A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Facile Preparation and Photoactivation of Prodrug-Dye Nanoassemblies
In This Article
Summary
This protocol describes the fabrication and characterization of a photoresponsive prodrug-dye nanoassembly. The methodology for drug release from the nanoparticles by light-triggered disassembly, including the light irradiation setup, is explicitly described. The drugs released from the nanoparticles following light irradiation exhibited excellent anti-proliferation effects on human colorectal tumor cells.
Abstract
Self-assembly is a simple yet reliable method for constructing nanoscale drug delivery systems. Photoactivatable prodrugs enable controllable drug release from nanocarriers at target sites modulated by light irradiation. In this protocol, a facile method for fabricating photoactivatable prodrug-dye nanoparticles via molecular self-assembly is presented. The procedures for prodrug synthesis, nanoparticle fabrication, physical characterization of the nanoassembly, photocleavage demonstration, and in vitro cytotoxicity verification are described in detail. A photocleavable boron-dipyrromethene-chlorambucil (BC) prodrug was first synthesized. BC and a near-infrared dye, IR-783, at an optimized ratio, could self-assemble into nanoparticles (IR783/BC NPs). The synthesized nanoparticles had an average size of 87.22 nm and a surface charge of -29.8 mV. The nanoparticles disassembled upon light irradiation, which could be observed by transmission electronic microscopy. The photocleavage of BC was completed within 10 min, with a 22% recovery efficiency for chlorambucil. The nanoparticles displayed enhanced cytotoxicity under light irradiation at 530 nm compared with the non-irradiated nanoparticles and irradiated free BC prodrug. This protocol provides a reference for the construction and evaluation of photoresponsive drug delivery systems.
Introduction
Chemotherapy is a common cancer treatment that employs cytotoxic agents to kill cancer cells and thus inhibits tumor growth1. However, patients may suffer from side effects such as cardiotoxicity and hepatotoxicity due to the off-target absorption of the chemotherapy drugs2,3,4. Therefore, localized drug delivery through the spatiotemporal control of drug release/activation in tumors is essential to minimize drug exposure in normal tissues.
Prodrugs are chemically modified drugs that exhibit reduced toxicity in normal tissues while retaining their action in diseased lesions upon activation5,6. Prodrugs can be responsive to a variety of stimuli, such as pH7,8, enzymes9,10, ultrasound11,12, heat13, and light14,15,16, and release their parent drugs specifically in the lesions. Nevertheless, many prodrugs exhibit inherent drawbacks, such as poor solubility, incorrect absorption rate, and early metabolic destruction, which may limit their development17. In this context, the formation of prodrug nanoassemblies offers advantages like decreased side effects, in situ drug release, better retention, and the combination of treatment and imaging, indicating great application potential for these nanoassemblies. Many prodrug nanoassemblies have been developed for disease treatment, including doxorubicin prodrug nanospheres, curcumin prodrug micelles, and camptothecin prodrug nanofibers18.
In this protocol, we present a simple method for the preparation of prodrug-dye nanoassemblies that exhibit high prodrug content, good water dispersibility, long-term stability, and sensitive responding ability. IR783 is a water soluble near-infrared dye that can serve as stabilizer of the nanoassemblies19. The other component of the nanoassembly is boron-dipyrromethene-chlorambucil (BODIPY-Cb, BC), a prodrug that was designed for two main reasons. As chlorambucil (Cb) displays systemic toxicity in vivo, the prodrug form can decrease its toxicity20. The BC prodrug can be photocleaved using 530 nm light irradiation directed at disease lesions, enabling the local release of Cb. On the other hand, Cb is prone to hydrolysis in aqueous environments, and can be protected by transforming it into a prodrug form21. Thus, the co-assembly of the BC prodrug and IR-783 dye was expected to form a stable and effective drug delivery nanosystem (Figure 1A). This prodrug-dye nanoassembly improves the dispersibility and stability of the prodrug molecules, suggesting its potential for application in light-controllable drug delivery. The photocleavage of the BC prodrug enables the disassembly of nanoparticles and the light-controlled release of Cb in the lesions (Supplemental Figure 1).
Protocol
1. Synthesis of boron-dipyrromethene-chlorambucil (BC) prodrug (Figure 2)22
- Synthesis of BODIPY-OAc
- Weigh 1.903 g of 2,4-dimethyl pyrrole and dissolve it in 20 mL of anhydrous dichloromethane (DCM) in a round-bottom flask under a nitrogen atmosphere. Weigh 1.638 g of acetoxy acetyl chloride and add it dropwise into the solution. Keep stirring for 10 min at room temperature and then reflux the solution for 1 h at 40 °C.
- Cool the mixture to room temperature. Weigh 5.170 g of N,N-diisopropylethylamine (DIPEA) and add it dropwise into the mixture under stirring. After 30 min, weigh 5.677 g of boron trifluoride diethyl etherate (BF3·OEt2), add it dropwise into the solution, and keep stirring for an additional 30 min.
- Add 10 g of silica gel (200-400 mesh) into the mixture and remove the solvent by rotary evaporation at 45 °C. Stop the evaporation when the silica gel returns to dry powder.
- Add a frit into the bottom of a cartridge (see Table of Materials). Fill the silica gel (from step 1.1.3) into the cartridge and then add another frit into the cartridge on the top of the filled gel.
- Fix the cartridge into the collar connected with the flash chromatography system (see Table of Materials) and turn it to lock it. Install the cartridge on top of the six-way valve in the flash chromatography system and install a flash column (see Table of Materials) under the valve.
- Start the chromatography instrument and set 515 nm and 365 nm as the detection wavelengths. Perform elution with 4/3 (v/v) hexane/DCM. Collect the eluent fractions as the 515 nm signal appears.
- Remove the solvent from the collected fractions by rotary evaporation at 40 °C until no more solvent is collected in the solvent collection flask. Place the solid product in a vacuum drying chamber overnight to remove the rest of solvent.
- Synthesis of BODIPY-OH
- Weigh 1.120 g of BODIPY-OAc (synthesized in step 1.1) and dissolve it in 70 mL of tetrahydrofuran (THF) at room temperature, fully covered with foil. Add 70 mL of 0.1 M LiOH aqueous solution dropwise into the BODIPY-OAc solution.
- Stir the mixture for 30 min and remove the solvent at 40 °C by rotary evaporation until no more solvent is collected in the solvent collection flask. Place the residue in a vacuum drying chamber overnight to remove water.
- Dissolve the dry residue in 30 mL of DCM and add 10 g of silica gel into the solution. Remove the solvent by rotary evaporation at 40 °C. Stop the evaporation when the silica gel returns to dry powder.
- Purify the product BODIPY-OH by column chromatography, following steps 1.1.4 to 1.1.6, with DCM alone as the eluent.
- Compare fractions collected at different elution time points with a THF solution of BODIPY-OAc on thin layer chromatography (TLC) and identify the product23.
- Spot 3-4 µL of the eluted fraction and the BODIPY-OAc solution separately on one edge of a TLC plate at the same height. Place the TLC plate in a glass chamber containing 1 mL of DCM, submerging the spotted edge into the DCM solvent but with the two spots out of the solvent.
- Take out the TLC plate when the DCM solvent reaches over half the height of the plate. Select the eluted fraction with a TLC spot at a different height from the BODIPY-OAc spot.
- Remove the solvent by rotary evaporation at 40 °C (step 1.1.7) to obtain the product BODIPY-OH.
- Synthesis of BODIPY-(Me)2-OH
- Weigh 313 mg of BODIPY-OH and dissolve it in 35 mL of anhydrous diethyl ether in the dark under a nitrogen atmosphere. Add 3.75 mL of methyl magnesium iodide (3 M in diethyl ether) dropwise into the solution and keep stirring for 3 h at room temperature.
- Quench the reaction by adding 3.5 mL of water dropwise.
- Extract the mixture with DCM and water.
- Transfer the mixture into a 125 mL separatory funnel. Add 20 mL of DCM into the mixture.
- Close the cap of the separatory funnel. Tilt the funnel at around 45° and slightly shake the funnel. Open the cap to deflate. Repeat this step 3 times, and allow to stand for 3 min.
- Open the bottom valve and collect the lower organic phase in a beaker.
- Add 30 mL of DCM to the aqueous phase. Repeat the extraction (steps 1.3.3.2 and 1.3.3.3) 3 times with 30 mL of DCM each time.
- Add 10 g of solid Na2SO4 into the collected organic phase to dry the organic phase overnight.
- Link a filtering flask to a vacuum pump with a rubber tube. Lay a piece of filter paper on the Büchner funnel and insert the funnel into the top of the flask. Wet the filter paper with 1 mL of DCM, transfer the mixture into the funnel, and turn on the vacuum pump. Collect the organic solution in the flask.
- Add 10 g of silica gel into the organic solution. Remove the organic solvent by rotary evaporation at 40 °C until the silica gel returns to dry powder. Purify the product BODIPY-(Me)2-OH by column chromatography (steps 1.1.4 to 1.1.6) with hexane/DCM = 1/1 (v/v) as the eluent.
- Select the eluted fractions containing the product using TLC analysis as described in step 1.2.5 (TLC spot at a different height from BODIPY-OH). Remove the solvent by rotary evaporation at 40 °C to obtain the product as described in step 1.1.7.
- Synthesis of BODIPY-(Me)2-I2-OH
- Weigh 41 mg of BODIPY-(Me)2-OH and dissolve it in 2.5 mL of anhydrous THF in the dark under a nitrogen atmosphere. Weigh 74 mg of N-iodosuccinimide and dissolve it in 1 mL of anhydrous THF.
- Add the N-iodosuccinimide solution dropwise into the BODIPY-(Me)2-OH solution. After stirring for 3.5 h at room temperature, remove the solvent by rotary evaporation at 40 °C until no more solvent is collected in the solvent collection flask on the rotary evaporator.
- Dissolve the residue in 10 mL of DCM and wash it 3 times with 30 mL of water each time, as described in step 1.3.3. Dry the organic phase with Na2SO4 (steps 1.3.4 and 1.3.5). Remove the solvent by rotary evaporation at 40 °C (step 1.1.7) to obtain the product BODIPY-(Me)2-I2-OH.
- Synthesis of BODIPY-Cb
- Weigh 85 mg of chlorambucil and dissolve it in 2 mL of anhydrous DCM in the dark under a nitrogen atmosphere. Weigh 69 mg of N,N'-dicyclohexylcarbodiimide and dissolve it in 1 mL of anhydrous DCM. Add it dropwise into the chlorambucil solution with stirring for 10 min.
- Dissolve 1.7 mg of 4-dimethylaminopyridine in 0.5 mL of anhydrous DCM. Add this solution into the mixture and keep stirring for 10 min at room temperature. Then add 73 mg of BODIPY-(Me)2-I2-OH dissolved in 2 mL of anhydrous DCM and keep stirring for 2 h.
- Add 10 g of silica gel into the mixture and remove the solvent by rotary evaporation at 40 °C. Stop the evaporation when the silica gel returns to dry powder. Purify the product BODIPY-Cb by column chromatography (steps 1.1.4 to 1.1.6, 540 nm and 365 nm signal wavelength) with hexane/DCM = 7/3 (v/v) as the eluent.
- Select the eluted fractions containing the product different from BODIPY-(Me)2-I2-OH using TLC analysis (step 1.2.5). Remove the solvent by rotary evaporation at 40 °C (step 1.1.7) to obtain the product BODIPY-Cb.
2. Preparation of IR783/BC NPs by the flash precipitation method
- Weigh 10 mg of the BC prodrug (BODIPY-Cb) and dissolve it in 1 mL of DMSO in a 1.5 mL microtube to obtain a 10 mg/mL stock solution. Cover the BC solution with foil.
- Prepare 300 µL of 0.4 mg/mL IR-783 in filtered deionized water in a 1.5 mL microtube. Place this microtube on a vortex mixer at 1,500 rpm.
- Add 20 µL of the BC solution in DMSO to the IR-783 solution over 10 s at a constant rate using a 20 µL pipette. The end of the pipette tip should touch the inner wall of the microtube (Figure 1B).
- Keep the microtube on the vortex mixer for an additional 30 s to obtain the IR783/BC NP solution. Then, place the nanoparticle solution on a rack fully covered with foil.
- Centrifuge the resulting IR783/BC NP solution for 10 min at 2,000 x g and 4 °C to remove aggregates. Collect the supernatant, leaving ~20 µL in the tube to avoid disturbing the pellet. Discard the pellet.
- Centrifuge the supernatant twice for 30 min at 30,000 x g and 4 °C and collect the nanoparticle precipitate from both the centrifugations. Resuspend the nanoparticles in 300 µL of 1x PBS.
NOTE: When the hydrophobic BC prodrug in DMSO is dispersed in water with vortexing, the DMSO is dissolved by water, and the prodrug molecules tend to form nanoscale assemblies to keep themselves stable under the local supersaturation situation24. - Quantify the content of IR-783 and BC by high performance liquid chromatography (HPLC), using the elution method shown in Table 1.
NOTE: The HPLC sample is prepared by mixing equal volumes of nanoparticle solution and acetonitrile. The injection volume is 20 µL. The detection wavelength for chlorambucil and BC prodrug is 260 nm, and the detection wavelength for IR783 is 783 nm. The HPLC column is an analytical 4.6 mm (inner diameter) x 100 mm (length) C18 column, with a 2.7 µm particle size and 120 Å pore size. - Calculate the prodrug encapsulation efficiency (EE%) and loading capacity (LC%) according to the following equations:

| Time (min) | Acetonitrile (%) | Water (%) |
| 0 | 20 | 80 |
| 5 | 20 | 80 |
| 30 | 95 | 5 |
| 35 | 95 | 5 |
Table 1: HPLC method for qualitative and quantitative analysis of BC prodrug and its photocleavage. Reproduced with permission25. Copyright 2022, Wiley.
3. Characterization of IR783/BC NPs
- Measure the average size of the IR783/BC NPs with a dynamic light scattering (DLS) instrument (see Table of Materials). Add 200 µL of IR783/BC NP solution in a cuvette and insert the cuvette in the holder for measurement. Set the measurement type as 'size' and measurement temperature as 25 °C. Perform three measurements with a duration of 20 s for each measurement.
- Measure the surface charge of the IR783/BC NPs with the DLS instrument using a zeta-potential test cuvette.
- Dilute 25 µL of IR783/BC NP solution with 725 µL of deionized water in a 1.5 mL microtube and add the solution into a zeta-potential test cuvette. Place the cuvette in the sample groove. Cap the sample groove.
- Set the measurement type as 'zeta-potential' and measurement temperature as 25 °C. Perform 10 measurements.
- Prepare the samples for transmission electron microscopy (TEM) imaging. Add 10 µL of IR783/BC NP solution on a piece of holey carbon film on a copper grid (300 mesh) and remove 7 µL. Leave 3 µL of solution on the film overnight for auto-evaporation.
NOTE: Adding 10 µL of the NP solution followed by the removal of 7 µL allows the droplet to cover a broader area on the film.
4. Photoactivation of IR783/BC NPs
- Set up an LED lamp (530 nm; see Table of Materials) with an iron stand so that the light directly faces the operating floor. Place an integrating sphere photodiode photometer (see Table of Materials) directly under the LED lamp.
NOTE: To prevent the influence of environmental light, all light irradiation experiments are conducted in a darkroom. - Turn on the LED lamp and open the cap of the photometer. Record the irradiance and set the lamp parameters using the associated software (see Table of Materials). Adjust the input current (mA) to set the irradiance as 50 mW/cm2.
NOTE: The irradiance is also affected by the distance between the LED lamp and photometer. In the setup used here (Figure 3A,B), the distance is fixed at 5 cm. - Dilute the IR783/BC NP solution with deionized water to 50 µM based on BC concentration. Add 200 µL of the IR783/BC NP solution into a 1.5 mL microtube. Place the tube on a foam block with a groove fitting size of the microtube and the same height as the photometer in step 4.1 (Figure 3C,D).
- Open the cap of the tube. Turn on the LED lamp and irradiate the nanoparticle solution for 1, 2, 3, 5, 7, and 10 min.
- Quantify BC consumption and Cb release by HPLC after light irradiation. Calculate the percentage of remaining BC and Cb release using the following equations:

5. Testing cytotoxicity of IR783/BC NPs with and without light irradiation
- Culture HCT116 cells (human colorectal tumor cell line) in RPMI 1640 medium containing 10% fetal bovine serum and 1% Penicillin-Streptomycin (complete medium) in a 5% CO2 atmosphere at 37 °C (~2 x 106 cells per dish in a 90 mm cell culture dish). Routinely subculture the cells every 2-3 days.
NOTE: HCT116 is a human colon cell line. Compared to other cancer cells such as HeLa, MCF7, and A549 cells, HCT-116 cells express a higher level of caveolin-125, which can be targeted by IR783 and enhance the cellular uptake of IR783/BC NPs. - Plate the HCT116 cells in 96-well plates with RPMI 1640 complete medium at a density of 104 cells per well.
- Aspirate the medium from the culture dish when the cell confluency exceeds 50%. Wash the cells with 1x PBS and remove the PBS. Add 1 mL of 0.25% trypsin solution and incubate at 37 °C in a 5% CO2 incubator.
- After 3 min, add 2 mL of complete medium to quench trypsin digestion. Resuspend the cells, transfer the cell suspension into a 15 mL centrifugation tube, and centrifuge at 300 x g for 3 min. Discard the supernatant and resuspend the cell pellet in 1 mL of complete medium.
- Dilute 10 µL of the cell suspension to 200 µL with complete medium. Place 10 µL on a hemocytometer and cap it with a coverslip.
- Observe the hemocytometer under a microscope (eyepiece: 10x; objective lens: 4x). Count and record the cell numbers at the four corner squares and the center. Calculate the cell concentration using the formula:
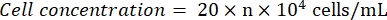
Where n = the average of the cell numbers of the five squares. - Dilute the cell suspension to 1 x 105 cells/mL. Add 100 µL of the cell suspension per well in a 96-well plate to seed the cells. Add 100 µL of PBS per well in the unseeded wells.
- Treat the cells with (1) 0.1-150 µM free BC, (2) 0.1-150 µM IR783/BC NPs (based on BC concentration), (3) 0.1-150 µM free BC with light irradiation, or (4) 0.1-150 µM IR783/BC NPs (based on BC concentration) with light irradiation. Incubate the cells at 37 °C in a 5% CO2 incubator for 6 h.
NOTE: The free BC and IR783/BC NP solutions are diluted from their respective stock solutions with complete medium. - After 6 h of incubation, replace the prodrug/nanoparticles-containing medium with fresh complete medium. Incubate the non-irradiation Groups 1 and 2 in the dark for 24 h. For Groups 3 and 4, irradiate the cells with a 530 nm LED lamp (50 mW/cm2) for 5 min, and incubate for 24 h.
NOTE: Cell plates are placed on a foam block to ensure the same height as the photometer in step 4.1. - Determine the cell viability with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
- After the BC or nanoparticle treatment, add 10 µL of MTT (10 mg/mL in PBS) to each well and incubate the plates at 37 °C for 3 h. Then, remove the medium and add 100 µL of DMSO to each well. Read the absorbance with a microplate reader at 490 nm, 570 nm, and 630 nm.
- Calculate the cell viability using the following equation:

NOTE: Four independent experiments (n = 4) of each group are conducted for analysis. OD490 can be replaced by OD570-OD630 in calculation of cell viability.
Results
IR783/BC NPs were successfully fabricated in this study using a flash precipitation method. The synthesized IR783/BC NPs presented as a purple solution, while the aqueous solution of IR783 was blue (Figure 4A). As shown in Figure 4B, the IR783/BC NPs exhibited an average size of approximately 87.22 nm with a polydispersity index (PDI) of 0.089, demonstrating a narrow size distribution. The surface charge of the IR783/NPs was approximately -29.8 mV (
Discussion
This protocol outlines a facile flash precipitation method for the fabrication of prodrug-dye nanoparticles, which offers a simple and convenient approach for nanoparticle formation. There are several critical steps in this method. Firstly, for all steps of synthesis, fabrication, and characterization, containers like microtubes should be covered with foil to avoid unnecessary photocleavage of the BC prodrug by environmental light. Moreover, in the flash precipitation step, the microtube containing the IR-783 solution sh...
Disclosures
A PCT application has been filed with No. PCT/CN2021/081262.
Acknowledgements
We acknowledge assistance from the University of Hong Kong Li Ka Shing Faculty of Medicine Faculty Core Facility. We thank Professor Chi-Ming Che at the University of Hong Kong for providing the human HCT116 cell line. This work was supported by Ming Wai Lau Centre for Reparative Medicine Associate Member Program and the Research Grants Council of Hong Kong (Early Career Scheme, No. 27115220).
Materials
| Name | Company | Catalog Number | Comments |
| 1260 Infinity II HPLC | Agilent Technologies | ||
| 2,4-Dimethyl pyrrole | J&K Scientific | 315305 | |
| 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide(MTT) | Gibco | M6494 | |
| 4-Dimethylaminopyridine (4-DMAP) | J&K Scientific | 212279 | |
| 90 mm Petri Dish Clear Treated Sterile | SPL | 11090 | |
| 96-well Tissue Culture Plate Clear Treated Sterile | SPL | 30096 | |
| Acetoxyacetyl chloride | J&K Scientific | 192001 | |
| Boron trifluoride diethyl etherate | J&K Scientific | 921076 | |
| Büchner funnel | AS ONE | 3-6466-01 | |
| Chlorambucil | J&K Scientific | 321407-1G | |
| CM100 Transmission Electron Microscope | Philips | ||
| CombiFlash RF chromatography system | Teledyne ISCO | ||
| Dichloromethane | DUKSAN Pure Chemicals | JT9315-88 | |
| Dimethyl sulfoxide | DUKSAN Pure Chemicals | 2762 | |
| Disposable cuvette | Malvern Panalytical | DTS1070 | Zeta potential measurement |
| Disposable cuvette | Malvern Panalytical | ZEN0040 | |
| Empty Disposable Sample Load Cartridges | Teledyne ISCO | 693873225 | can hold up to 65 g |
| Fetal bovine serum | Gibco | 10270106 | |
| Filtering flask | AS ONE | 3-7089-03 | |
| Hexane | DUKSAN Pure Chemicals | 4198 | |
| Holey carbon film on copper grid | Beijing Zhongjingkeyi Technology Co.,Ltd | BZ10023a | |
| HPLC column (InfinityLab Poroshell 120) | Agilent Technologies | 695975-902T | |
| Integrating sphere photodiode power sensor | Thorlabs | S142C | |
| IR783 | Tokyo Chemical Industry (TCI) Co., Ltd | I1031 | |
| LED | Mightex | LCS-0530-15-11 | |
| LED Driver Control Panel V3.2.0 (Software) | Mightex | ||
| Lithium Hydroxide Anhydrous | TCI | L0225 | |
| Methylmagnesium iodide, 3M solution in diethyl ether | Aladdin | M140783 | |
| N,N-Diisopropyl ethyl amine (DIPEA) | J&K Scientific | 203402 | |
| N,N'-Dicyclohexylcarbodiimide (DCC) | J&K Scientific | 275928 | |
| penicillin–streptomycin | Gibco | 15140122 | |
| Phosphate-buffered saline (10×) | Sigma-Aldrich | P5493 | |
| Power and energy meter | Thorlabs | PM100 USB | |
| Rotavapor | BUCHI Rotavapor R300 | ||
| RMPI 1640 | Gibco | 21870076 | |
| Separatory funnel (125 mL) | Synthware | F474125L | |
| Silver Silica Gel Disposable Flash Columns, 40 g | Teledyne ISCO | 692203340 | |
| Sodium sulfate, anhydrous | Alfa Aesar | A19890 | |
| SpectraMax M4 | Molecular Devices LLC | ||
| Tetrahydrofuran (THF), anhydrous | J&K Scientific | 943616 | |
| Trypsin-EDTA (0.25%), phenol red | Gibco | 25200056 | |
| Vortex | DLAB Scientific Co., Ltd | MX-S | |
| Zetasizer Nano ZS90 | Malvern Instrument |
References
- Chabner, B. A., Roberts, T. G. Chemotherapy and the war on cancer. Nature Reviews Cancer. 5 (1), 65-72 (2005).
- Monsuez, J. -. J., Charniot, J. -. C., Vignat, N., Artigou, J. -. Y. Cardiac side-effects of cancer chemotherapy. International Journal of Cardiology. 144 (1), 3-15 (2010).
- Floyd, J., Mirza, I., Sachs, B., Perry, M. C. Hepatotoxicity of chemotherapy. Seminars in Oncology. 33 (1), 50-67 (2006).
- Bar-Joseph, H., Stemmer, S. M., Tsarfaty, I., Shalgi, R., Ben-Aharon, I. Chemotherapy-induced vascular toxicity-real-time in vivo imaging of vessel impairment. Journal of Visualized Experiments. (95), e51650 (2015).
- Denny, W. A. Prodrug strategies in cancer therapy. European Journal of Medicinal Chemistry. 36 (7-8), 577-595 (2001).
- Kastrati, I., Delgado-Rivera, L., Georgieva, G., Thatcher, G. R. J., Frasor, J. Synthesis and characterization of an aspirin-fumarate prodrug that inhibits NFκB activity and breast cancer stem cells. Journal of Visualized Experiments. (119), e54798 (2017).
- Mao, J., et al. A simple dual-pH responsive prodrug-based polymeric micelles for drug delivery. ACS Applied Materials & Interfaces. 8 (27), 17109-17117 (2016).
- Li, S. -. Y., et al. A pH-responsive prodrug for real-time drug release monitoring and targeted cancer therapy. Chemical Communications. 50 (80), 11852-11855 (2014).
- Andresen, T. L., Thompson, D. H., Kaasgaard, T. Enzyme-triggered nanomedicine: Drug release strategies in cancer therapy (Invited Review). Molecular Membrane Biology. 27 (7), 353-363 (2010).
- Xu, G., McLeod, H. L. Strategies for enzyme/prodrug cancer therapy. Clinical Cancer Research. 7 (11), 3314-3324 (2001).
- Luo, W., et al. Dual-targeted and pH-sensitive doxorubicin prodrug-microbubble complex with ultrasound for tumor treatment. Theranostics. 7 (2), 452 (2017).
- Gao, J., et al. Ultrasound triggered phase-change nanodroplets for doxorubicin prodrug delivery and ultrasound diagnosis: An in vitro study. Colloids and Surfaces B: Biointerfaces. 174, 416-425 (2019).
- Brade, A. M., Szmitko, P., Ngo, D., Liu, F. -. F., Klamut, H. J. Heat-directed suicide gene therapy for breast cancer. Cancer Gene Therapy. 10 (4), 294-301 (2003).
- Long, K., et al. One-photon red light-triggered disassembly of small-molecule nanoparticles for drug delivery. Journal of Nanobiotechnology. 19 (1), 357 (2021).
- Liu, Y., Long, K., Kang, W., Wang, T., Wang, W. Optochemical control of immune checkpoint blockade via light-triggered PD-L1 dimerization. Advanced NanoBiomed Research. 2 (6), 2200017 (2022).
- Wang, T., et al. Optochemical control of mTOR signaling and mTOR-dependent autophagy. ACS Pharmacology & Translational Science. 5 (3), 149-155 (2022).
- Abet, V., Filace, F., Recio, J., Alvarez-Builla, J., Burgos, C. Prodrug approach: An overview of recent cases. European Journal of Medicinal Chemistry. 127, 810-827 (2017).
- Li, G., et al. Small-molecule prodrug nanoassemblies: an emerging nanoplatform for anticancer drug delivery. Small. 17 (52), 2101460 (2021).
- Shamay, Y., et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nature Materials. 17 (4), 361-368 (2018).
- Sinoway, P. A., Callen, J. P. Chlorambucil. Arthritis & Rheumatism. 36 (3), 319-324 (1993).
- Owen, W. R., Stewart, P. J. Kinetics and mechanism of chlorambucil hydrolysis. Journal of Pharmaceutical Sciences. 68 (8), 992-996 (1979).
- Lv, W., et al. Upconversion-like photolysis of BODIPY-based prodrugs via a one-photon process. Journal of the American Chemical Society. 141 (44), 17482-17486 (2019).
- Silver, J. Let us teach proper thin layer chromatography technique. Journal of Chemical Education. 97 (12), 4217-4219 (2020).
- Saad, W. S., Prud'homme, R. K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today. 11 (2), 212-227 (2016).
- Long, K., et al. Photoresponsive prodrug-dye nanoassembly for in-situ monitorable cancer therapy. Bioengineering & Translational Medicine. 7 (3), 10311 (2022).
- Zhong, T., et al. A self-assembling nanomedicine of conjugated linoleic acid-paclitaxel conjugate (CLA-PTX) with higher drug loading and carrier-free characteristic. Scientific Reports. 6 (1), 36614 (2016).
- Long, K., et al. Green light-triggered intraocular drug release for intravenous chemotherapy of retinoblastoma. Advanced Science. 8 (20), 2101754 (2021).
- Lv, W., Wang, W. One-photon upconversion-like photolysis: a new strategy to achieve long-wavelength light-excitable photolysis. Synlett. 31 (12), 1129-1134 (2020).
- Rwei, A. Y., Wang, W., Kohane, D. S. Photoresponsive nanoparticles for drug delivery. Nano Today. 10 (4), 451-467 (2015).
- Grzelczak, M., Vermant, J., Furst, E. M., Liz-Marzán, L. M. Directed self-assembly of nanoparticles. ACS Nano. 4 (7), 3591-3605 (2010).
- Gnanasammandhan, M. K., Idris, N. M., Bansal, A., Huang, K., Zhang, Y. Near-IR photoactivation using mesoporous silica-coated NaYF4:Yb,Er/Tm upconversion nanoparticles. Nature Protocols. 11 (4), 688-713 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved