A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Two-Photon Calcium Imaging of Forebrain Activity in Behaving Adult Zebrafish
In This Article
Summary
Here, we present a protocol to perform two-photon calcium imaging in the dorsal forebrain of adult zebrafish.
Abstract
Adult zebrafish (Danio rerio) exhibit a rich repertoire of behaviors for studying cognitive functions. They also have a miniature brain that can be used for measuring activities across brain regions through optical imaging methods. However, reports on the recording of brain activity in behaving adult zebrafish have been scarce. The present study describes procedures to perform two-photon calcium imaging in the dorsal forebrain of adult zebrafish. We focus on steps to restrain adult zebrafish from moving their heads, which provides stability that enables laser scanning imaging of the brain activity. The head-restrained animals can freely move their body parts and breathe without aids. The procedure aims to shorten the time of head restraint surgery, minimize brain motion, and maximize the number of neurons recorded. A setup for presenting an immersive visual environment during calcium imaging is also described here, which can be used to study neural correlates underlying visually triggered behaviors.
Introduction
Calcium fluorescence imaging with genetically encoded indicators or synthetic dyes has been a powerful method of measuring neuronal activity in behaving animals, including non-human primates, rodents, birds, and insects1. The activity of hundreds of cells, up to approximately 800 µm below the brain surface, can be measured simultaneously using multi-photon imaging2,3. The activity of specific cell types can also be measured by expressing calcium indicators in genetically defined neuronal populations. Application of the imaging method for small vertebrate models opens up new possibilities in the field of neuronal computation across brain regions.
Zebrafish are a widely used model system in neuroscience research. Larval zebrafish at around 6 days post-fertilization have been used for calcium imaging due to their miniature brain and transparent body4. Juvenile zebrafish (3-4 weeks old) are also used for studying the neural mechanisms underlying sensorimotor pathways5,6. However, the maximal performance level for complex behaviors, including associative learning and social behaviors, is reached at an older age7,8. Thus, a reliable protocol is required to study multiple cognitive functions in the brains of adult zebrafish using imaging methods. While zebrafish larva and juvenile zebrafish can be embedded in agarose for in vivo imaging, adult zebrafish at 2 months or older suffer from hypoxia in such conditions and are physically too strong to be restrained by agarose. Therefore, a surgical procedure is required to stabilize the brain and enable the animal to breathe freely through the gills.
Here, we describe a head restraint protocol that involves a novel design of a single head bar. The reduced surgery time of 25 min is twice as fast as the previous method9. We also describe the design of the recording chamber (semi-hexagonal tank), the head stage and a quick-lock mechanism to combine the two parts9. Finally, the setup to present an immersive visual stimulus to study visually triggered brain activity and behaviors is also described. Overall, the procedures described here can be used to perform two-photon calcium imaging in genetically defined cell populations in a head-restrained adult zebrafish, enabling the investigation of brain activities during various behavioral paradigms.
Protocol
All animal procedures were approved and carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Academia Sinica. Details of the research tools can be found in the Table of Materials.
1. Preparation of recording chamber
- Prepare a semi-hexagonal tank, a base plate, and a head stage (Figure 1A; Supplementary Files 1-3). The head stage consists of two metal posts attached to a circular plate. The circular plate contains grooves that can be locked onto the base plate. After being locked together, the head stage and the base plate are laid at the bottom of the semi-hexagonal tank.
- Place the semi-hexagonal tank on the experimental platform of the microscope. The platform should be able to move in the X and Y directions without hitting the limit of the translation stage.
- Aim the 810 nm infrared (IR) light and the camera to the head stage for behavioral recording. Ensure that the field of view of the camera is large enough to fit an adult zebrafish.
- To prevent the two-photon laser and the visual stimulus from interfering with behavioral recording, set up an 875 nm short-pass filter and a 700 nm long-pass filter in front of the camera, respectively.
- To present the visual stimulus, attach back-projection films to the inner side of the three walls of the semi-hexagonal tank (Figure 1A).
- Aim the three projectors at the three surfaces of the tank. The three images should be aligned to form a continuous visual scene. To prevent projector lights from contaminating the calcium fluorescence signal, place a neutral density filter and a red color filter (600 nm, long-pass) in front of each projector, and place a band-pass filter (510/80 nm) in front of the photomultiplier tube (PMT).
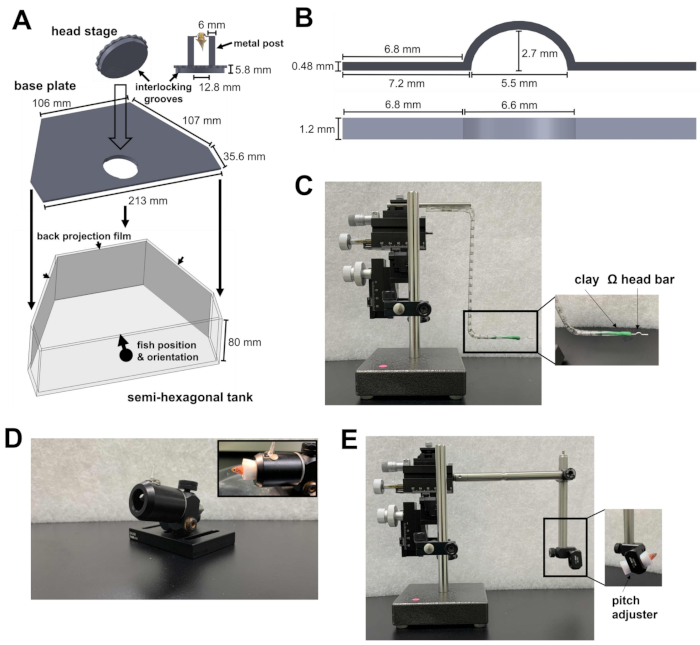
Figure 1: Instruments required for head restraint surgery. (A) The quick-lock mechanism between the circular plate of the head stage and the base plate inside the semi-hexagonal tank. Computer-aided design (CAD) files of the custom-made parts can be found in Supplementary Files 1-4. (B) Ω-shaped head bar for the head restraint. (C) The three-axis micromanipulator used to position the head bar to the attachment site. Inset: orientation of the head bar in the clay. (D) Cannon to hold the fish during surgery. Inset: orientation of fish within the cannon. (E) Fish loading module and the micromanipulator used to load the fish onto the head stage. Inset: orientation of fish within the module. Please click here to view a larger version of this figure.
2. Head restraint surgery
- Prepare an Ω-shaped head bar (Figure 1B; Supplementary File 4) for the zebrafish head restraint. To do this, attach a piece of oil-based modeling clay to a three-axis micromanipulator. Ensure that the clay is solid enough to hold the head bar, but soft enough to detach from the head bar after head restraint.
- Insert an arm of the head bar into the clay and orient the head bar horizontally (Figure 1C). This will ensure that the later attachment of the head bar onto the zebrafish will be level.
NOTE: The head bar can be made of titanium (24 mg) or stainless steel (43 mg) and can be reused for multiple experiments. The head bar material does not affect the behavior of adult zebrafish (weight ranges from 300-1,000 mg) under a head restraint. - Prepare the cannon, a hollow tube for holding the fish body in place during surgery (Figure 1D).
- Prepare 0.03% and 0.01% tricaine methanesulfonate (TMS; see Table of Materials) in fish tank water. This will be used to anesthetize and maintain the fish in a sedated state during surgery.
- Prepare four tissue swabs to remove excess skin and water from the attachment sites on the skull. To prepare each swab, cut a paper towel into a 3 cm square and fold along its diagonal to produce a tube-like structure. Twist the ends of the tube into a fine point. The attachment site is very small, so a fine point allows finer control of the swab.
- Prepare a fish loading module for the micromanipulator (Figure 1E).
NOTE: The module consists of two steel posts held together by a right-angle post clamp. One post attaches to the micromanipulator, while the other post holds a rotating post clamp that carries the fish. The module is used to position the fish onto the head stage with fine control. The rotating post clamp serves as a pitch adjuster to change the pitch angle of the fish. - Anesthetize the fish with 0.03% TMS in fish tank water. Throughout the following steps, use a syringe to deliver 0.01% TMS in fish tank water to the mouth in short pulses every 90 s. Water flow from the perfusion should induce gill movement. This will allow the fish to survive for more than 40 min during surgery.
NOTE: Tg[neuroD:GCaMP6f] is used due to its broad expression of the calcium indicator in the forebrain at both the larval and adult stages10.
NOTE: Optionally, use gravity perfusion to provide constant water flow to the mouth during periods in surgery where both hands are occupied. - Wrap the fish into a capsule (Figure 2A) that holds the fish inside the cannon.
- Wrap the anesthetized fish in a piece of moist paper tissue starting from the tip of the tail to around 1 mm caudal to the gill. Ensure that the wrapping is tight to prevent sliding of the fish out of the tissue.
- Wrap a soft plastic tubing with a longitudinal slit (e.g., a medium-sized heat-shrink tubing cut open) around the paper tissue to cover the fish from the end of the tail to around 2 mm caudal to the gill. The tubing ensures that the fish's body remains straight throughout the surgery.
- Wrap a paper towel around the tubing to a diameter that is roughly similar to the diameter of the cannon.
- Wrap a soft plastic tube cut from the bulb of a plastic transfer pipette around the paper towel. This ensures that the capsule can be loaded smoothly into the cannon.
- Load the fish into the cannon.
- Use a scalpel to remove the skin covering the attachment sites, two triangular areas of the skull rostrolateral to the cerebellum and above the gills (Figure 2B), then remove the skin covering the region between the attachment sites. Use tissue swabs to dry out the attachment sites and clear out any remaining skin.
- Use an adjustable platform to support the head during skin removal, but the platform must not contact the eyes to avoid eye injury during the operation.
NOTE: Thorough removal of the skin at the attachment sites is crucial in reducing motion artifacts during imaging.
- Use an adjustable platform to support the head during skin removal, but the platform must not contact the eyes to avoid eye injury during the operation.
- Apply a drop of tissue glue (see Table of Materials) at the center of each attachment site using a 10 µL pipette tip.
NOTE: Tissue glue covers the attachment sites and dries out quickly. The tissue glue provides a bonding interface between the skull and the dental cement used to glue the head bar, and prevents water from the gills from reaching the attachment site. - Put the fish body in an upright position and position the head bar slightly above and caudal to the attachment sites in preparation for head bar gluing.
- Prepare dental cement (see Table of Materials) and use it immediately to glue the head bar to the skull (Figure 2C). The procedure is time sensitive and must be done within 45 s.
- Mix one small spoon of polymer with four drops of quick monomer and one drop of catalyst V. Stir the mixture evenly for 15 s.
- Use a micropipette and a 10 µL pipette tip to apply the mixture onto the attachment sites and the region between the sites. Avoid covering the gills and the eyes.
- Gently press the head bar onto the cement using the micromanipulator. Cover the head bar with the remaining cement.
- Wait for 12 min to allow curing of the dental cement. Avoid water contact with the cement.
- To improve optical access to the forebrain for calcium imaging, remove the skin above the telencephalon. Skin removal can be done in 3 min with five cuts using a scalpel (Figure 2D). Make sure that lipid droplets adhering to the surface of the skulls are removed.
- After curing, remove the clay from the head bar.
- Transfer the entire capsule from the cannon to the pitch adjuster in the fish loading module of the micromanipulator.
- Use the micromanipulator to position the fish. The head bar should be positioned on top of the metal posts of the head stage. Slightly increase the pitch angle of the fish so that the surface of the forebrain can be better aligned to the optical plane during two-photon imaging.
- Glue the head bar to the metal posts with ultraviolet (UV)-curable glue. A 15 s UV exposure is sufficient for curing.
- Pull out the fish from the capsule and lock the head stage onto the base plate within the semi-hexagonal tank.
- Immerse the animal in fish tank water. The fish should start breathing and recover from anesthesia within 1-2 min.
NOTE: Optionally, use syringe perfusion to deliver fresh water to the mouth.
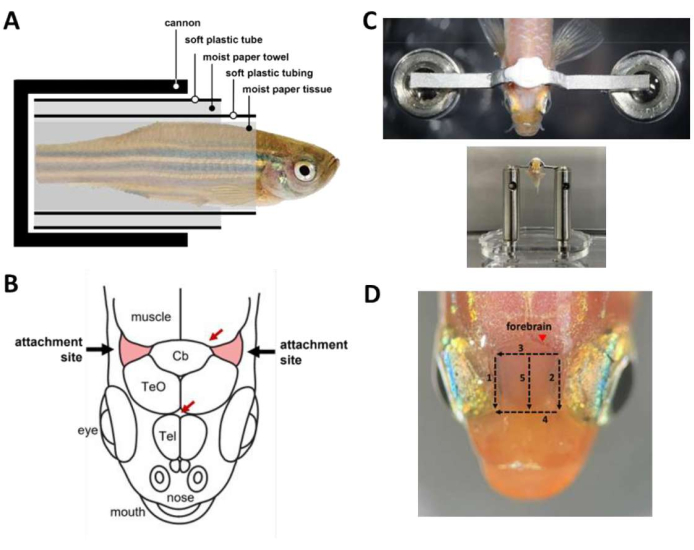
Figure 2: Key steps during head restraint surgery. (A) Composition of the capsule inside the cannon. (B) Attachment sites on the skull (red). Red arrows specify blood vessel sites. (C) Top: head bar attached to the fish skull. Bottom: fish loaded onto the head stage. (D) Cuts needed to remove the skin above the forebrain. Numbers denote the cutting sequence. Avoid skin removal at the marked site (arrowhead) to prevent bleeding of the animal. Please click here to view a larger version of this figure.
3. Two-photon imaging
- Turn on the laser and wait for 30 min for the power to stabilize. Set the wavelength to 920 nm and power to around 50 mW at the sample.
NOTE: The laser beam controlled by the resonant scanner moves slowly at the turnaround points of the scan path. To prevent tissue damage, a Pockels cell is used to reduce laser power at the turnaround points. - Place the recording chamber containing the head-restrained zebrafish on the experimental platform (Figure 3A). The platform should be able to move in the X and Y directions without hitting the limit of the translation stage.
- Place the objective lens as close as possible to the forebrain surface. The objective lens should be aimed at the front edge of the pupil (Figure 3B).
- Open the laser shutter and enable the PMT. Gradually elevate the objective lens (~1 mm) until the dorsal forebrain is revealed in the fluorescence image (Figure 3C).
- To increase the number of neurons recorded, use a piezo actuator to acquire images at multiple depths (six image planes, 15 µm apart). This will increase the data yield at the expense of the frame rate. Enable fast Z-axis scanning to control the piezo actuator (uniform mode, number of slices = 6, step size = 15 µm, waveform = sawtooth, flyback time = 4 ms, actuator lag = 8 ms).
- For behavior recording, turn on the 810 nm infrared (IR) lights on both sides of the tank. Adjust the angle to illuminate the entire body, which should be clearly visible in the camera.
- Turn on the projectors.
- Start recording data.
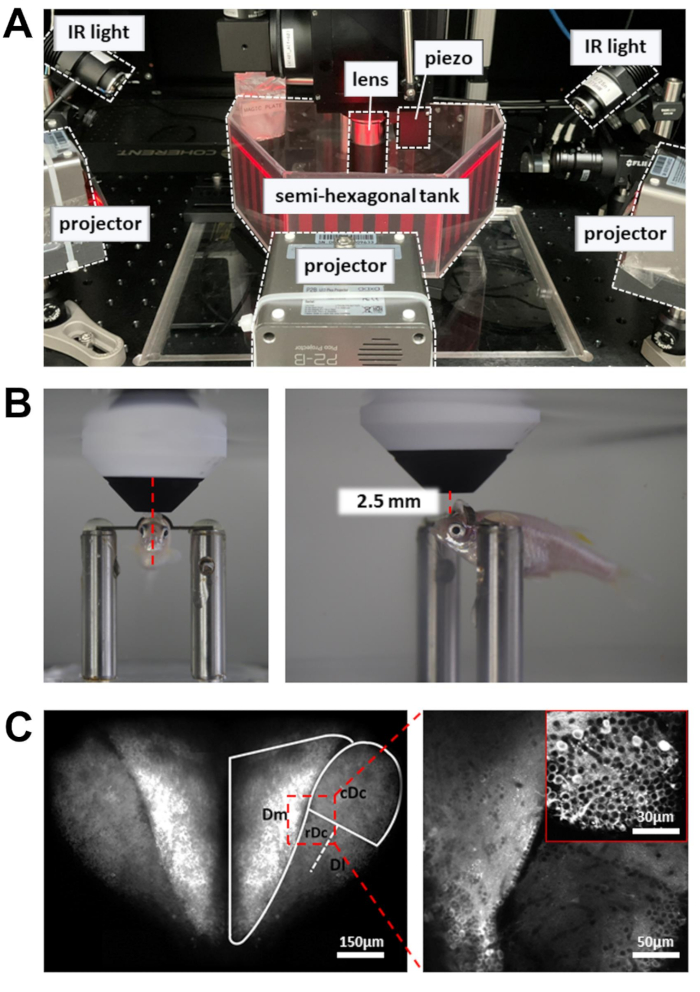
Figure 3: Setup to perform calcium imaging, behavior recording, and visual stimulus display. (A) Three projectors present a visual stimulus onto the walls of the semi-hexagonal tank. IR lights on the side are used to illuminate the body of the zebrafish. (B) Positioning of the objective lens. Left: front view. Right: side view. The distance between the 16x objective lens and the targeted brain region is around 2.5 mm. (C) Example two-photon image. Left: maximum projection of the entire dorsal forebrain in Tg[neuroD:GCaMP6f]. Right: zoomed-in image to reveal neurons across multiple brain regions. Inset: a higher magnification from a different brain region. Images are averages of 10 s of data recorded at 5Hz. Please click here to view a larger version of this figure.
Results
The protocol consists of two parts: head restraint surgery and two-photon calcium imaging of neuronal activities in the forebrain. The success of surgery is defined by the survival of the animal and the stability of the head restraint. The survival rate can be greatly improved by frequent perfusion of 0.01% TMS solution through the mouth during surgery. Fish should recover from anesthesia and breathe actively within 1-2 min after being immersed into fish tank water. Two-photon calcium imaging enables activity recording o...
Discussion
Here, we describe a detailed protocol to restrain the head of adult zebrafish for two-photon calcium imaging. There are two critical steps to achieve a head restraint that is stable enough for laser scanning imaging. First, the head bar has to be glued to the specific attachment sites of the skulls. Other parts of the skull are often too thin to provide mechanical stability and may even be fractured during strong body movements. Second, the skin above the attachment sites has to be thoroughly removed. Residual water...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by the Institute of Molecular Biology, Academia Sinica, and National Science and Technology Council, Taiwan. The Machine Shop at the Institute of Physics, Academia Sinica helped to fabricate custom-designed parts. We also want to thank P. Argast (Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland) for the design of the quick-lock mechanism of the head stage.
Materials
| Name | Company | Catalog Number | Comments |
| Acquisition card | MBF Bioscience | Vidrio vDAQ | Microscope |
| Back-projection film | Kimoto | Diland screen - GSK | present visual stimulus |
| Band-pass filter (510/80 nm) | Chroma | ET510/80m | Microscope |
| Base plate for the semi-hexagonal tank | custom made | see supplemental files | recording chamber |
| Camera filter (<875 nm) | Edmund optics | #86-106 | Behavior recording |
| Camera filter (>700 nm) | Edmund optics | #43-949 | Behavior recording |
| Camera lens | Thorlabs | MVL50M23 | Behavior recording |
| Chameleon Vision-S | Coherent | Vision-S | Laser |
| Circular plate for the head stage | custom made | see supplemental files | recording chamber |
| Controller for piezo actuator | Physik Instrumente | E-665. CR | Microscope |
| Current amplifier | Thorlabs | TIA60 | Microscope |
| Elitedent Q-6 | Rolence Enterprise | Q-6 | Surgery: UV lamp |
| Emission Filter 510/80 nm | Chroma | ET510/80m | Microscope |
| Head bar | custom made | see supplemental files | recording chamber |
| Infrared light | Thorlabs | M810L3 | Behavior recording |
| LED projector | AAXA | P2B LED Pico Projector | present visual stimulus |
| Moist paper tissue (Kimwipe) | Kimtech Science | 34155 | Surgery: moist paper tissue |
| Motorized XY sample stage | Zaber | X-LRM050 | Microscope |
| Neutral Density Filters (50% Transmission) | Thorlabs | NE203B | present visual stimulus |
| Ø1/2" Post Holder | ThorLabs | PH1.5V | Surgery: hollow tube for cannon |
| Ø1/2" Stainless Steel Optical Post | ThorLabs | TR150/M | Surgery: fish loading module |
| Objective lens 16x, 0.8NA | Nikon | CF175 | Microscope |
| Oil-based modeling clay | Ly Hsin Clay | C4086 | Surgery: head bar holder |
| Optical adhesive | Norland Products | NOA68 | Surgery: UV curable glue |
| Photomultiplier tube | Hamamatsu | H11706P-40 | Microscope |
| Piezo actuator | Physik Instrumente | P-725.4CA PIFOC | Microscope |
| Pockels Cell | Conoptics | M350-80-LA-BK-02 | Microscope |
| Red Wratten filter (> 600 nm) | Edmund optics | #53-699 | present visual stimulus |
| Resonant-Galvo Scan System | INSS | RGE-02 | Microscope |
| Right-Angle Clamp for Ø1/2" Post | ThorLabs | RA90/M | Surgery: fish loading module |
| Rotating Clamp for Ø1/2" Post | ThorLabs | SWC/M | Surgery: fish loading module |
| ScanImage | MBF Bioscience | Basic version | Microscope |
| Semi-hexagonal tank | custom made | see supplemental files | recording chamber |
| Super-Bond C&B Kit | Sun Medical Co. | Super-Bond C&B | Surgery: dental cement |
| Tricaine methanesulfonate | Sigma Aldrich | E10521 | Surgery: anesthetic |
| USB Camera | FLIR | BFS-U3-13Y3M-C | Behavior recording |
| Vetbond | 3M | 1469SB | Surgery: tissue glue |
References
- Grienberger, C., Konnerth, A. Imaging calcium in neurons. Neuron. 73 (5), 862-885 (2012).
- Chow, D. M., et al. Deep three-photon imaging of the brain in intact adult zebrafish. Nature Methods. 17 (6), 605-608 (2020).
- Mittmann, W., et al. Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nature Neuroscience. 14 (8), 1089-1093 (2011).
- Friedrich, R. W., Jacobson, G. A., Zhu, P. Circuit neuroscience in zebrafish. Current Biology. 20 (8), R371-R381 (2010).
- Kappel, J. M., et al. Visual recognition of social signals by a tectothalamic neural circuit. Nature. 608 (7921), 146-152 (2022).
- Bartoszek, E. M., et al. Ongoing habenular activity is driven by forebrain networks and modulated by olfactory stimuli. Current Biology. 31 (17), 3861-3874 (2021).
- Valente, A., Huang, K. H., Portugues, R., Engert, F. Ontogeny of classical and operant learning behaviors in zebrafish. Learning & Memory. 19 (4), 170-177 (2012).
- Buske, C., Gerlai, R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Developmental Psychobiology. 54 (1), 28-35 (2012).
- Huang, K. H., et al. A virtual reality system to analyze neural activity and behavior in adult zebrafish. Nature Methods. 17 (3), 343-351 (2020).
- Rupprecht, P., Prendergast, A., Wyart, C., Friedrich, R. W. Remote z-scanning with a macroscopic voice coil motor for fast 3D multiphoton laser scanning microscopy. Biomedical Optics Express. 7 (5), 1656-1671 (2016).
- Papadopoulos, I. N., Jouhanneau, J. -. S., Poulet, J. F. A., Judkewitz, B. Scattering compensation by focus scanning holographic aberration probing (F-SHARP). Nature Photonics. 11 (2), 116-123 (2017).
- Torigoe, M., et al. Zebrafish capable of generating future state prediction error show improved active avoidance behavior in virtual reality. Nature Communications. 12 (1), 5712 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved