A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Simple Continuous Glucose Monitoring in Freely Moving Mice
In This Article
Summary
Here, we describe a simple method to implant a commercial continuous glucose monitor designed for patients onto mice and provide the scripts to analyze the results.
Abstract
Mice are a common model organism used to study metabolic diseases such as diabetes mellitus. Glucose levels are typically measured by tail-bleeding, which requires handling the mice, causes stress, and does not provide data on freely behaving mice during the dark cycle. State-of-the-art continuous glucose measurement in mice requires inserting a probe into the aortic arch of the mouse, as well as a specialized telemetry system. This challenging and expensive method has not been adopted by most labs. Here, we present a simple protocol involving the utilization of commercially available continuous glucose monitors used by millions of patients to measure glucose continuously in mice as a part of basic research. The glucose-sensing probe is inserted into the subcutaneous space in the back of the mouse through a small incision to the skin and is held in place tightly using a couple of sutures. The device is sutured to the mouse skin to ensure it remains in place. The device can measure glucose levels for up to 2 weeks and sends the data to a nearby receiver without any need to handle the mice. Scripts for the basic data analysis of glucose levels recorded are provided. This method, from surgery to computational analysis, is cost-effective and potentially very useful in metabolic research.
Introduction
Diabetes mellitus (DM) is a devastating disease characterized by high blood glucose levels. Type 1 DM can be a result of an autoimmune attack on the insulin-producing beta cells in the pancreas. Type 2 DM and gestational DM, on the other hand, are characterized by a failure of the beta cells to secrete sufficient insulin in response to a rise in glucose levels1. The mouse is a common model organism used to study DM since it has similar physiology, and its normal glucose levels are close to those of humans. Furthermore, specific mouse strains may develop DM due to mutations in key signaling pathways or following exposure to specific diets, which enables disease modeling2,3,4.
Blood glucose is commonly measured in mice using glucometers designed for patients by extracting a small drop of blood (1-2 µL) from the tip of the tail of the mouse. This method causes stress and requires handling of the mouse, which affects the glucose levels and prohibits the measurement of blood glucose levels in freely behaving mice or when the researcher is not close by5. Bleeding the mice may cause stress to nearby mice, particularly to mice of the same cage whose glycemia has not been measured yet, thus affecting the results. Mice respond differently depending on the handler, and the person measuring glucose may affect the glucose levels of the mice. These pitfalls call for careful experimental design and underlie some inconsistencies between experiments.
It is possible to measure glucose in freely moving mice with no bleeding by implanting glucose sensors into the aortic arch of the mice using state-of-the-art telemetry6. The resulting measurements are very good and can be sustained over a long period, but it is challenging to implant these sensors, and the telemetry system is expensive, leading to a moderate adoption of this methodology and no adoption in non-specialized labs. Subcutaneous or other glucose sensors that are tailored to the dimensions of the mice and their physiology have been developed in recent years, but these again require highly skilled experts and are in some cases costly6,7,8,9,10.
Commercial continuous glucose monitors (CGMs) that were originally developed to monitor the glucose levels of DM patients offer another option to measure glucose in freely moving mice, with lower cost and technical expertise requirements than implanted probes. Such probes have been used in basic research by a few labs5,11,12,13,14,15 including our colleagues who used this protocol16. These devices typically include a sensor, a mounting device, a receiver, and a software application. The sensor has a cannula guiding the enzymatic glucosensor, adhesive tape, an energy source, short-term memory, and a wireless communication module that stores and sends the data to the receiver. The receiver can show the current glucose levels and sends the data to a server; this receiver can be a cellphone. The software application provides data for the patient and the medical care team on the glycaemia of the patient. In patients, the sensor is attached easily using the mounting device. The cannula is inserted subcutaneously by pressing the mounting device against the skin, and the sensor stays in place with the help of adhesive tape.
This is a detailed protocol for adapting a commercial CGM device to measure glucose levels in mice. This protocol describes how to surgically insert the glucose sensor and attach it to the mouse. Scripts for basic data analysis and data visualization are provided. The potential pitfalls, troubleshooting, and examples of standard results are provided. The protocol below is specific for a certain CGM but can be easily adapted to other types of commercial CGMs as they become available.
Protocol
The experiments were approved by the Hebrew University's Institutional Animal Care and Use Committee (IACUC).
NOTE: All tools must be sterilized, and handling of the cannula must be performed using a sterile technique. The protocol below is fine-tuned to a specific CGM. The protocol can be adapted to other CGMs.
1. Analgesic administration before the procedure
- Administer 5% dextrose and 0.45% saline with meloxicam at 5 mg/kg body weight subcutaneously.
2. Anesthesia administration
- Place the mouse in the induction chamber, closing the lid tightly. Set the induction of anesthesia in the induction chamber at 3% isoflurane at a flow rate of 500 mL/min.
- Once the mouse is unresponsive, remove the mouse from the chamber, and fit the nose cone to the mouse. Confirm the anesthesia level with an interdigital pinch. Set the concentration to 1%-1.5% isoflurane and the flow rate to 100 mL/min in a mouse weighing 30 g.
- Apply ophthalmic ointment to the eyes to prevent dryness during anesthesia.
3. Sensor preparation
- Mount the sensor onto the sensor mounting device to expose the sensor's tape and cannula side (Figure 1A). Be cautious since the needle is inserted into the cannula and exposed.
- Suture two 5-0 taper point sutures to the tape on both sides of the cannula (Figure 1A).
4. Hair removal and disinfection
- Shave an area of approximately 4 cm x 4 cm on the midline of the back of the mouse.
- Administer a depilatory cream to the shaved area to ensure complete hair removal.
- Wipe the skin, and disinfect it using an antiseptic solution containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
5. Dorsal skin preparation
- Make a 2 mm incision in the center of the shaved area above the spine using sharp scissors (Figure 1B).
- Briefly, insert small forceps with a blunt edge under the skin to form a small subcutaneous pocket so that the cannula can be inserted easily into the subcutaneous pocket (Figure 1B).
- Pass a suture from step 3.2 through the skin on each side of the incision (Figure 1C).
6. Sensor insertion
- Remove the sensor completely from the sensor mounting device (the cannula is void from the needle), and hold the sensor with forceps to keep the surrounding tape from sticking to itself.
- Carefully insert the cannula into the subcutaneous pocket.
- Pull the sutures on each side, and tighten and tie them to attach the sensor firmly in place, thus preventing the cannula from slipping out from the subcutaneous pocket once the adhesive tape loosens over time.
7. Sensor attachment and suturing
- Attach the sensor to the back firmly by tying the inner sutures and using the adhesive tape surrounding the sensor.
- Make eight discontinuous sutures around the sensor, attaching the border of the sensor's tape to the skin (Figure 1D).
8. Activation of the reader
- Once the sensor has been inserted, activate the reader by turning on the reader, pressing Start New Sensor, and swiping the sensor according to the manufacturer's instructions.
- The first reading can only be taken a few minutes after installing the CGM. In the case of this CGM, the first reading can be taken after 60 min.
9. Reading results
- Place the reader close to the mouse (there is no need to touch it). All data stored in the sensor are transmitted to the reader.
NOTE: Different CGM devices may differ in the period of historic data saving capacity. In the case of this CGM, a maximum of 8 h can be stored between two readings.
10. Removing the sensor
- Anesthetize the mouse (see section 2).
- Cut the sutures connecting the sensor to the back of the mouse using sharp scissors.
- Remove and cut the sutures at the incision by gently removing the sensor.
- If necessary, use a single suture to close the incision in the back of the mouse.
11. Data analysis
- Data download: Download the data as per the instructions provided by the CGM manufacturer.
NOTE: Each CGM has a different format, which may or may not be easily accessible to the user. This is an important consideration in choosing the CGM. - For analysis with the software provided, format the data according to the instructions in the readme file on Github (https://github.com/mika-littor/CGM-in-Mice-Analysis.git).
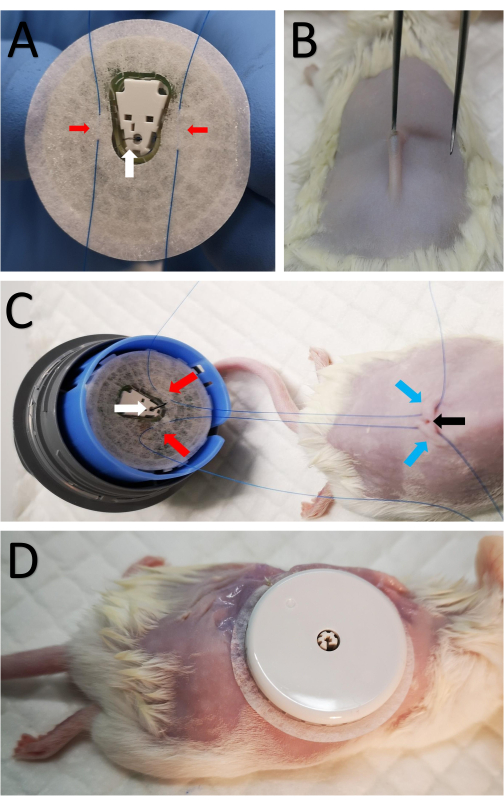
Figure 1: Attachment of the sensor to the mouse. (A) Two sutures marked by red arrows are passed through the sensor tape on both sides of the cannula on the bottom side of the CGM sensor, marked by a white arrow. (B) A small 2 mm incision is made in the center of the shaved area along the spine using sharp scissors. Small forceps with a blunt edge are briefly inserted under the skin to form a small subcutaneous pocket so that the cannula can be inserted subcutaneously. (C) The same sutures from A are passed subcutaneously on each side of the incision. The red arrows mark the sutures attached to the sensor as in A, the blue arrows mark the location the sutures passed through to the skin in the back of the mouse, and the black arrow shows the incision. (D) After the cannula is inserted, the inner sutures are tightened and tied close to the incision to secure the CGM. The sensor is then sutured to the skin. Please click here to view a larger version of this figure.
Results
Surgical outcome
Results from eight HSD:ICR mice (aged 8 weeks) fed a high-fat high-sucrose diet (HFHS) for 18 weeks and five lean HSD:ICR mice (aged 12 weeks) are shown. The device we used stores data for up to 8 h. Access to the local animal facility was restricted to 07:00-19:00, thus prohibiting data collection during the late P.M. hours, when the mice are active. The mice were, therefore, placed in a room with reverse lighting for 7 days before the surgical procedure, with dark hours between 8...
Discussion
This protocol offers a simple, inexpensive method to monitor glucose levels in mice that does not require challenging microsurgery and does not involve bleeding or handling the mice. The method is easy to implement in every facility and does not cause mortality, pain, or excessive discomfort to the mice. The most critical step in the protocol is inserting the cannula of the glucose sensor under the skin of the mouse. The addition of a few sutures allows the cannula to stay in place for a longer time. The sensors are smal...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank Dvir Mintz DVM and the veterinary and husbandry staff in the animal facility, as well as members of our group, for fruitful discussions. This study was supported by an Israel Science Foundation grant 1541/21 awarded to D.B.Z. D.B.Z. is a Zuckerman STEM faculty.
Materials
| Name | Company | Catalog Number | Comments |
| 2% Chlorhexidine Gluconate and 70% Isopropyl Alcohol | 3M | ID 7000136290 | |
| 5% Dextrose and 0.45% Sodium Chloride Injection, USP | Braun | L6120 | |
| Castroviejo needle holder | FST | 12061-02 | |
| Extra Fine Bonn scissors | FST | 14084-08 | |
| FreeStyle Libre 1 reader | Abbott | ART27543 | |
| FreeStyle Libre sensor | Abbott | ART36687 | |
| FreeStyle Libre sensor applicator | Abbott | ART36787 | |
| Gauze pads | Sion medical | PC912017 | |
| Graefe Forceps | FST | 11052-10 | |
| Hair Removal Cream | Veet | 3116523 | |
| High-fat high-sucrose diet | Envigo Teklad diets | TD.08811 | |
| Isoflurane, USP Terrell | Piramal | 26675-46-7 | |
| Meloxicam 5 mg/mL | Chanelle Pharma | 08749/5024 | |
| MiniARCO Clipper kit | Moser | CL8787-KIT | |
| PROLENE Polypropylene Suture 5-0 | Ethicon | 8725H | |
| Puralube Opthalmic Ointment | Perrigo | 574402511 | |
| Q-tips | B.H.W | 271676 | |
| SomnoSuite Low-Flow Anesthesia System | Kent Scientific | SOMNO |
References
- Polonsky, K. S. The past 200 years in diabetes. New England Journal of Medicine. 367 (14), 1332-1340 (2012).
- Rees, D. A., Alcolado, J. C. Animal models of diabetes mellitus. Diabetic Medicine. 22 (4), 359-370 (2005).
- Pearson, J. A., Wong, F. S., Wen, L. The importance of the non-obese diabetic (NOD) mouse model in autoimmune diabetes. Journal of Autoimmunity. 66, 76-88 (2016).
- Heydemann, A. An overview of murine high fat diet as a model for Type 2 diabetes mellitus. Journal of Diabetes Research. 2016, 2902351 (2016).
- Kennard, M. R., et al. The use of mice in diabetes research: The impact of experimental protocols. Diabetic Medicine. 38 (12), 14705 (2021).
- Klueh, U., et al. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technology and Therapeutics. 8 (3), 402-412 (2006).
- Wuyts, C., Simoens, C., Pinto, S., Philippaert, K., Vennekens, R. Continuous glucose monitoring during pregnancy in healthy mice. Scientific Reports. 11, 4450 (2021).
- Korstanje, R., et al. Continuous glucose monitoring in female NOD mice reveals daily rhythms and a negative correlation with body temperature. Endocrinology. 158 (9), 2707-2712 (2017).
- Han, B. G., et al. Markers of glycemic control in the mouse: Comparisons of 6-h-and overnight-fasted blood glucoses to Hb A1c. American Journal of Physiology - Endocrinology and Metabolism. 295 (4), 981-986 (2008).
- Xie, X., et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nature Biomedical Engineering. 2 (12), 894-906 (2018).
- Van Der Meulen, T., et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 21 (7), 769-776 (2015).
- Peterson, Q. P., et al. A method for the generation of human stem cell-derived alpha cells. Nature Communications. 11, 2241 (2020).
- Klueh, U., Liu, Z., Feldman, B., Kreutzer, D. Interstitial fluid physiology as it relates to glucose monitoring technologies: Importance of Interleukin-1 and Interleukin-1 receptor antagonist in short-term glucose sensor function in vivo. Journal of Diabetes Science and Technology. 4 (5), 1073 (2010).
- Klueh, U., Antar, O., Qiao, Y., Kreutzer, D. L. Role of interleukin-1/interleukin-1 receptor antagonist family of cytokines in long-term continuous glucose monitoring in vivo. Journal of Diabetes Science and Technology. 7 (6), 1538 (2013).
- Klueh, U., Kaur, M., Qiao, Y., Kreutzer, D. L. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials. 31 (16), 4540-4551 (2010).
- Kogot-Levin, A., et al. Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition. JCI Insight. , 164296 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved