A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Chimeric Antigen Receptor T Cell Manufacturing on an Automated Cell Processor
In This Article
Summary
This article details the manufacturing process for chimeric antigen receptor T cells for clinical use, specifically using an automated cell processor capable of performing viral transduction and cultivation of T cells. We provide recommendations and describe pitfalls that should be considered during the process development and implementation of an early-phase clinical trial.
Abstract
Chimeric antigen receptor (CAR)-T cells represent a promising immunotherapeutic approach for the treatment of various malignant and non-malignant diseases. CAR-T cells are genetically modified T cells that express a chimeric protein that recognizes and binds to a cell surface target, resulting in the killing of the target cell. Traditional CAR-T cell manufacturing methods are labor-intensive, expensive, and may carry the risk of contamination. The CliniMACS Prodigy, an automated cell processor, allows for manufacturing cell therapy products at a clinical scale in a closed system, minimizing the risk of contamination. Processing occurs semi-automatically under the control of a computer and thus minimizes human involvement in the process, which saves time and reduces variability and errors.
This manuscript and video describes the T cell transduction (TCT) process for manufacturing CAR-T cells using this processor. The TCT process involves CD4+/CD8+ T cell enrichment, activation, transduction with a viral vector, expansion, and harvest. Using the Activity Matrix, a functionality that allows ordering and timing of these steps, the TCT process can be customized extensively. We provide a walk-through of CAR-T cell manufacturing in compliance with current Good Manufacturing Practice (cGMP) and discuss required release testing and preclinical experiments that will support an Investigational New Drug (IND) application. We demonstrate the feasibility and discuss the advantages and disadvantages of using a semi-automatic process for clinical CAR-T cell manufacturing. Finally, we describe an ongoing investigator-initiated clinical trial that targets pediatric B-cell malignancies [NCT05480449] as an example of how this manufacturing process can be applied in a clinical setting.
Introduction
Adoptive transfer of T cells engineered to express a chimeric antigen receptor (CAR) has shown remarkable efficacy in treating patients with refractory B-cell malignancies1,2,3,4,5. However, the traditional manufacturing methods for CAR-T cells are labor-intensive, time-consuming, and require highly trained technicians to carry out highly specialized steps. For example, the traditional manufacturing process of an autologous CAR-T cell product involves density gradient centrifugation, elutriation or magnetic separation to enrich T cells, activation, and transduction with a viral vector in a sterile flask, and expansion in a bioreactor prior to harvest and formulation. Various systems have emerged recently that aim to partially automate this process. For example, the Miltenyi CliniMACS Prodigy (hereafter referred to as the "processor") is an automated cell processing device that can perform many of these steps in an automated fashion6,7,8,9. An in-depth discussion of traditional and automated CAR-T manufacturing methods is presented in a recent review article10.
The processor builds upon the functionality of the CliniMACS Plus, a U.S. Food and Drug Administration (FDA)-approved medical device for the processing of hematopoietic progenitor cells. The processor includes a cell cultivation unit that allows for automated washing, fractionation, and cultivation of cells (Figure 1). The T cell transduction (TCT) process is a preset program within the processor device that largely replicates manual CAR-T cell manufacture. TCT allows for customizable cell processing using a graphical user interface (the "Activity Matrix," Figure 2). Because the processor automates many steps and consolidates the functionality of multiple devices into one machine, it requires less training and specialized troubleshooting skills from technologists. Because all steps are performed within a closed, single-use tubing set, the processor may be operated in facilities with less stringent air-handling infrastructure than would be considered acceptable for an open manufacturing process. For example, we are operating the processor in a facility certified as ISO class 8 (comparable to EU grade C).
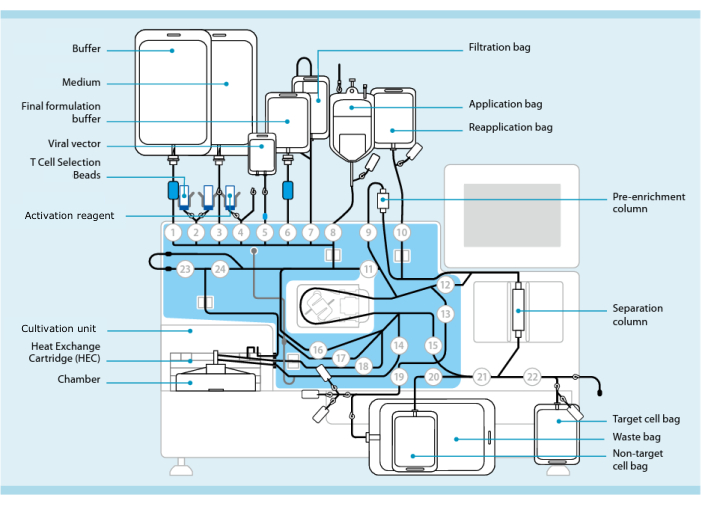
Figure 1: CAR-T cell manufacturing using the T cell transduction system. Shown is the processor with the tubing set installed. The tubing set allows for connecting other components such as bags containing processing buffer, culture medium, and lentiviral vector via sterile welding. Once the leukapheresis product is added to the Application bag, it can be labeled with T Cell Selection Beads, passed through the Separation column, and then transferred into the Reapplication bag. Selected cells are then directed to the Cultivation unit of the instrument for culture and activated with the Activation reagent (see Table of Materials). The final product is collected in the Target cell bag. Throughout the process, it is possible to remove samples for quality control aseptically. Grey numbers inside of circles represent the numbered valves on the processor that direct the liquid path through the tubing set. Reproduced with permission from 11. Please click here to view a larger version of this figure.
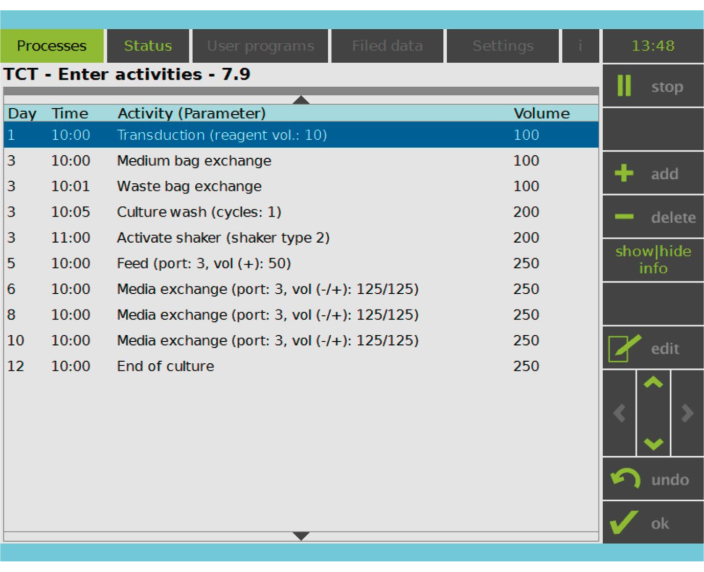
Figure 2: Activity Matrix. After T cell selection and activation, the remainder of the CAR-T cell manufacturing process is fully customizable. Activities can be added or deleted and scheduled for the appropriate day and time, and the culture volume after the activity can be specified (Volume). For example, the Transduction activity was configured to begin at 10:00 AM on Day 1, and the culture volume at the end of the activity was set as 100 mL. The Activity Matrix can be edited throughout the cultivation period. The status of the process can be monitored on the integrated screen of the processing device. Please click here to view a larger version of this figure.
The aim of this manuscript is to provide a detailed walk-through of manufacturing CAR-T cells using the processor and additionally provide guidance on the in-process and product release testing that will likely be required by regulators to approve an investigational new drug (IND) application. The presented protocol stays close to the vendor's recommended approach and is the underlying protocol for IND 28617, which is currently being evaluated in a single-center investigator-initiated phase I/II clinical trial. This trial aims to determine the safety and efficacy of using this processor to manufacture humanized CD19-directed autologous CAR-T cells for patients with B cell acute lymphoblastic leukemia (B-ALL) or B-lineage lymphoblastic lymphoma (B-Lly) [NCT05480449]. The trial started in September 2022 and is planned to enroll up to 89 patients ages 0-29 years with B-ALL or B-Lly. We report some manufacturing results from the trial in the manuscript.
We would like to point out that although the manuscript is presented as a protocol with steps to follow, it should be considered a starting point for others to begin optimizing their own CAR-T cell manufacturing process. A non-comprehensive list of possible variations to the presented protocol includes: using fresh instead of cryopreserved T cells as starting material; using a different method of T cell enrichment or omitting it altogether; using different media and cytokine cocktails such as IL7/IL15 instead of IL2; varying the concentration of human AB serum or omitting it altogether; timing of transduction; using "multi-hit" transductions; varying agitation, culture volumes, and feeding schedule; using different methods of genetic transfer including electroporation of nucleic acids or non-lentiviral vectors; using a different final formulation buffer and/or cryoprotectant; and infusing CAR-T cells fresh instead of cryopreserving for infusion at a later time. These variations may have a significant impact on the cellular composition and potency of the therapeutic product.
| Overall Process Step | Process Day | Technical Details | |||
| Cell Enrichment | Day 0 | Selection of CD4+/CD8+ T cells | |||
| Cell Activation | T cell culture seeding and activation | ||||
| Cell Transduction | Day 1 | Lentiviral transduction (100 mL culture volume) | |||
| Cell Expansion (followed by cell formulation) | Day 2 | -- | |||
| Day 3 | Culture Wash (1 cycle); Shaker activated; Culture volume increases to 200 mL | ||||
| Day 4 | -- | ||||
| Day 5 | Feed (50 mL); Culture volume reaches final volume of 250 mL | ||||
| Day 6 | In-process sample; Media exchange (-125 mL / +125 mL) | ||||
| Day 7 | Media exchange (-150 mL / +150 mL) or Harvest | ||||
| Day 8 | In-process sample; Media exchange (-150 mL / +150 mL) or Harvest | ||||
| Day 9 | Media exchange (-180 mL / +180 mL) or Harvest | ||||
| Day 10 | In-process sample; Media exchange (-180 mL / +180 mL) or Harvest | ||||
| Day 11 | Media exchange (-180 mL / +180 mL) or Harvest | ||||
| Day 12 | Media exchange (-180 mL / +180 mL) or Harvest | ||||
| Day 13 | Harvest | ||||
Table 1: Process timeline and overview. This table summarizes the TCT process steps employed in a current clinical trial [NCT05480449]. The process starts with T cell enrichment by CD4+/CD8+ selection, culture seeding, and activation on Day 0, followed by transduction on Day 1. Cells rest for 48 h, followed by a culture wash, an increase of the culture volume to 200 mL, and agitation using a shaking mechanism. On Day 6, the first in-process sample is taken. Cells are harvested once sufficient cells are available for at least three full doses of CAR-T cells (5 × 106 CAR-T cells/kg if the patient is <50 kg, otherwise 2.5 × 108 CAR-T cells) and quality control testing (~2 × 106 CAR-T cells); or once the culture reaches a total of 4-5 x 109 cells. Abbreviations: TCT = T cell transduction; CAR-T = chimeric antigen receptor T cells; MACS = magnetic-activated cell sorting.
Protocol
All research was performed in compliance with institutional guidelines with approval by the hospital's Institutional Review Board (IRB), and all subjects have provided informed consent for publication of the data collected within the context of the trial.
NOTE: The first section of the Protocol provides a high-level overview of the CAR-T manufacturing process. The remaining sections provide the step-by-step instructions. The protocol describes the workflow using TCT software version 1.4, which is the current version as of this writing. The user interface of other versions of the TCT software may vary.
1. Process timeline and overview (Table 1)
- Prepare for the procedure on a Monday (Day -1) with preflight checks. Ensure that the processor and other equipment are running as expected and that all reagents and consumables are available and in-date for the entire manufacturing run.
- On day 0, install the tubing set on the machine. Thaw previously cryopreserved T cells in a dry bath and connect them by sterile welding to the tubing set installed on the instrument.
NOTE: This protocol assumes that autologous T cells were collected via apheresis, cryopreserved, and stored until the start of manufacturing. While it is possible to use freshly collected T cells, this increases the logistical burden as it requires coordinating apheresis collection with CAR-T cell manufacturing. We strongly recommend using a dry-thaw process instead of a water bath to minimize the risk of bacterial contamination. - Label T cells with CD4 and CD8 reagents (see Table of Materials) and enrich by magnetic selection.
NOTE: It is possible to omit T cell enrichment or perform it prior to loading cells onto the processor. See section 5 of the Protocol. - After enrichment of CD4+/CD8+ cells, take a sample for a cell count. Seed the culture with 1-2 × 108 cells in an initial volume of 70 mL of medium (see Table of Materials) supplemented with 5% human AB serum and recombinant human IL2 (25 ng/mL).
- Add a vial of activation reagent (a colloidal polymeric nanomatrix conjugated to anti-CD3 and anti-CD28 antibodies; see Table of Materials) to activate T cells and incubate the culture without agitation for 24 h at 37 ºC in a 5% CO2 atmosphere. Cryopreserve any leftover CD4+/CD8+ cells as backup, if desired.
NOTE: In case of manufacturing failure, leftover CD4+/CD8+ selected cells may be used as starting material. If the failure is purely technical, such as contamination or operator error, and sufficient cells remain, then using leftover cells to perform an additional manufacturing run can be considered. If the quality of starting material is of concern, then a new apheresis procedure could be warranted; however, this is ultimately a clinical decision. In either case, a manufacturing failure is a significant event that should be investigated, and the sponsor and possibly regulators should be informed. - After 24 h of activation, transduce T cells with lentiviral vector at an appropriate multiplicity of infection (MOI).
NOTE: It is critical to determine the lentiviral vector titer and establish an appropriate MOI before starting to manufacture clinical products. The vector titer should be determined by performing a small-scale experiment in which human primary T cells are transduced at various concentrations of the vector. Considerations for the appropriate MOI include the cost of the vector, the desired transduction efficiency, and an acceptable vector copy number. This protocol uses an MOI of 30-50% to minimize the cost of the vector and keep the average vector copy number below 8 copies per transduced cell. - On Day 3, trigger the Culture Wash activity, and start low-level agitation. Increase the culture volume to 200 mL.
- On Day 5, add 50 mL of medium to the culture, increasing the culture volume to 250 mL.
- On Day 6 of cultivation, take a sample from the culture, and enumerate CAR-T cells by flow cytometry. Use this measurement to estimate the rate at which the culture is growing and identify the optimal time of harvest.
NOTE: Each in-process sampling yields 3 mL for testing and removes 7 mL total of culture volume. - From Day 7 to 13, if the culture has not been terminated, take up to two additional in-process samples on alternate days, and perform daily media exchanges to feed the growing culture. Harvest the product when the total nucleated cell (TNC) count reaches 5 × 109 and/or when sufficient cells are available for the required number of doses and release testing.
- On the day of harvest, take an in-process sample from the actively growing culture. Use this sample for mycoplasma, endotoxin, replication-competent lentivirus (RCL) testing, vector copy number (VCN) testing, cell count, cell size analysis, flow cytometry, and gram stain.
- Initiate the final harvest program, which triggers the removal of the culture medium and a cell wash with the final formulation buffer (a sterile isotonic crystalloid solution supplemented with 4% human serum albumin; see Table of Materials). After harvest is completed, the target cell bag will hold 100 mL of cell product in final formulation buffer. Add dimethyl sulfoxide (DMSO) to a concentration of 10% (v/v), aliquot the product into individual doses, and cryopreserve using a controlled-rate freezer.
2. Day -1: Preparation and preflight checks
- Verify that CO2 gas levels and compressed air are sufficient to produce an inlet pressure of at least 20 psi on each line.
- Turn on the processor and confirm that there are no errors on startup. If needed, set the clock to the correct time. Shut down the processor.
- Ensure cell count, volume, and total CD4+ and total CD8+ count of the T cell starting material are known.
- Ensure there are sufficient quantities of the viral vector, reagents, and consumables for the entire manufacturing run.
- Place a bottle of human AB serum in the refrigerator to thaw overnight.
3. Day 0: Tubing set installation
- Prepare 3 L of processing buffer (0.5 % (w/v) human serum albumin (HSA) in phosphate-buffered saline / ethylenediaminetetraacetic acid (PBS/EDTA) buffer).
- Prepare 2 L of culture medium (2 L of medium supplemented with 100 mL of human AB serum to a final concentration of 5% and two vials of recombinant human IL2 at 25 µg/vial; see Table of Materials for more information on these reagents). Transfer 10 mL culture medium into a 20 mL reagent bag and store at 4 ºC overnight.
- Turn on the instrument and select the T Cell Transduction Process (TCT) from the touch screen interface. Click Run to start the TCT process; let the instrument guide the user through the procedure using on-screen instructions and prompts.
- On the Parameter Input screen, enter the operator's initials, the tubing set lot number, and its expiration date when prompted.
- The Process Setup screen presents four different processes. Choose Full process (1).
NOTE: Other available processes include starting from CD4+/CD8+ selected T cells (see section 5 below) and restarting a previously aborted manufacturing run. - When prompted, choose two vials of selection reagent to reflect the CD4+/CD8+ selection method.
- Install the tubing set per the on-screen instructions. Ensure all Luer connections are tight and that there are no defects in the tubing set.
- Follow the on-screen instructions to initiate the automated upper and lower integrity tests.
NOTE: An integrity test may fail due to a faulty tubing set, an incorrectly installed tubing set, or a defect in the machine's peristaltic pump. We strongly recommend having at least one additional tubing set on hand for production runs. We also recommend that a second technologist verify the correct installation of the tubing set. - Follow the on-screen instructions for attaching the medium and processing buffer bags.
- Begin the automatic priming of the tubing set.
NOTE: The TCT process must continue within 3 h after priming. Ensure T cell starting material will be ready.
4. T cell enrichment
- When the Transfer cell product screen appears, begin thawing the cryopreserved T cell product.
NOTE: The acceptable volume of T cells that can be added to the tubing set ranges from 50 to 280 mL. The maximum number of target (sum of CD4+ and CD8+) cells is 3 × 109, and the maximum TNC count is 2 × 1010. - Transfer the thawed cells to a 150 mL transfer bag. Sterile weld the transfer bag to the Application bag of the tubing set.
- Remove a sample from the Application bag using the QC pouch and perform a cell count.
- Connect the CD4 and CD8 reagent vials.
- Start the selection (T cell enrichment) process.
- After enrichment, remove a sample of the CD4+/CD8+ selected cells from the Reapplication bag's QC pouch for cell count, flow cytometry, and cell size analysis.
NOTE: The cell count result is needed to proceed to the next step.
5. Alternative: Starting with CD4+/CD8+ selected cells
- Prepare the Medium as described in step 3.2. No processing buffer is needed.
- Turn on the processor and select T cell cultivation with TS installation (3) on the Process Setup screen. Follow the on-screen instructions and prompts.
- Following the on-screen instructions, prime the tubing set with medium instead of processing buffer.
- When the "Prepare cultivation-Connect cell product" screen appears, begin thawing the CD4+/CD8+ selected T cells.
NOTE: The minimum number of T cells (sum of CD4+ and CD8+ T cells) for the process is 1.0 × 108. - Transfer cells to a 150 mL transfer bag and dilute with medium to a final volume of 50 mL.
- Sterile weld the cell suspension to the instrument's Reapplication bag.
- Remove a sample of the CD4+/CD8+ selected cells from the Reapplication bag's QC pouch for cell count and cell size analysis.
6. Culture setup and programming of the Activity Matrix
- Enter the cell concentration and desired starting number (1-2 × 108 T cells).
NOTE: The instrument will automatically pump the appropriate volume from the Reapplication bag into the culture chamber and adjust the final volume to 70 mL. - Attach a vial of the activation reagent per the on-screen instructions.
- Enter 5% for CO2 concentration and 39 °C for culture chamber temperature.
NOTE: The manufacturer recommends entering 39 °C or a value specifically calibrated for the instrument. - Set up the Activity Matrix. Use the Enhanced feeding protocol as a starting point and modify individual steps within the protocol to user-defined specifications (Figure 2).
- Ensure that the time of Transduction activity is 24 h after seeding (Day 1).
- Ensure that the time of Culture Wash activity is 48 h after Transduction (Day 3).
- Set the time of Activate Shaker (shaker type 2) to 30 min after the start of the Culture Wash.
- Delete any Medium Bag Exchange and Waste Bag Exchange activities.
- Touch ok on the screen to begin cultivation.
- Cryopreserve any leftover CD4+/CD8+ selected cells in the Application bag for future use, if needed.
7. Day 1: T cell transduction
- Calculate the volume of the lentiviral vector to use based on the desired MOI.
- Retrieve the 20 mL reagent bag containing 10 mL culture medium that was stored at 4 ºC overnight (step 3.2). Thaw the vector vial and transfer the volume calculated in 7.1 into the 20 mL reagent bag.
NOTE: We recommend freezing any leftover vector for retention or for research and development. - Modify the Activity matrix to set the time of Transduction to 2 min into the future. When prompted, touch ok to start the Transduction activity.
- Sterile weld the vector bag to the tubing set following the on-screen instructions.
- Modify the Activity matrix based on the actual time that the Transduction activity was started.
NOTE: We suggest starting transduction within 20-24 h after seeding to ensure hands-on activities are performed during normal working hours.- Ensure that the time of Culture Wash is 48 h after Transduction (Day 3).
- Set the time of Activate Shaker (shaker type 2) to 30 min after Culture Wash (Day 3).
- Ensure that the time of Media Exchange is in the afternoon (1 PM) of Day 6.
8. Day 6: First in-process sample
- Touch the Sample button and follow the on-screen instructions to obtain a QC sample from the active culture.
- Perform cell count, flow cytometry, and cell size analyses. Send 1 mL of the sample for a Gram stain.
NOTE: Cell counts should be repeated approximately every other day to monitor culture growth. - Prepare another 2 L of culture medium (see step 3.2).
- Add a Medium Bag Exchange activity to the Activity Matrix, timed to start 2 min into the future. Adjust the Media Exchange activity to start 20 min into the future. Follow on-screen instructions.
NOTE: Media Exchange is the replacement of the culture medium in the cultivation chamber. Medium Bag Exchange is the replacement of the culture medium bag hung on the instrument.
9. Day of harvest (anywhere from day 7 to 13): Harvest and cryopreservation
- Touch the Sample button and follow the on-screen instructions to obtain a QC sample from the active culture.
- Separate the QC sample into three aliquots of 1 mL each. Use 1 mL for flow cytometry, cell count, and cell size analyses. Use 1 mL for mycoplasma, vector copy number, replication-competent lentivirus, and endotoxin testing. Send the final 1 mL for Gram stain.
- Prepare the final formulation buffer (a sterile isotonic crystalloid solution supplemented with 4 % HSA in a 2 L bag, see Table of Materials). Save 100 mL of this buffer to prepare the cryoprotectant solution at a later step.
- Modify the Activity Matrix to set the time of End of Culture to 2 min into the future and delete all other remaining activities. Follow the on-screen instructions to attach the final formulation buffer to the tubing set and begin harvest.
NOTE: The processor will automatically transfer the cells into the Target cell bag. The volume will be 100 mL. - Take a 0.5 mL sample from the Target cell bag QC pouch and perform a cell count.
- Seal off the Target cell bag and remove it from the tubing set. Divide the CAR-T product into appropriate doses and cryopreserve them in a controlled-rate freezer. Store cells in the vapor phase of liquid nitrogen storage tank at ≤ -150 ºC.
NOTE: Final formulation and aliquoting of doses are specific to the protocol. We provide here an example in which three equal doses of CAR-T cells and QC vials are cryopreserved. See section 10 for details. - Download the process data from the instrument, remove and dispose of the tubing set, and perform a shutdown.
10. Cryopreservation of CAR-T cells
NOTE: This protocol assumes CAR-T cells are cryopreserved after manufacture and stored until the patient is ready for infusion. While it is possible to infuse freshly manufactured CAR-T cells, this increases the logistical burden as it requires coordinating CAR-T cell manufacturing with CAR-T cell infusion. This may be problematic in case of a manufacturing failure. Especially if the clinical protocol requires lymphodepleting chemotherapy prior to CAR-T infusion, we strongly suggest cryopreserving, because a manufacturing failure may expose the patient to the risk of unnecessary chemotherapy. Regulators may require investigators to demonstrate the product passes all release testing prior to infusion, which may be difficult to achieve without cryopreservation.
- From the final 100 mL of harvested product, determine and remove the volume required to meet the desired CAR-T doses and quality control (QC) vials for performing additional release testing (e.g., viability), retention, and a sterility sample.
NOTE: It is critical to develop a fully defined strategy for aliquoting the final product. We suggest manufacturing multiple doses of the product to allow for reinfusions, given that sufficient material is available. A product volume of 10-20 mL in a bag allows for rapid thawing and easy infusion by syringe push. It is recommended to fill each product to the desired CAR-T cell dose (e.g., 5 × 106 CAR-T cells per kg or 2.5 × 108 CAR-T cells) as this will avoid the need for dose calculations on the day of infusion. Also recommended are cryopreserving at least four QC vials and accounting for the possibility that there might be leftover material; we suggest saving this material as it can be useful for research and development purposes. - Centrifuge the cells to reduce the volume.
- Bring up the product(s) to half of the desired final volume using the portion of final formulation buffer put aside in step 9.3.
- Prepare 2x cryoprotectant by creating a 20% DMSO solution in final formulation buffer.
NOTE: Cryoprotectant formulation and DMSO concentration can be modified. - Add 2x cryoprotectant to cells at equal volume for a final DMSO concentration of 10%.
NOTE: Duration of exposure of the product to cryoprotectant should be minimized. - Fill the dose bags and QC vials. Send 1 mL for sterility.
NOTE: Regulators commonly require a 14-day sterility assay performed on the final product. However, rapid sterility assays are emerging; at this time, the compendial 14-day method is the most common. - Cryopreserve products using a controlled-rate freezer.
NOTE: The controlled-rate freezer program should be validated to produce a cooling rate of ~ -1 ºC/min to ~ -45 ºC, with compensation for the eutectic point. - Store the product in the vapor phase of a liquid nitrogen ultralow freezer at ≤ -150 °C.
11. Examination of procedure performance
NOTE: Throughout the TCT process, several QC samples are drawn from the active culture. Table 2 provides a grid that can help the reader organize the results for reference and calculate procedure performance metrics. The terms below consisting of a letter and a number (e.g., "B4") refer to cells in the grid of this table. The following values are used in the performance calculations: B3 = total nucleated cells (TNC) pre-enrichment; B4 = TNC post-enrichment; E2 = The sum of CD4+ and CD8+ T cells as a percentage of the total cells of the initial apheresis product; E4 = The sum of the CD4+ and CD8+ T cells as a percentage of the total cells post-enrichment; G2 = CD19+ cells as a percentage of the total cells of the initial product; G4 = CD19+ cells as a percentage of the total cells after CD4+/CD8+ enrichment; B10 = TNC of the Active Culture on day of harvest.
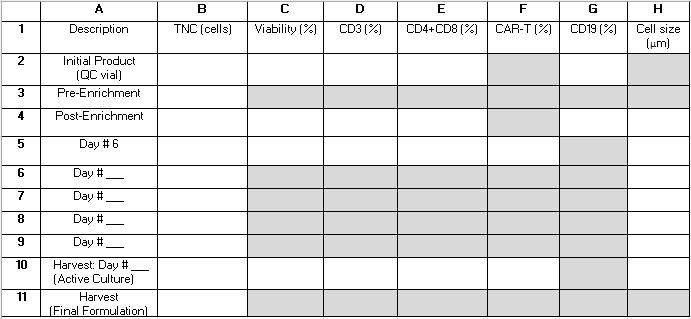
Table 2: Procedure performance grid. We provide this grid to help organize in-process test reults needed to calculate procedure performance statistics. Rows represent samples analyzed at various time points during the procedure and are labeled with numbers 1-11. Rows 6-9 can be used to capture results from samples taken after day 6 of cultivation but prior to the day of harvest. Columns represent measured parameters and are labeled with letters A-H. Fields that are shaded gray do not apply. Some additional fields may not apply depending on whether CD4+/CD8+ enrichment is performed as part of the procedure and the length of the cultivation. We suggest writing "N/A" into those fields. Abbreviations: TNC = total nucleated cell count; QC = quality control. Please click here to download this Table.
- Calculate recovery of CD4+/CD8+ cells after selection by dividing the total number of CD4+ plus CD8+ T cells post-enrichment by the total number of CD4+ plus CD8+ T cells prior to enrichment using equation (1).
CD4+/CD8+ T cell recovery =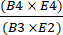 (1)
(1) - Calculate depletion of CD19+ cells after enrichment by dividing the total number of CD19+ cells post CD4+/CD8+ enrichment by the total number of CD19+ cells prior to enrichment. Since this number is expected to be very small, report the logarithm of this fraction as shown in equation (2):
Log CD19 cell depletion = log10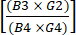 (2)
(2) - Calculate the total fold growth by dividing the total number of cells in active culture at harvest by the number of cells seeded using equation (3):
Total fold growth =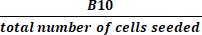 (3)
(3) - Calculate the average daily growth by taking the root of the total fold growth with respect to the day of harvest using equation (4):
Average daily growth = day of harvest √ (total fold growth) (4)
Results
Results from the initial three CAR-T manufacturing runs of the NCT05480449 trial are presented below in Table 3. The starting material, vector, culture cytokines, and AB serum concentrations were kept consistent for each run. Products were harvested on day 7 or 8. The average daily cell growth was 46% (increase in total cell count), indicating that the TCT process was effective in promoting cell expansion. These results suggest that the processor can produce consistent and reproducible CAR-T cell product...
Discussion
CAR-T cell therapy has emerged as a promising treatment approach for B-cell and other malignancies. However, traditional CAR-T cell manufacturing methods have several limitations, such as high cost, labor-intensive production, and open steps that increase the risk of contamination. Recently, several semi-automated platforms, including the Miltenyi CliniMACS Prodigy (the "processor"), have emerged to address these limitations. The T cell transduction (TCT) process, integrated into the processor described in this m...
Disclosures
S.K., S.G., and Y.W. have received research support from Miltenyi Biotec.
Acknowledgements
The authors would like to acknowledge the contributions of several individuals and organizations to this work. The Cell and Gene Therapy Laboratory and the Penn Translational and Correlative Studies Laboratory provided valuable assistance with process development and preparation for IND submissions. Melissa Varghese and Amanda DiNofia contributed to the process development and preparation for IND submissions that underly this manuscript. This work was supported by an Acceleration Grant of the Cell and Gene Therapy Collaborative of the Children's Hospital of Philadelphia. The authors would also like to thank Miltenyi Biotec for their technical and research support. Figure 1 is covered by copyright © 2023 Miltenyi Biotec B.V. & Co. KG; all rights reserved.
Materials
| Name | Company | Catalog Number | Comments |
| 12 x 75 borosilicate tubes | Charles River | TL1000 | |
| 20 mL Reagent Bag | Miltenyi Biotec | 170-076-631 | |
| 50 mL Conical Tube | Fisher | 05-539-10 | |
| 150 mL Transfer Set | Fenwal | 4R2001 | |
| 2,000 mL Transfer Set | Fenwal | 4R2041 | |
| 7AAD | Fisher Scientific | BDB559925 | |
| Alcohol Prep | Tyco/Healthcare | ||
| Bag Access | Medline | 2300E-0500 | |
| CD19 APC-Vio770 REAfinity | Miltenyi Biotec | 130-113-643 | |
| CD19 CAR Detection Reagent Biotin | Miltenyi Biotec | 130-129-550 | |
| CD19 PE | BD | 555413 | |
| CD3 APC | BD | 340440 | |
| CD4 VioBright FITC REAfinity | Miltenyi Biotec | 130-113-229 | |
| CD45 VioBlue REAfinity | Miltenyi Biotec | 130-110-637 | |
| CD8 APC-Vio770 REAfinity | Miltenyi Biotec | 130-110-681 | |
| Cellometer Reference Beads 10um | Nexcelom | B10-02-020 | |
| Cellometer Reference Beads 15um | Nexcelom | B15-02-010 | |
| Cellometer Reference Beads 5um | Nexcelom | B05-02-050 | |
| Cellometer Slides | Nexcelom | CHT4-SD100-002 | |
| CliniMACS CD4 GMP MicroBeads | Miltenyi Biotec | 276-01 | The CD4 reagent |
| CliniMACS CD8 GMP MicroBeads | Miltenyi Biotec | 275-01 | The CD8 reagent |
| CliniMACS PBS/EDTA Buffer | Miltenyi Biotec | 130-021-201 | The buffer |
| DMSO | Origen | CP-10 | |
| Freezing Bag 50 mL | Miltenyi Biotec | 200-074-400 | |
| Freezing Vial, 1.8 mL | Nunc | 12565171N | |
| Freezing Vial, 4.5 mL | Nunc | 12565161N | |
| Human AB serum | Valley Biomedical | Sterile filtered, heat inactivated | |
| Human Serum Albumin 25% | Grifols | 68516-5216-1 | |
| Human Serum Albumin 5% | Grifols | 68516-5214-1 | |
| MACS GMP Recombinant Human IL-2 | Miltenyi Biotec | 170-076-148 | The cytokines |
| MACS GMP T Cell TransAct | Miltenyi Biotec | 200-076-202 | The activation reagent |
| MycoSeq Mycoplasma Detection Kit | Life Technologies | 4460623 | |
| Needles, Hypodermic 14G | Medline | SWD200573 | |
| Needles, SlideSafe 18G | BD | B-D305918 | |
| Pipet tips, 2-200 μL, individually wrapped | Eppendorf | 022492209 | |
| Pipet tips, 50-1000 μL, individually wrapped | Eppendorf | 022492225 | |
| Pipets 10 mL | Fisher | 13-678-27F | |
| Pipets 25 mL | Fisher | 13-675-30 | |
| Pipets 5 mL | Fisher | 13-678-27E | |
| Plasmalyte-A | Baxter | 2B2544X | The electrolyte solution |
| Prodigy TS520 Tubing Set | Miltenyi Biotec | 170-076- 600 | The tubing set |
| Sterile Field | Medline | NON21001 | |
| Streptavidin PE-Vio770 | Miltenyi Biotec | 130-106-793 | |
| Syringe 1 mL | BD | 309628 | |
| Syringe 10 mL | BD | 302995 | |
| Syringe 3 mL | BD | 309657 | |
| Syringe 30 mL | BD | 302832 | |
| Syringe 50 mL | BD | 309653 | |
| TexMACS GMP Medium | Miltenyi Biotec | 170-076-306 | The medium |
| Triple Sampling Adapter | Miltenyi Biotec | 170-076-609 | |
| Viral Vector | CHOP Clinical Vector Core | huCART19 | |
| Equipment | |||
| Biological Safety Cabinet | The Baker Co | ||
| Cellometer Auto 2000 | Nexcelom | ||
| CliniMACS Prodigy | Miltenyi Biotec | 200-075-301 | The processor |
| Controlled Rate Freezer | Planer/Kryosave | ||
| Endosafe nexgen-PTS150K | Charles River | ||
| Mettler Balance | Mettler | ||
| Refrigerated Centrifuge | Thermo Fisher | ||
| Refrigerated Centrifuge | Fisher Sci | ||
| SCD Sterile Tubing Welder | Terumo | ||
| Sebra Tube Sealer | Sebra | ||
| Varitherm | Barkey | The dry thaw device | |
| XN-330 Hematology Analyzer | Sysmex |
References
- Maude, S. L., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine. 378 (5), 439-448 (2018).
- Shah, N. N., et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nature Medicine. 26 (10), 1569-1575 (2020).
- Maude, S. L., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New England Journal of Medicine. 371 (16), 1507-1517 (2014).
- Grupp, S. A., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 368 (16), 1509-1518 (2013).
- Maude, S. L., et al. Efficacy of humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children and young adults with relapsed/refractory acute lymphoblastic leukemia. Blood. 128 (22), 217 (2016).
- Mock, U., et al. Automated manufacturing of CAR-T cells for adoptive immunotherapy using CliniMACS Prodigy. Cytotherapy. 18 (8), 1002-1011 (2016).
- Fernández, L., et al. GMP-compliant manufacturing of NKG2D CAR memory T cells using CliniMACS Prodigy. Frontiers in Immunology. 10 (10), 2361 (2019).
- Zhu, F., et al. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T Cells using CliniMACS Prodigy device at an academic medical center. Cytotherapy. 20 (3), 394-406 (2018).
- Zhang, W., Jordan, K. R., Schulte, B., Purev, E. Characterization of clinical grade CD19 chimeric antigen receptor T cells produced using automated CliniMACS prodigy system. Drug Design, Development and Therapy. 12 (12), 3343-3356 (2018).
- Abou-El-Enein, M., et al. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discovery. 2 (5), 408-422 (2021).
- Miltenyi Biotec. . CliniMACS Prodigy User Manual. , (2021).
- Ghassemi, S., et al. Rapid manufacturing of non-activated potent CAR T cells. Nature Biomedical Engineering. 6 (2), 118-128 (2022).
- U.S. Department of Health and Human Services, Food and Drug Administration. . Chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs) guidance for industry. , (2020).
- U.S. Department of Health and Human Services, Food and Drug Administration. . Considerations for the development of chimeric antigen receptor (CAR) T cell products draft guidance for industry. , (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved