90% humidity.
- When the CAM is fully developed (from ED7), place a sterilized silicone ring (6 mm in diameter and approximately 1.5 mm in thickness) on the CAM surface, along the small capillaries. Avoid major blood vessels when placing the ring.
NOTE: The ring defines the workspace, helps mark the place of substance application, and prevents the liquid contents from leaking out. - Terminate the cultivation of the embryos according to the country's legislation.
- Importantly, wear gloves or keep hands disinfected with 70% ethanol during work. Aspirate improperly tipped embryos or unfertilized eggs with a vacuum aspirator.
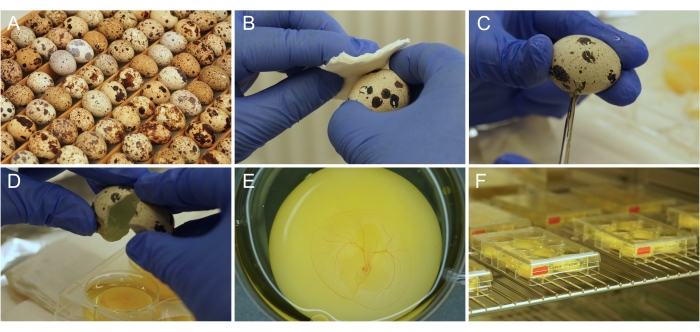
Figure 1: Ex ovo culture preparation. (A) Japanese quail eggs as they are stored and incubated. (B) Egg surface disinfection with ethanol. (C) Eggshell is cut with scissors. (D) The content of the egg is emptied into the well. (E) Properly prepared 3-day old embryo, with developing CAM vasculature. (F) Culture plates stored in an incubator. Please click here to view a larger version of this figure.
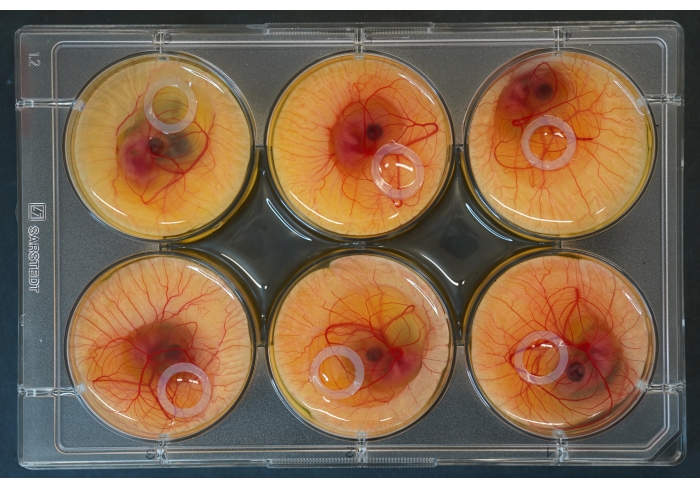
Figure 2: 6-well culture plate with embryos and silicone rings placed on top. Please click here to view a larger version of this figure.
3. Inoculation of tumor cells
NOTE: All procedures require the use of a sterile laminar flow cabinet.
- Culture different types of adherent cells in flasks according to respective culture protocols.
- Remove the old medium from the flasks and rinse the cell layer with sterile phosphate-buffered saline (PBS) to remove traces of the medium.
- After trypsinization, inhibit the trypsin by adding the culture medium and harvest the cells into centrifuge tubes. Centrifuge the cells and resuspend the cell pellet in a fresh culture medium. Count the cells and resuspend them in a cell culture medium at the desired concentration.
NOTE: It is also possible to produce 3D cell cultures, i.e., spheroids using, for example, the hanging drop method21.
- Implant 1 x 105-1 x 106 tumor cells or 5-15 spheroids (per CAM) in 30 µL of cell medium solution into the silicone ring on top of the CAM.
NOTE: The volume depends on the size of the silicone ring used. If a silicone ring with a larger diameter is used, a larger volume is needed to cover the entire area. Depending on the cell type, or for different types of experiments, various cell concentrations may be used. Sometimes, fine scraping of CAM is used to improve the adhesion of the embedded cells. - Return the CAMs with inoculated cells to the incubator (37 °C and 80%-90% humidity).

Figure 3: Inoculation of CAM with tumors. (A) Aspiration of spheroids with a pipette, and (B) implantation on the CAM surface. Please click here to view a larger version of this figure.
4. Application of photosensitizer
- Application of hypericin
- Under dim lighting, prepare a 2 mM stock solution of hypericin by dissolving it in 100% dimethyl sulfoxide (DMSO). Prepare a 79 µM working solution of hypericin shortly before the experiment with sterile PBS or saline solution. Ensure the final concentration of DMSO in all solutions is always under 0.2%, which does not affect the development of the embryo.
- Under sterile conditions, apply an appropriate volume of hypericin solution to the silicone ring. A volume of 30 µL is sufficient to fill a ring with a diameter of 6 mm.
- Keep embryos in an incubator at 37 °C and 80%-90% humidity. Hypericin in an aqueous solution is photoactive after its monomerization in the tissue, approximately 3 h after its application on the CAM.
- Application of hypericin with LDL, HDL, or nanoparticles as transport systems
NOTE: These transport systems are used to improve the penetration of hypericin into cells and cell structures.- Due to the photosensitivity, perform all the steps, including the preparation of solutions, under dim lighting and store the solutions in the dark.
- Prepare the hypericin-LDL and hypericin-HDL formulations by mixing appropriate volumes of lipoproteins and hypericin stock solutions in PBS according to reference15. The concentration of LDL or HDL to hypericin is 20:115.
- Synthesize polymer nanoparticles by living cationic ring-opening polymerization according to references12,13. Adjust the concentration of hypericin in polymer nanoparticles with PBS to 10 μM12,13.
5. PDD and PDT
- Conduct PDD
- Under sterile conditions, apply photosensitizer (hypericin, hypericin-loaded polymeric nanoparticles, or hypericin with lipoprotein carrier) on the CAM surface, with or without tumor cells (ED 7-9).
- Illuminate the CAM using violet excitation light (custom-made circular LED light of 405 nm wavelength) and record the fluorescence of hypericin in CAM tissue and tumor cells with a digital camera at time intervals of 0, 1, 3, 5, 24, and 48 h after hypericin administration.
- If the research requires an image of the CAM in white light, record the CAM before hypericin administration and at the end of the experiment, shortly before the tissue fixation, as the light affects the photoactivation of hypericin.
- Evaluate the fluorescence intensity using an image processing and analysis program (e.g., ImageJ22).
- Split RGB image channels into separate red, green, and blue images. Analyze the red intensity image in 8-bit form. Evaluate the red intensity from the area inside the ring and approximate it as the hypericin fluorescence. Obtain whole image profile plots (using ImageJ's Profile Plot plugin) at different time intervals after hypericin administration (i.e., from 0 h, right after administration, up to 48 h).
- Conduct PDT
- Perform the PDT at least 3 h after hypericin application.
- Place the optical fiber above the surface of the CAM for the laser ray to cover the entire area within the silicone ring.
- Perform in vivo irradiation (1-2 min) with a 405 nm laser light at a fluence rate of 285 mW/cm2.
- Record CAM using white light and 405 nm fluorescent light before and after photodynamic treatment (24 h and 48 h).
- Detect photodamage 24 h and 48 h after PDT from the images taken in white light. Evaluate vascular damage by a semiquantitative score16: 1 - no destruction; 2 - partial closure of capillaries (diameter ≤10 µm) with no destruction of medium or larger (diameter ≥50 µm) and smaller vessels (diameter 10-50 µm); 3 - partial closure of smaller vessels with capillaries vanishing; 4 - partial closure of larger vessels with smaller vessels and capillaries vanishing; and 5 - total photo thrombosis with most of the vessels vanishing.
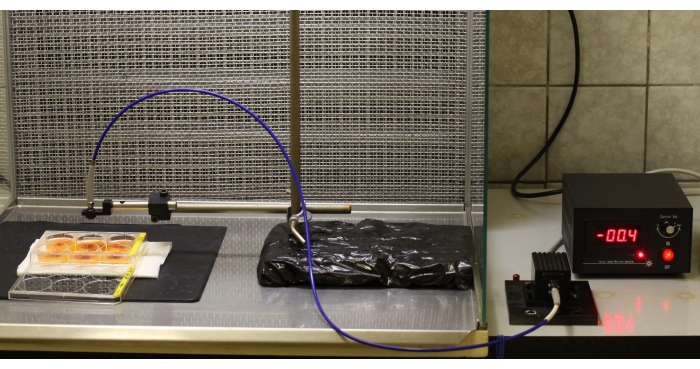
Figure 4: Treatment of CAM with laser light. This picture was taken for illustrative purposes. For PDD or PDT, the room must be dark. Please click here to view a larger version of this figure.
6. Preparing CAM for further evaluation
- Paraffin embedding
- Fix the CAM tissue in a cultivation plate with 4% paraformaldehyde (PFA) in PBS for a minimum of 2 h and a maximum of overnight.
- Remove the PFA and carefully cut out from the CAM a part of the tissue within the silicone ring.
- Immediately dehydrate the CAM tissue in ascending alcohol series as follows. Place the CAM tissue in 70% ethanol for 3 min, eosin solution for 2 min (for easier location of the tissue in the paraffin block), 96% ethanol for 3 x 5 min (always in a new Petri dish), and 100% ethanol for 5 min and xylene for 2 x 10 min.
- Using a spatula or thin brush, transfer the samples as quickly as possible to the dissolved paraffin in Petri dishes (59 °C). After 24 h, place the tissue in histological mold, fill it with paraffin embedding medium, and allow it to solidify in the refrigerator. Cut the solidified CAM from the embedding medium, turn it in the tray by 90°, fill it again with the embedding medium, and let it solidify.
- Prepare 5-10 µm sections on a microtome for histopathological analysis to determine PDT-induced damage.
- Preparation of frozen CAM sections for histology
NOTE: For some methodologies including histology, frozen sections prepared on cryostat microtome are more suitable. Follow the below steps for preparing the frozen CAM sections.- Carefully mount native or 4% paraformaldehyde-fixed CAM on the glass slide.
- Fill embedding mold to half with optimal cutting temperature compound (OCT) and freeze in liquid nitrogen or a mixture of dry ice and ethanol.
- After freezing, carefully tilt and slide the CAM from the glass slide onto the top of the frozen OCT medium. Place again in the mold, cover with OCT medium, and freeze as in Step 6.2.2.
- Fractal analysis of quail CAM
NOTE: Vasculature changes caused by PDT can be assessed by calculating the fractal dimension coefficient19.- In the laminar flow cabinet, overflow CAM in a cultivation plate with a pre-warmed (37 °C) fixation solution of 4% paraformaldehyde and 2% glutaraldehyde in PBS.
- Remove the fixation solution after 48 h. Carefully separate the CAM from the embryo with micro-scissors and a fine brush and wash it in PBS.
- Mount the washed CAM on a glass slide and let it slowly dry.
- Photograph the slide using a digital camera and a transilluminator as a source of homogenous white light.
- Process digital images using ImageJ software22. For the analysis, select a square region (512 × 512 pixels) from the area with distal arterial branches. Binarize and skeletonize the images and calculate the fractal dimension coefficient (Df) following the procedures described in reference31.
- Molecular analysis of CAM tissue
- For molecular analysis, carefully separate native CAM, freeze in liquid nitrogen, and store at -80 °C.
- Determine gene expression according to standard protocols for RNA isolation23, reverse transcription into cDNA, and quantitative PCR24.
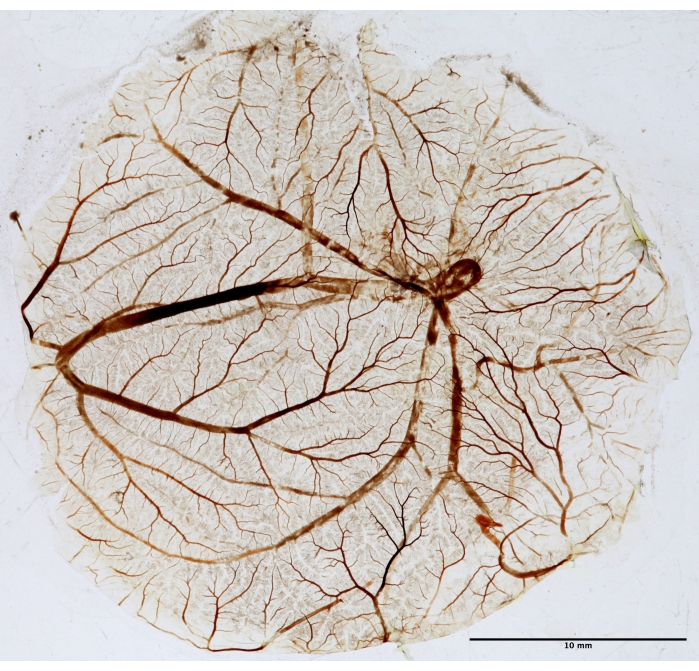
Figure 5: CAM tissue for fractal analysis. After PDT, CAM is fixed, mounted on a slide, and dried for fractal analysis. The picture was taken in white light using a transilluminator. Please click here to view a larger version of this figure.
Results
The localization of the tumor on the CAM surface is difficult in white light. Photosensitizer (here, hypericin) used in PDD is expected to be taken up selectively by the tumor and helps visualize the tumor. The addition of hypericin and the use of fluorescent light (e.g., 405 nm) showed the tumor (squamous cell carcinoma TE1) position very well (Figure 6A). Histological analysis showed vital tumor cells invading healthy tissues. Concentric structures of abnormal squamous cells, often de...
Discussion
For successful ex ovo cultivation, it is important to follow the protocol above. Moreover, if the eggs are not opened carefully enough or there is insufficient humidity during the cultivation, the yolk sack sticks to the shell and often ruptures. The start of an ex ovo cultivation at the time of about 60 h of egg incubation ensures the high survival rate of the embryos, as they are already large enough to survive the handling. At the later developmental stages, the CAM becomes thinner and adheres to the...
Disclosures
The authors have no conflicts of interest.
Acknowledgements
The work was supported by VEGA 2/0042/21 and APVV 20-0129. The contribution of V. Huntošová is the result of the project implementation: Open scientific community for modern interdisciplinary research in medicine (Acronym: OPENMED), ITMS2014+: 313011V455 supported by the Operational Program Integrated Infrastructure, funded by the ERDF.
Materials
| Name | Company | Catalog Number | Comments |
| 6-Well Cell Culture Plate | Sarstedt | 83.392 | Transparent polystyrene, sterile |
| CO2 Incubator ESCO CCL-0508 | ESCO, Singapore | CCL-050B-8 | CO2 cell culture incubator |
| cryocut Leica CM 1800 | Reichert-Jung, USA | ||
| digital camera Canon EOS 6D II | Canon, Japan | ||
| diode laser 405 nm | Ocean Optics, USA | ||
| DMSO | Sigma-Aldrich | 67-68-5 | dimethyl sulfoxid |
| eosin | Sigma-Aldrich | 15086-94-9 | |
| ethanol | Sigma-Aldrich | 64-17-5 | |
| fine brush size 2 | Faber-Castell | 281802 | brush for CAM separation and manipulation |
| glutaraldehyde | Sigma-Aldrich | 111-30-8 | |
| hematoxylin | Sigma-Aldrich | 517-28-2 | |
| hypericin | Sigma-Aldrich | 84082-80-4 | |
| incubator Bios Midi | Bios Sedl any, Czech Republic any, Czech Republic | Forced draught incubator for initial incubation | |
| incubator Memmert IF160 | Memmert, Germany | Forced air circulation incubator for CAM incubation | |
| Kaiser slimlite plano, LED light box | Kaiser, Germany | 2453 | Transilluminator |
| LED light 405 nm | custom made circular LED light | ||
| macro lens Canon MP- E 65 mm f/2.8 | Canon, Japan | ||
| microscope Kapa 2000 | Kvant, Slovakia | optical microscope | |
| microtome Auxilab 508 | Auxilab, Spain | manual rotary microtome | |
| paraformaldehyde | Sigma-Aldrich | 30525-89-4 | |
| Paraplast Plus | Sigma-Aldrich | P3683 | parafin medium for tissue embedding |
| PBS | Sigma-Aldrich | P4417 | Phosphate saline buffer |
| scissors Castroviejo | Orimed | OR66-108 | micro scissors for CAM separation |
| software ImageJ 1.53 | public domain | image processing and analysis program | |
| stock solution HDL | Sigma-Aldrich | 437641-10MG | high density lipoproteins |
| stock solution LDL | Sigma-Aldrich | 437644-10MG | low density lipoproteins |
| Tissue-Tek O.C.T. Compound | Sakura Finetek | 4583 | Optimal Cutting Temperature Compound 118 mL squeeze bottles |
References
- Nowak-Sliwinska, P., van Beijnum, J. R., van Berkel, M., vanden Bergh, H., Griffioen, A. W. Vascular regrowth following photodynamic therapy in the chicken embryo chorioallantoic membrane. Angiogenesis. 13 (4), 281-292 (2010).
- van Leengoed, H. L. L. M., vander Veen, N., Versteeg, A. A. C., Ouellet, R., van Lier, J. E., Star, W. M. In-vivo photodynamic effects of phthalocyanines in a skin-fold observation chamber model: role of central metal ion and degree of sulfonation. Photochemistry Photobiology. 58 (4), 575-580 (1993).
- Ainsworth, S. J., Stanley, R. L., Evans, D. J. R. Developmental stages of the Japanese quail. Journal of Anatomy. 216 (1), 3 (2010).
- De Fouw, D. O., Rizzo, V. J., Steinfeld, R., Feinberg, R. N. Mapping of the microcirculation in the chick chorioallantoic membrane during normal angiogenesis. Microvascular Research. 38 (2), 136-147 (1989).
- Sandau, K., Kurz, H. Modelling of vascular growth processes: a stochastic biophysical approach to embryonic angiogenesis. Journal of Microscopy. 175 (3), 205-213 (1994).
- Kurz, H., Ambrosy, S., Wilting, J., Marmé, D., Christ, B. Proliferation pattern of capillary endothelial cells in chorioallantoic membrane development indicates local growth control, which is counteracted by vascular endothelial growth factor application. Developmental Dynamics. 203 (2), 174-186 (1995).
- Huss, D., Poynter, G., Lansford, R. Japanese quail (Coturnix japonica) as laboratory animal model. Lab Animal. 37 (11), 513-519 (2008).
- Gottfried, V., Lindenbaum, E. S., Kimel, S. The chick chorioallantoic membrane (CAM) as an in-vivo model for photodynamic therapy. Journal of Photochemistry and Photobiology, B: Biology. 12 (2), 204-207 (1992).
- Miškovský, P. Hypericin - a new antiviral and antitumor photosensitizer: mechanism of action and interaction with biological molecules. Current Drug Targets. 3 (1), 55-84 (2002).
- Čavarga, I., et al. Photodynamic effect of hypericin after topical application in the ex ovo quail chorioallantoic membrane model. Planta Medica. 80 (1), 56-62 (2014).
- Martinez-Poveda, B., Quesada, A. R., Medina, M. A. Hypericin in the dark inhibits key steps of angiogenesis in vitro. Europan Journal of Pharmacology. 516 (2), 97-103 (2005).
- Datta, S., et al. Unravelling the excellent chemical stability and bioavailability of solvent responsive curcumin-loaded 2-ethyl-2-oxazoline-grad-2-(4-dodecyloxyphenyl)- 2-oxazoline copolymer nanoparticles for drug delivery. Biomacromolecules. 19 (7), 2459-2471 (2018).
- Huntošová, V., et al. Alkyl Chain length in poly(2-oxazoline)-based amphiphilic gradient copolymers regulates the delivery of hydrophobic molecules: a case of the biodistribution and the photodynamic activity of the photosensitizer hypericin. Biomacromolecules. 22 (10), 4199-4216 (2021).
- Buríková, M., et al. Hypericin fluorescence kinetics in the presence of low density lipoproteins: study on quail CAM assay for topical delivery. General Physiology and Biophysic. 35 (4), 459-468 (2016).
- Lenkavska, L., et al. Benefits of hypericin transport and delivery by low- and high-density lipoproteins to cancer cells: From in vitro to ex ovo. Photodiagnosis and Photodynamic Therapy. 25, 214-224 (2019).
- Rück, A., Böhmler, A., Steiner, R. PDT with TOOKAD studied in the chorioallantoic membrane of fertilized eggs. Photodiagnosis and Photodynamic Therapy. 2 (1), 79-90 (2005).
- Gottfried, V., Davidi, R., Averbuj, C., Kimel, S. In vivo damage to chorioallantoic membrane blood vessels by porphycene-induced photodynamic therapy. Journal of Photochemistry and Photobiology, B: Biology. 30 (2-3), 115-121 (1995).
- Buzzá, H. H., Silva, L. V., Moriyama, L. T., Bagnato, V. S., Kurachi, C. Evaluation of vascular effect of Photodynamic Therapy in chorioallantoic membrane using different photosensitizers. Journal of Photochemistry and Photobiology B: Biology. 138, 1-7 (2014).
- Dougherty, T. J., et al. Photodynamic therapy. Journal of the National Cancer Institute. 90, 889-905 (1998).
- Xiang, L., et al. Real-time optoacoustic monitoring of vascular damage during photodynamic therapy treatment of tumor. Journal of Biomedical Optics. 12 (1), 01400-01408 (2007).
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of Visualized Experiments. (51), 2720 (2011).
- Abramoff, M. D., Magelhaes, P. J., Ram, S. J. Image Processing with ImageJ. Biophotonics International. 11 (7), 36-42 (2004).
- Chomczynski, P., Sacchi, N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 162 (1), 156-159 (1987).
- Máčajová, M., Čavarga, I., Sýkorová, M., Valachovič, M., Novotná, V., Bilčík, B. Modulation of angiogenesis by topical application of leptin and high and low molecular heparin using the Japanese quail chorioallantoic membrane model. Saudi Journal of Biological Sciences. 27 (6), 1488-1493 (2020).
- Mangir, N., Dikici, S., Claeyssens, F., MacNeil, S. Using Ex Ovo chick chorioallantoic membrane (CAM) assay to evaluate the biocompatibility and angiogenic response to biomaterials. ACS Biomaterials Science Engineering. 5 (7), 3190-3200 (2019).
- Marshall, K. M., Kanczler, J. M., Oreffo, R. O. C. Evolving applications of the egg: chorioallantoic membrane assay and ex vivo organotypic culture of materials for bone tissue engineering. Journal of Tissue Engineering. 11, 1-25 (2020).
- Merlos Rodrigo, M. A., et al. Extending the applicability of in ovo and ex ovo chicken chorioallantoic membrane assays to study cytostatic activity in neuroblastoma cells. Frontiers in Oncology. 11, 1-10 (2021).
- Meta, M., Kundeková, B., Bilčík, B., Máčajová, M. The effect of silicone ring application on CAM vasculature in Japanese Quail (Coturnix japonica). Proceedings of the Student Scientific Conference Faculty of Natural Sciences of Comenius University, Bratislava, Slovakia. , 385-390 (2019).
- Kohli, N., et al. Pre-screening the intrinsic angiogenic capacity of biomaterials in an optimised ex ovo chorioallantoic membrane model. Journal of Tissue Engineering. 11, 1-15 (2020).
- Kundeková, B., Máčajová, M., Meta, M., Čavarga, I., Bilčík, B. Chorioallantoic membrane models of various avian species differences and applications. Biology-Basel. 10 (4), 301 (2021).
- Parsons-Wingerter, P., Elliott, K. E., Clark, J. I., Farr, A. G. Fibroblast growth factor-2 selectively stimulates angiogenesis of small vessels in arterial tree. Arteriosclerosis, Thrombosis and Vascular Biology. 20 (5), 1250-1256 (2000).
- Buzzá, H. H., Zangirolami, A. C., Davis, A., Gómez-García, P. B., Kurachi, C. Fluorescence analysis of a tumor model in the chorioallantoic membrane used for the evaluation of different photosensitizers for photodynamic therapy. Photodiagnosis and Photodynamic Therapy. 19, 78-83 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved