A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
T4 Bacteriophage and E. coli Interaction in the Murine Intestine: A Prototypical Model for Studying Host-Bacteriophage Dynamics In Vivo
In This Article
Summary
Bacteriophages (phages), viruses that infect bacteria, are an integral component of the gut microbiome. Though these symbiotic inhabitants drive bacterial fitness and population dynamics, little is understood about how they impact gut homeostasis and disease. This protocol studies isolated T4 phages within a mouse model, adaptable to other phage-bacterial pairs.
Abstract
Bacteriophages (phages) are viruses that infect bacteria with species- and strain-level specificity and are the most abundant biological entities across all known ecosystems. Within bacterial communities, such as those found in the gut microbiota, phages are implicated in regulating microbiota population dynamics and driving bacterial evolution. There has been renewed interest in phage research in the last decade, in part due to the host-specific killing capabilities of lytic phages, which offer a promising tool to counter the increasing threat of antimicrobial resistant bacteria. Furthermore, recent studies demonstrating that phages adhere to intestinal mucus suggest they may have a protective role in preventing bacterial invasion into the underlying epithelium. Importantly, like bacterial microbiomes, disrupted phageomes have been associated with worsened outcomes in diseases such as inflammatory bowel disease. Previous studies have demonstrated that phages can modulate the microbiome of animals and humans through fecal filtrate transplants, benefiting the host's health. With this recent wave of research comes the necessity to establish and standardize protocols for studying phages in the context of the gut microbiome. This protocol provides a set of procedures to study isolated T4 phages and their bacterial host, Escherichia coli, in the context of the murine gastrointestinal tract. The methods described here outline how to start from a phage lysate, administer it to mice and assess effects on bacterial host and phage levels. This protocol can be modified and applied to other phage-bacterial pairs and provides a starting point for studying host-phage dynamics in vivo.
Introduction
Bacteriophages, or phages, are viruses that infect and kill bacteria with species and strain-level specificity1. Phages play important roles within complex bacterial communities such as the gut microbiota, where they have been implicated in regulating population dynamics and driving bacterial fitness2. Throughout the last decade, there has been renewed interest in phage research owing to the rise of antimicrobial resistant pathogens3, and the potential for phage therapy as an alternative treatment strategy. In recent years, lytic phage cocktails have been used intravenously with some success in serious, antibiotic-resistant bacterial septic infections in humans3,4. Oral phage therapy has also been proposed as a potential alternative to antibiotics to treat intestinal infections and inflammation. Furthermore, phages have been implicated in the success of fecal filtrate transplants (FFT), which are fecal microbiota preparations that have been filtered to remove bacteria, in the treatment of recurrent Clostridioides difficile infection (rCDI)5,6, inflammatory bowel disorders (IBD)7,8 and necrotizing enterocolitis in pre-term pigs9. Given these results, it is important to consider interactions both between phages and the gut microbiota, and phages and the mammalian host, as the addition of novel phages into a preexisting community may have indirect effects on the community as a whole, and not only its target bacteria2,10.
The study of phage interactions with their target bacteria in vitro has proven useful for understanding the mechanisms and impacts of phage and bacteria interactions in the gut. In this setting, it has been shown that Escherichia coli-specific T4 phages of the order Caudovirales require immunoglobulin (Ig)-like domains located within highly antigenic outer capsid (Hoc) proteins on the virion surface to adhere to intestinal mucus11. Additionally, transwell assays have shown that T4 phages are capable of interacting with epithelial cell cultures and translocating through cell layers by macropinocytosis12,13. These results support the hypothesis that phages can interact with their metazoan host, even though they are incapable of infecting eukaryotic cells. These models, while useful, lack the full range of complex interactions that occur in a gut ecosystem that are required for a comprehensive exploration of the tripartite interaction between phages, bacteria and the metazoan host.
Mouse models are an important tool for investigating phages within complex environments. A desirable application of phage administration is as an alternative strategy to treat antimicrobial resistant infections or pathobionts associated with chronic inflammatory diseases, including IBD. However, emerging literature suggests that phage behavior in vitro does not fully represent in vivo functions. Buttimer et al.14 demonstrated that a phage cocktail was able to deplete the targeted bacteria in a simplified human microbiota consortium in vitro, but could not be replicated in vivo in gnotobiotic mice colonized with the same bacteria-phage consortium. Furthermore, in a conventional mouse microbiome, T7 phage led to selective depletion of its target gut bacteria, although gradual recovery was observed over time, indicative of evolved resistance15. Other studies have demonstrated co-existence of orally administered phages and their target bacterial strains in vivo2,16. Indeed, beyond phage/bacteria co-existence, phage administration led to widespread changes in overall microbiota community composition and function2,16. This is relevant in disease settings as several studies have found associations between increased relative abundance of Caudovirales and IBD7,8,17 that were independent of changes in bacterial abundance7. It remains unknown whether this is a driver or consequence of disease pathogenesis.
The historic focus of phage investigation has been around the relationship between a phage and its target bacterium. However, it is also important to consider potential interactions between phage and the mucosa, epithelium, and immune system of the metazoan host. These interactions all play an important role in the overall response to intestinal phage infection. To demonstrate this, phages have been studied using germ-free (GF) mice to elucidate their impact on the immune system without interference by the microbiota8. In this system, phage nucleic acids were detected by Toll-like receptors (TLRs) located within endosomes of phagocytic immune cells (macrophages and dendritic cells). This activated downstream signaling and stimulated T cell dependent production of interferon (IFN)-γ8 or type I IFNs18. Moreover, Fluckiger et al.19 implicated memory CD8+ T cells in the recognition of phage-encoded (prophage) antigens, which resulted in T cell cross-reactivity with tumor antigens, resulting in reduced tumor burden. Finally, phage-specific antibody production has been documented in mouse studies where phages were delivered to animal models in a continual manner through drinking water8,20, or by repeated oral gavage over several months20, demonstrating the capacity for phage proteins to promote humoral immune responses. Although these modes of phage inoculation allow for optimal and continual priming of the immune system, they may not represent the naturally occurring interactions between phages and the intestinal environment, nor the kinetics of orally applied phage therapy. Thus far, a limited number of studies have examined the interactions of phage with a single bacterial species in monocolonized mouse models21. However, monocolonized mice proved critical in deciphering microbe-specific effects of individual species on gastrointestinal (GI) tract and immune development22,23,24, and they may yet prove useful in understanding tripartite interactions between phages, their target bacteria, and the metazoan host.
Excitingly, there is still much to learn about the interactions between intestinal phage and gut commensal bacteria, as well as the interactions that occur between the metazoan host and the phages that reside within it. This protocol provides a set of procedures to study isolated T4 phage and its bacterial counterpart, E. coli (K-12, BW25113), using a gnotobiotic mouse model. These standardized procedures also provide a foundation for optimizing other phage/bacteria dyads by adapting the growth parameters to the pairs of interest. The methods described here outline: (1) Preparation of T4 phage and vehicle lysates for oral gavage of mice; (2) Oral administration of T4 phage to E. coli monocolonized gnotobiotic mice; (3) Monitoring T4 phage levels in mouse feces and tissues over time.
For the representative results presented here, purified T4 phage lysates were propagated from phage bank stocks maintained by the Rohwer Lab. The Phage-on-Tap method for propagating T4 phage was adapted25, as referenced in this protocol. The method yields high titer, endotoxin-low phage stocks within three days. Utilizing this approach, 10 mL of ≥ 1010 plaque forming units (pfu)/mL of T4 phage with < 0.5 endotoxin units (EU)/mL were routinely collected. The recommended endotoxin levels for oral or intravenous administration into mice are ≤ 20 EU/mL and ≤ 5 EU/kg/h (or 0.1 EU administered over 1 h for a 20 g mouse), respectively, making this a suitable method of phage preparation for in vivo inoculation. All phage stocks were stored at 4 °C in saline magnesium (SM) phage buffer (recipe provided in step 1.1.5.1). E. coli was cultivated in LB media. For various phage-bacteria pairs, diverse culture media and growth conditions may be adapted from this protocol. Phages can also be sourced from the environment, such as wastewater, marine water, soil and intestinal contents and can be isolated and purified as per Sambrook and Russell26 prior to preparation using the appropriate growth and propagation conditions for each phage-host pair of interest25. Alternatively, phages can be obtained from commercial sources (see Table of Materials) or from phage banks.
Protocol
All experiments were conducted in accordance with the guidelines established by the UBC Animal Care Committee and Biosafety Committee-approved protocols (A23-0113, B19-0038). Mice were housed at the University of British Columbia under pathogen-free conditions at the Center for Disease Modelling. C57BL/6 mice were bred within the facility in a sterile flexible film isolator, provided with sterile mouse diet, water, bedding, and nesting material. The mice were maintained on a 12 h day/night cycle. Experimental mice, both male and female, were age-matched within each experiment, ranging between 6 to 12 weeks of age and weighing 15-30 g for all experiments.
1. Preparation of phage and vehicle lysates for oral gavage into mice
- Phage purification and stock generation
NOTE: In this study, T4 phages are grown and titered by plating onto a lawn of bacteria using the single agar layer method, described below. The double agar layer method has been described previously25,27 and can also be used with similar efficacy. The single agar layer method was selected for this protocol as it provides improved plaque visibility28.- Grow E. coli in 5 mL of sterile LB media in sterile polystyrene or glass culture tubes (with caps). Incubate tubes at 37 °C with shaking at 200 rpm overnight, until stationary phase is reached.
- Prepare soft agar in a glass media bottle by autoclaving LB with 0.5% agar and cooling to 50 °C or below (while remaining a liquid) prior to supplementation. Prepare enough soft agar for 15 mL per plate.
- Supplement soft agar with MgSO4 and CaCl2 to a final concentration of 1 mM for each. Add 100 µL of overnight E. coli culture for every 3 mL of soft agar. Stir gently using a magnetic stir bar to homogenize the supplements and culture in the soft agar.
NOTE: Consistency in the volume and density of E. coli that is added to the soft agar preparation is critical (outlined in the representative results section). - Add 15 mL of soft agar + E. coli per Petri dish (15 cm diameter) using a serological pipette. Allow plates to set at room temperature for same-day use.
NOTE: Plates will set in approximately 20 min with lids open. The agar will remain soft but will not shift when plates are inverted. - Prepare 8-10 serial dilutions of the source T4 phage in factors of 10 by diluting in SM buffer. Spot 5 µL of each dilution onto plates. Allow spots to dry, invert and incubate plates at 37 °C overnight.
- To prepare Saline Magnesium (SM) phage buffer, in 1 L of deionized H2O, dissolve 100 mM NaCl, 8 mM MgSO4·7H2O, and 50 mM Tris-HCl (pH 7.4). Autoclave and store at room temperature.
NOTE: The next day, E. coli should have grown into a lawn throughout the soft LB agar. Plaques, or cleared areas of growth visible in the E. coli lawn, will appear where phage killing of infected E. coli hosts occurred. Each plaque represents a plaque forming unit (pfu).
- To prepare Saline Magnesium (SM) phage buffer, in 1 L of deionized H2O, dissolve 100 mM NaCl, 8 mM MgSO4·7H2O, and 50 mM Tris-HCl (pH 7.4). Autoclave and store at room temperature.
- Pick a single plaque from the soft agar plate by pushing a sterile pipette tip into the center of the plaque, creating an agar plug at the end of the tip. Resuspend the plaque plug into 1 mL of SM buffer in a 1.7 mL microcentrifuge tube. Vortex the tube at max speed for 1 min to mix.
- Centrifuge the tube at 4000 x g for 5 min at room temperature to remove any debris from the lysate. Transfer the supernatant into a new microcentrifuge tube.
- To propagate the isolated phage, repeat steps 1.1.1-1.1.3. Add 100 µL of the isolated phage to a 15 mL aliquot of soft agar containing E. coli in a conical tube. Invert the tube three times to mix and pour the agar into a petri dish.
- Allow plates to dry before inverting and incubating overnight at 37 °C. Prepare a lawn control plate without phage to confirm E. coli viability.
NOTE: After overnight incubation, the control plate should have a lawn of E.coli while the phage-containing plate should be completely lysed. - To extract the phage from the plate, add 5 mL of SM buffer to the surface of the phage-cleared plate and shake at 70 rpm on a rocker for 15 min at room temperature.
- Collect the buffer from the plate into a 50 mL conical centrifuge tube and centrifuge at 4000 x g for 5 min at room temperature to pellet any debris from the agar plate.
- Collect the T4 phage stock lysate into a new tube and store at 4 °C until titration and propagation.
NOTE: T4 phages are stable for over 10 years25; however, stability will depend on the type of phage and storage conditions. Phage titer will also vary with storage time. Phages are sensitive to freezing. Therefore, cryopreservation is recommended in liquid nitrogen and avoiding freeze-thaw cycles as this may damage phages, reducing phage titer25. - Titer the T4 phage stock lysate to determine the concentration in pfu/mL, as previously described25,29,30. A high titer T4 phage stock will contain 108 pfu/mL or more.
- Preparation of experimental phage lysates
- Culture E. coli in 5 mL of LB media in sterile polystyrene or glass culture tubes (with caps). Incubate tubes overnight at 37 °C with shaking at 200 rpm, until stationary phase is reached.
- Subculture the overnight E. coli culture 1:50 in 100 mL LB media in a glass conical flask. Incubate at 37 °C with shaking at 200 rpm until the bacteria have reached early to mid-exponential phase (approx. 1.5 h).
NOTE: An initial growth curve can be performed to determine the optical density at 600 nm (OD600) range at which the bacterial culture is in exponential phase. Culture in a glass conical tube is suggested as recent evidence has found that phages are able to adhere to plastics such as polypropylene31. - Add 100 µL of the high titer T4 phage stock from step 1.1 into the E. coli subculture and incubate at 37 °C with shaking for 3 h, or until the new lysate is no longer cloudy. Collect the T4 phage lysate into 50 mL conical tubes and either proceed directly to clean-up steps or, if required, store at 4 °C until the next day.
NOTE: A larger phage burst size (indicating a higher average number of virions released per replication cycle) necessitates a longer incubation period for the bacterial subculture in step 1.2.2. This provides increased bacteria density prior to spiking with phage stocks. - Centrifuge lysates at 4000 x g for 20 min at room temperature to pellet any remaining bacteria and cellular debris. Filter-sterilize the resulting supernatant using a 0.22 µm nylon filter and transfer the filtrate to new 50 mL conical centrifuge tubes.
- Add 0.1 volumes of chloroform to each volume of filtered T4 phage lysate to kill any remaining bacteria and prevent bacterial growth. Vortex briefly to mix and incubate at room temperature for 10 min.
NOTE: Lipid enveloped phages are sensitive to chloroform, which may reduce phage titer25. Skip this step if required.
CAUTION: Chloroform is a toxic organic solvent that is dangerous when inhaled, ingested, or absorbed through the skin. Use a fume hood and appropriate personal protective equipment (PPE) when working with chloroform. Use glass instead of polystyrene serological pipettes when working with chloroform, as it is not compatible with most plastics. Chloroform can be placed in polypropylene conical centrifuge tubes for steps 1.2.5-1.2.6 but should not be stored long term in plastic tubes. - Centrifuge the lysate at 4000 x g for 5 min at room temperature to separate the chloroform from the lysate. Use a serological pipette to carefully transfer the top lysate layer into a new 50 mL conical tube without disturbing the underlying chloroform layer. Discard the chloroform waste into the appropriate hazardous liquid waste container. Store lysates at 4 °C until concentration and buffer exchange the following day.
- Concentrate phage lysates in a 100 kDa centrifugal filter device (see Table of Materials) by adding 13 mL of phage lysate to the upper reservoir of the device and centrifuging at 4000 x g for 5 min, or until most of the lysate has passed through the filter into the lower reservoir.
NOTE: Aim to have approximately 2 mL of lysate at the end of the spin time. As the phage concentration increases in the filter with the addition of lysate, spin times will likely increase. Centrifugal filter devices have a physical dead stop to prevent them from spinning dry32. Do not let the filter membrane dry out if continued use is intended (i.e., by removing all liquid from the upper reservoir). Spin times may vary based on phage type and titer. - Using a P200 or P1000 pipette, gently pipette the remaining ~2 mL of lysate up and down within the upper reservoir to unclog the filter membrane after each spin. Discard the filtrate from the lower reservoir into a waste container, leaving the concentrated phage in the upper reservoir.
- Repeat steps 1.2.7-1.2.8 until the entire volume of phage lysate has been passed through the filter device, with ~2 mL of concentrated phage retained in the upper reservoir after each spin.
- Unclog the filter membrane after the last spin by pipetting the remaining lysate (~2 mL) in the upper reservoir up and down. Wash the phage (buffer exchange) by adding 12 mL of SM buffer to the upper reservoir and centrifuge at 4000 x g for 10 min, or until most of the buffer has passed through the filter.
- Discard the filtrate and repeat the wash step (step 1.2.10). Resuspend the remaining 2 mL of lysate in SM buffer to a final volume of 10 mL (or less), for long-term storage. Transfer the lysate to a 50 mL conical centrifuge tube and store at 4 °C until endotoxin removal.
NOTE: It is optional to titer the phage at this stage to ensure that the phage lysate has been concentrated (> 108 pfu/mL), and to determine phage loss during the endotoxin removal process. - Remove contaminating endotoxins from the T4 phage lysate by adding 0.4 volumes of 1-octanol (see Table of Materials) to the total volume of lysate.
NOTE: Endotoxins are removed as they are highly immunostimulatory, and therefore their presence may lead to the induction of a phage-independent innate immune response25.
CAUTION: 1-Octanol is an aromatic, organic, flammable compound with a strong odor and is an eye irritant. Wear appropriate PPE when working with 1-octanol. Perform all work in a fume hood to avoid vapor inhalation. Use sealing film to prevent the leaking of tubes when working outside of the fume hood. - Seal the lid of the conical centrifuge tube with sealing film to prevent leaking. Shake at 120 rpm on a platform or rocking shaker at room temperature for 1 h, followed by incubation at 4 °C for 1.5 h, without shaking.
- Centrifuge at 4000 x g for 10 min to separate the endotoxin-cleared lysate from the 1-octanol. The 1-octanol layer will float on top of the lysate. Use a P1000 pipette to carefully remove as much 1-octanol as possible and discard into the appropriate hazardous/flammable liquid waste container.
- Use an 18 G needle and 10 mL syringe to collect the phage lysate beneath the remaining 1-octanol layer, taking care not to collect the 1-octanol layer. Store lysate at 4 °C until speed vacuum.
- Transfer 1 mL aliquots of T4 phage lysate into sterile 1.5 mL microcentrifuge tubes. Speed vacuum with lids open at 4000 x g at room temperature to evaporate residual 1-octanol from the lysate. Speed vacuum for 3 h or until the phage lysate volume has been reduced by 30%25. Store at 4 °C until titration.
- Titer the phage lysate to determine the concentration in pfu/mL. Dilute the resulting T4 phage lysate in SM buffer to the desired concentration and re-titer to confirm.
- Quantify endotoxins present in the T4 phage lysate using a chromogenic endotoxin quantitation kit as per manufacturer instructions (see Table of Materials). Compare endotoxin levels in the final phage lysate with a pre-endotoxin-removal sample, vehicle controls, buffer, and mouse drinking water.
NOTE: The chromogenic endotoxin quantification kit is inactivated by 1-octanol25. Perform speed vacuum steps to remove 1-octanol (step 1.2.16) before quantifying endotoxins. The endotoxin content for distilled water is estimated at 20 EU/mL25; however, the endotoxin content of mouse drinking water may vary between facilities. If the endotoxin levels in the phage lysate exceed the amount present in drinking water, consider repeating endotoxin removal steps (1.2.12-1.2.16). - Store concentrated, endotoxin-cleared T4 phage lysates in SM buffer at 4 °C.
- Preparation of phage-free bacterial lysate for vehicle controls
NOTE: A phage-free bacterial lysate can be prepared as a vehicle to control for any effects due to bacterial contaminants (e.g., endotoxin) generated in the production of phage lysate in step 1.2, which could impact experimental outcomes when inoculated into mice.- From an overnight culture of E. coli, subculture the E. coli 1:50 into 100 mL of LB media. Without adding phage, continue to culture the bacteria while shaking at 37 °C for 3 h, or matching the incubation time for the phage lysate produced in step 1.2.
- Transfer the E. coli culture to 50 mL conical tubes and lyse cells using a sonicator probe (see Table of Materials) on ice at 30 kHz for 30 s pulses (3x) to manually lyse bacterial cells.
- Follow the rest of the protocol as per step 1.2, starting at step 1.2.4, including all clean up, wash and endotoxin removal steps.
NOTE: During concentration using the centrifugal filter device, the vehicle lysate will flow through the filter more quickly than the phage lysate, due to the absence of phage. Therefore, shorten centrifugation times from 5 mins to 2-5 min to ensure that the ultrafilter membrane does not dry out during prolonged centrifugation. - Titer the vehicle lysate to confirm that it does not contain phage.
- Use a chromogenic endotoxin quantitation kit as per manufacturer instructions to measure endotoxin levels in the vehicle lysate. Dilute the vehicle lysate in SM buffer to match the endotoxin levels in the phage lysate.
- Alternative experimental controls: preparation of heat-inactivated phage lysate
NOTE: An alternative to the phage-free bacterial lysate is a heat-inactivated phage lysate. Heat-inactivation dissociates phage virions, while residual endotoxins such as lipopolysaccharides (LPS) are heat stable8,33. This method was used by Gogokhia et al.8 to determine whether intact, viable phage virions were required for immune activation. Researchers are encouraged to test both methods (phage-free vs. heat-inactivated) and determine which control is most appropriate for their experimental needs. Whichever is chosen, it is important to acknowledge that a buffer control is unlikely to be appropriate due to the significant manipulation that is required to concentrate and clean-up a phage stock.- Transfer the required volume of cleaned, purified, and diluted T4 phage lysate from step 1.2 (100 µL per mouse) to sterile microcentrifuge tubes fitted with lid locks.
- Heat lysates on a heat block at 95 °C for 15 min16.
- OPTIONAL: If the removal of phage/bacterial nucleic acids is required, perform DNase I and RNase A treatment as per Jakočiūnė and Moodley34. Briefly:
- Add 50 µL DNase I 10x buffer, 1µL DNase I (1 U/µL) and 1 µL RNase A (10 mg/mL) (see Table of Materials) to 450 µL of heat-inactivated phage lysate.
- Incubate lysates at 37 °C for 1.5 h in a heat block, without shaking.
- Inactivate the DNase I and RNase A by adding 20 µL 0.5 M ethylenediaminetetraacetic acid (EDTA)34.
- Hold lysates at room temperature for 10 min to cool and combine lysates if multiple tubes are used. Store at 4 °C until titration.
- Perform a phage titer to confirm that no viable T4 phage is present in the lysate. Store heat-inactivated lysates at 4 °C.
- Use a chromogenic endotoxin quantitation kit as per manufacturer instructions to measure endotoxin levels in the heat-inactivated lysate. Dilute the heat-inactivated lysate in SM buffer according to the endotoxin levels present in the matched phage lysate.
2. Administration and monitoring of T4 phage in E. coli monocolonized mice
- Monocolonization of mice with E. coli
- Prepare E. coli for administration into GF mice8 by culturing E. coli picked from a single colony in LB media overnight.
- Under strict, sterile conditions35, administer 200 µL of E. coli culture to GF mice housed in either sterile vinyl isolators or bioexclusion airtight cages by oral gavage. If using aerotolerant bacteria, mice may also be colonized by application of 200 µL of bacterial culture to the backs of each mouse.
NOTE: The inoculation dose will be strain dependent, as different bacteria demonstrate different pH survival ability36,37. Zucoloto et al.35 recommend inoculation with 1 x 108 cfu per animal. Representative results shown here were generated from mice inoculated with an overnight culture (cfu was not determined). Pilot experiments can be performed to determine a dose that results in reliable colonization of the mouse strain of interest. The volume of bacteria to be administered by oral gavage may be impacted by the age and weight of the mice. Consult individual lab or institutional animal ethics protocol for maximum allowable doses. The volume suggested here is based on a gavage allowance of 10% of the mouse body weight (e.g., 200 µL maximum for a 20 g mouse). Some bacterial species will not tolerate the acidity of the stomach and fail to colonize the gut. Consider first gavaging with 100 µL of 1 M NaHCO3 to neutralize stomach acids2. If using a strict anaerobe to colonize, the microbe will need to be grown under anaerobic conditions and transferred to the gnotobiotic animal facility in individually prepared air-tight containers (1 for each mouse). Perform each gavage quickly once the container is opened to limit loss of microbial viability due to oxygen exposure. - Monitor mice for adverse health effects.
NOTE: In the event of adverse health effects, refer to the standards set by the relevant animal care facility. Possible adverse health effects include, but are not limited to: (1) Aspiration of gavage fluid: symptoms include expulsion of bubbles via the nose, "open mouth" breathing/gasping. (2) Perforation of the esophagus: symptoms include rapidly ailing health, hunching, lethargy, leading to death of the animal within 24 h. (3) Inflammation of the esophagus due to repeated gavages: symptoms include difficulty inserting the gavage needle. (4) Diarrhea due to alterations to the microbiome. - Confirm bacterial colonization in fecal pellets by culturing at least once per week and/or by 16S rRNA sequencing35.
NOTE: If colonizing breeders within a gnotobiotic isolator for production of experimental mice, plan at least 9 weeks in advance for generation of 6-week-old first generation of offspring (F1) experimental mice. Alternatively, GF adult mice can be monocolonized. In this approach, waiting 7 weeks post-colonization prior to phage inoculation is recommended, as this is the time needed for the intestinal mucosa to stabilize after introduction of a complex microbiota into GF mice38.
- Oral inoculation of T4 phage into E. coli monocolonized mice
- Dilute T4 phage lysates and vehicle controls to a predetermined concentration in SM buffer. As per Hsu et al.2, dilute phage lysates to allow for administration of 2 x 106 pfu per mouse2.
NOTE: A higher or lower concentration of phage may be used depending on the stability of the phage in vivo. Doses of 2 x 102, 2 x 104 and 2 x 106 pfu of T4 phage per mouse resulted in stable and long-term colonization in the gut that did not appear to be dose-dependent (shown in representative results section). Phage-bacteria kinetics should therefore be piloted in vivo prior to large scale experiments. - Under sterile, gnotobiotic conditions, gavage each mouse with 100 µL of autoclaved 1 M NaHCO3 to neutralize stomach acids. Wait 10 min, then gavage with 100 µL of T4 phage lysate or vehicle control.
- Monitor mice for adverse health effects.
- Dilute T4 phage lysates and vehicle controls to a predetermined concentration in SM buffer. As per Hsu et al.2, dilute phage lysates to allow for administration of 2 x 106 pfu per mouse2.
3. Monitoring T4 phage levels in vivo
NOTE: Once mice have been inoculated with phage, the concentration of both phages and target bacteria can be measured in fecal or tissue samples. This provides information on the kinetics of the phage infection and colonization dynamics of both organisms.
- Spot plating T4 phage to determine concentration in fecal pellets
- Collect fecal pellets from each mouse into sterile, pre-weighed, microcentrifuge tubes to measure T4 phage and E. coli levels. Store tubes on ice until plating.
NOTE: Place samples on ice in the interim between collection and plating to slow the growth of aerotolerant bacteria. Over several hours, there may still be growth of aerobic bacteria, or some death of anaerobic bacteria due to oxygen exposure which could lead to skewed bacteria counts. Therefore, bacteria and phage plating should be performed as soon as possible after sample collection. Obligate anaerobic bacteria will not tolerate oxygen exposure. To preserve the sample, collect samples into sealed tubes and transfer tubes to an anaerobic chamber as soon as possible after collection. If no growth is detected in fecal samples, consider alternatives such as 16S rRNA qPCR for bacterial quantification. - Record final weights of each tube and calculate the sample weight by subtracting the initial tube weight. This will be used for normalizing T4 phage and E. coli concentration to sample weight (pfu/g or cfu/g, respectively).
- Add 1 mL of sterile SM buffer to each tube and vortex thoroughly at maximum speed (>1 min) to homogenize fecal pellets. If the sample is less than 15 mg, a smaller volume of SM buffer may be added. Record the volume of SM buffer added to each sample for calculations of cfu/g or pfu/g of sample.
- Prepare a series of 8 (or more depending on the expected phage concentration) serial dilutions of 20 µL of each sample in 180 µL SM buffer, in factors of 10. Vortex each homogenized sample briefly to mix before adding to the first tube/well. Pipette to mix in between each addition and change tips between each dilution to prevent inflating phage or bacteria counts via sample carryover.
NOTE: If fibers and debris present in the sample hinder pipetting, add additional buffer to the fecal slurry to further dilute the stock sample. - Spot 5 µL of each dilution onto LB soft agar plates containing E. coli (for phage plaque assays) or LB agar plates (1.5% agar, for bacterial colony assays) to determine T4 phage and E. coli concentration in each sample. For accuracy, spot each sample in triplicate.
NOTE: If preparing serial dilutions in a 96-well plate, an 8-channel P20 multichannel pipette may be used to pipette each column of serially diluted sample onto plates. Change tips between each dilution, even if moving from most dilute to most concentrated, as phage may adhere to the walls of the pipette tip and alter the amount of phage added to each new well31. - Allow each spot to dry before inverting the plate and placing it in the incubator. Incubate overnight at 37 °C.
- For each sample, select the dilution at which there are 3-30 countable plaques per spot. Count and record the number of plaques in the spot and the dilution used.
- Calculate pfu/g of sample by dividing the number of plaques by the volume plated in each spot to give pfu/µL. Multiply this by the dilution factor and by the volume of SM buffer added to each sample to give pfu/sample. Lastly, divide by the sample weight to give pfu/g30.

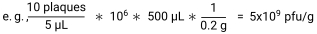
- Collect fecal pellets from each mouse into sterile, pre-weighed, microcentrifuge tubes to measure T4 phage and E. coli levels. Store tubes on ice until plating.
- Tissue sampling at experimental endpoints
- At each selected time point, euthanize mice as per approved institutional animal ethics protocol and collect cecal contents, small and large intestinal contents, and any tissues of interest into sterile, pre-weighed 2 mL round-bottom microcentrifuge tubes.
- Record final weights of each tube to calculate the sample weight. Store tissues and cecal contents on ice until same day plating.
- Add sterile SM buffer to each tube and record the volumes added.
- For cecal and intestinal contents, vortex samples thoroughly (>1 min) as per step 3.1.3.
- For tissue samples, add one sterile metal bead to each tube. Homogenize tissues using a tissue lyser (see Table of Materials) at 20 Hz for 5 min or until tissues are dissociated and the suspension is homogenous.
NOTE: If samples do not homogenize well, consider increasing the volume of SM buffer added, increasing the homogenization time, or increasing the homogenization frequency to 30 Hz. A tissue homogenizer may also be used to dissociate tissues while allowing for bacterial and phage recovery39,40. Homogenizers are operated at higher frequencies than tissue lysers while maintaining bacteria integrity. - Prepare serial dilutions of each sample in factors of 10 and perform spot plating to determine E. coli and T4 phage concentrations in each sample, as described in step 3.1.4-3.1.841.
Results
To investigate the interactions between the T4 phage/E. coli dyad in the murine intestine, T4 phage and vehicle lysates were prepared, cleaned, and purified (Figure 1A). T4 phage lysates were titered by plaque assay and diluted to 2 x 107 pfu/mL (2 x 106 pfu/mouse) in SM buffer. Vehicle lysates were also titered to confirm no viable phage presence and diluted in the same volume of SM buffer as the T4 phage lysate. Endotoxin levels were quantified in diluted lys...
Discussion
The study of phages in the microbiome presents a significant challenge compared to their bacterial counterparts. Specifically, phages do not contain a conserved phylogenetic marker common to all phages akin to the 16S and 18S ribosomal subunits that allow for the ease in sequencing and identification of prokaryotic and eukaryotic species, respectively42. However, with advances in next generation sequencing approaches, including increasing read lengths, throughput and decreasing costs, comes the ra...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge that the land they performed this research on is the traditional, ancestral, and unceded territory of the xwməθkwəy̓əm (Musqueam) Nation. The land it is situated on has always been a place of learning for the Musqueam people, who for millennia have passed on in their culture, history, and traditions from one generation to the next on this site. We encourage others to learn more about the native lands in which they live and work at https://native-land.ca. The authors acknowledge support from the Natural Sciences and Engineering Council of Canada (NSERC) Canadian Graduate Scholarships - Master's (N.P.), Michael Smith Health Research BC Trainee Award (RT-2023-3174, to MH), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants Program (RGPIN-2019-04591 to C.T., RGPIN-2016-04282 to LCO), Canadian Institute for Advanced Research / Humans and the Microbiome (FL-001253 Appt 3362, to C.T.), Michael Smith Foundation for Health Research Scholar Award (18239, to C.T.), Canadian Institutes for Health Research (PJT-159458 to LCO) and the Canadian Foundation for Innovation (34673 to LCO and 38277 to CT). We are grateful for technical support from the UBC Centre for Disease Modelling and ubcFLOW, which is supported by the UBC GREx Biological Resilience Initiative, and to members of the Osborne and Tropini labs for critical discussions and evaluation of the manuscript. Figure 1A and Figure 2A were created using Biorender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 1-octanol (99%) | Thermofisher | CAAAA15977-AP | |
| 50 ml PES Steriflip Sterile Disposable Vacuum Filter Units | Millipore Sigma | SCGP00525 | |
| Agarose (Low-EEO/Multi-Purpose/Molecular Biology Grade) | Fisher BioReagents | BP160-500 | |
| Amicon® 100kDa Ultra-15 centrifugal filter device, Ultracel-100 | Millipore Sigma | UFC910008 | |
| BD Microtainer® Tubes, SST | BD Medical | 365967 | |
| Bioexclusion airtight cages (ISO cages) | Techiplast | 1245ISOCAGE | |
| C1000 Touch™ Thermal Cycler with 96-Well Fast Reaction Module | BioRad | 1851196 | |
| Calcium Chloride Dihydrate (White Crystals to Powder) | Fisher BioReagents | BP510-500 | |
| Cap Locks For 1.5ML Tube 100/pk | Andwin Scientific | 16812612 | |
| Chloroform (Ethanol as Preservative/Certified ACS) | Fisher | C298-500 | |
| Copper coated steel beads (4.5 mm) | Crosman Corporation | 0767 | |
| DNeasy Blood & Tissue Kit (50) | Thermo Scientific | 69504 | |
| DreamTaq Green PCR Master Mix (2X) | Thermo Scientific | K1081 | |
| Ethylenediaminetetraacetic acid (EDTA) disodium salt solution, for molecular biology, 0.5 M in H2O | Sigma Aldrich | E7889 | |
| Fisher BioReagents™ Agar, Powder / Flakes, Fisher BioReagents™ | Fisher Bioreagents | BP1423-500 | |
| Fisher BioReagents™ Microbiology Media: LB Broth (Powder) - Lennox | Fisher Bioreagents | BP1427-500 | |
| GeneRuler 100 bp DNA Ladder | Thermo Scientific | SM0241 | |
| Green FastMix® qPCR mix, 1250 rxns | QuantaBio | 95072-012 | |
| HEPA filters for isocage lids, AUTOCLAVABLE H14 FILTERS FOR ISO LINE- IRRADIATED | Techiplast | UISOHEPAXTBOX-300 | |
| Magnesium sulfate heptahydrate | Fisher BioReagents | BP213-1 | |
| MaxQ 6000 Incubated Shaker | Thermo Scientific | 8354-30-0009 | |
| Microbiology Media: LB Broth (Powder) - Lennox | Fisher BioReagents | BP1427-500 | |
| Microcentrifuge Tubes with Locking Snap Cap, 2ml | Fisher | 14-666-315 | |
| Parafilm sealing film | Bemis | PM-996 | |
| Phage stocks | Carolina Biological Supply | n/a | |
| PicoLab® Mouse Diet 20 EXT | LabDiet | 5R58 | |
| Pierce™ Chromogenic Endotoxin Quant Kit | Thermo Scientific | A39552S | |
| RNase A (17,500 U) | Qiagen | 19101 | |
| RNase-free DNase Set | Qiagen | 79254 | |
| Sodium Bicarbonate (Fine White Powder) | Fisher Chemical | BP328-500 | |
| Sodium Chloride (Crystalline/Certified ACS) | Fisher Chemical | S271 | |
| Sonicator (probe model CL-18; power source model FB50) | Fisher scentific | n/a | |
| Sterile flexible film isolator | Class Biologically Clean | n/a | |
| SYBR™ Safe DNA Gel Stain | Invitrogen | S33102 | |
| T100 Thermal Cycler | BioRad | 1861096 | |
| T4 phage primer, forward (CCACACATAGCGCGAGTATAA) | IDT | n/a | |
| T4 phage primer, forward (GAAACTCGGTCAGGCTATCAA) | IDT | n/a | |
| TissueLyser II | Qiagen | 85300 | |
| Tris-HCl, 1M Solution, pH 8.0, Molecular Biology Grade, Ultrapure | Thermo Scientific | AAJ22638AE | |
| Water, (DNASE, RNASE free) | Fisher BioReagents | BP2484100 |
References
- Rohwer, F., Segall, A. M. A century of phage lessons. Nature. 528 (7580), 46-47 (2015).
- Hsu, B. B., et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host & Microbe. 25 (6), 803-814.e5 (2019).
- Gordillo Altamirano, F. L., Barr, J. J. Phage Therapy in the postantibiotic era. Clinical Microbiology Reviews. 32 (2), (2019).
- Schooley, R. T., et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy. 61 (10), (2017).
- Ott, S. J., et al. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology. 152 (4), 799-811.e7 (2017).
- Zuo, T., et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 67 (4), 634-643 (2018).
- Norman, J. M., et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 160 (3), 447-460 (2015).
- Gogokhia, L., et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host & Microbe. 25 (2), 285-299.e8 (2019).
- Brunse, A., et al. Fecal filtrate transplantation protects against necrotizing enterocolitis. The ISME Journal. 16 (3), 686-694 (2022).
- Duerkop, B. A., Clements, C. V., Rollins, D., Rodrigues, J. L. M., Hooper, L. V. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proceedings of the National Academy of Sciences of the United States of America. 109 (43), 17621-17626 (2012).
- Barr, J. J., et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proceedings of the National Academy of Sciences of the United States of America. 110 (26), 10771-10776 (2013).
- Nguyen, S., et al. Bacteriophage Transcytosis provides a mechanism to cross epithelial cell layers. mBio. 8 (6), (2017).
- Bichet, M. C., et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience. 24 (4), 102287 (2021).
- Buttimer, C., et al. Impact of a phage cocktail targeting Escherichia coli and Enterococcus faecalis as members of a gut bacterial consortium in vitro and in vivo. Frontiers in Microbiology. 13, 936083 (2022).
- Li, Y., et al. Bacteriophages allow selective depletion of gut bacteria to produce a targeted-bacterium-depleted mouse model. Cell Reports Methods. 2 (11), 100324 (2022).
- Reyes, A., Wu, M., McNulty, N. P., Rohwer, F. L., Gordon, J. I. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proceedings of the National Academy of Sciences of the United States of America. 110 (50), 20236-20241 (2013).
- Federici, S., et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 185 (16), 2879-2898.e4 (2022).
- Sweere, J. M., et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science (New York, N.Y.). 363 (6434), (2019).
- Fluckiger, A., et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science (New York, N.Y.). 369 (6506), 936-942 (2020).
- Majewska, J., et al. Induction of Phage-Specific Antibodies by Two Therapeutic Staphylococcal Bacteriophages Administered per os. Frontiers in Immunology. 10, 2607 (2019).
- Weiss, M., et al. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology. 393 (1), 16-23 (2009).
- Thomson, C. A., Morgan, S. C., Ohland, C., McCoy, K. D. From germ-free to wild: modulating microbiome complexity to understand mucosal immunology. Mucosal Immunology. 15 (6), 1085-1094 (2022).
- Al-Asmakh, M., Zadjali, F. Use of germ-free animal models in microbiota-related research. Journal of Microbiology and Biotechnology. 25 (10), 1583-1588 (2015).
- Ivanov, I. I., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139 (3), 485-498 (2009).
- Bonilla, N., et al. Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ. 4, e2261 (2016).
- Sambrook, J., Russell, D. W. . Molecular Cloning: A Laboratory Manual. 1, (2001).
- Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E., Johnson, R. P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods in Molecular Biology (Clifton, N.J). 501, 69-76 (2009).
- Manikantha, B., Karthika, R., Murugadas, V., Vishnuvinayagam, S., Rao, B. M. Comparison of the single agar and double agar layer methods for enumeration of bacteriophages. Fishery Technology. 59, 60-63 (2022).
- Sanders, E. R. Aseptic laboratory techniques: plating methods. Journal of Visualized Experiments. 63, e3064 (2012).
- Louten, J. Chapter 7 - Detection and diagnosis of viral infections. Essential Human Virology. , 111-132 (2016).
- Richter, &. #. 3. 2. 1. ;., et al. Adsorption of bacteriophages on polypropylene labware affects the reproducibility of phage research. Scientific Reports. 11 (1), 7387 (2021).
- . Amicon Ultra-15 Centrifugal Filter Devices Available from: https://www.emdmillipore.com/CA/en/product/Amicon-Ultra-15-Centrifugal-Filter-Unit (2018)
- Hecker, W., Witthauer, D., Staerk, A. Validation of dry heat inactivation of bacterial endotoxins. PDA Journal of Pharmaceutical Science and Technology. 48 (4), 197-204 (1994).
- Jakočiūnė, D., Moodley, A. A Rapid bacteriophage DNA extraction method. Methods and Protocols. 1 (3), 27 (2018).
- Zucoloto, A. Z., Yu, I. L., McCoy, K. D., McDonald, B. Generation, maintenance, and monitoring of gnotobiotic mice. STAR Protocols. 2 (2), 100536 (2021).
- Ng, K. M., et al. Single-strain behavior predicts responses to environmental pH and osmolality in the gut microbiota. mBio. 14 (4), e0075323 (2023).
- McCallum, G., Tropini, C. The gut microbiota and its biogeography. Nature Reviews. Microbiology. , (2023).
- Bergstrom, K., Xia, L. The barrier and beyond: Roles of intestinal mucus and mucin-type O-glycosylation in resistance and tolerance defense strategies guiding host-microbe symbiosis. Gut Microbes. 14 (1), 2052699 (2022).
- Askar, M., Ashraf, W., Scammell, B., Bayston, R. Comparison of different human tissue processing methods for maximization of bacterial recovery. European Journal of Clinical Microbiology & Infectious Diseases. 38 (1), 149-155 (2019).
- Redanz, S., Podbielski, A., Warnke, P. Improved microbiological diagnostic due to utilization of a high-throughput homogenizer for routine tissue processing. Diagnostic Microbiology and Infectious Disease. 82 (3), 189-193 (2015).
- Bhinder, G., et al. The Citrobacter rodentium mouse model: studying pathogen and host contributions to infectious colitis. Journal of Visualized Experiments. 72, e50222 (2013).
- Reyes, A., Semenkovich, N. P., Whiteson, K., Rohwer, F., Gordon, J. I. Going viral: next-generation sequencing applied to phage populations in the human gut. Nature Reviews Microbiology. 10 (9), 607-617 (2012).
- Camarillo-Guerrero, L. F., Almeida, A., Rangel-Pineros, G., Finn, R. D., Lawley, T. D. Massive expansion of human gut bacteriophage diversity. Cell. 184 (4), 1098-1109.e9 (2021).
- Reyes, A., et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 466 (7304), 334-338 (2010).
- Bach, M. S., et al. Filamentous bacteriophage delays healing of Pseudomonas-infected wounds. Cell Reports. Medicine. 3 (6), 100656 (2022).
- Filyk, H. A., Osborne, L. C. The multibiome: The intestinal ecosystem's influence on immune homeostasis, health, and disease. EBioMedicine. 13, 46-54 (2016).
- Rohwer, F., Merry, Y., Maughan, H., Hisakawa, N. Heather Life in Our Phage World: A Centennial Field Guide to the Earth's Most Diverse Inhabitants. Wholon. , (2014).
- Glonti, T., Pirnay, J. P. In Vitro techniques and measurements of phage characteristics that are important for phage therapy success. Viruses. 14 (7), 1490 (2022).
- Fraser, J. S., Yu, Z., Maxwell, K. L., Davidson, A. R. Ig-like domains on bacteriophages: a tale of promiscuity and deceit. Journal of Molecular Biology. 359 (2), 496-507 (2006).
- Li, H., et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nature Communications. 6, 8292 (2015).
- Bergström, A., et al. Nature of bacterial colonization influences transcription of mucin genes in mice during the first week of life. BMC Research Notes. 5, 402 (2012).
- Adams, M. H. . Bacteriophages. , (1959).
- Kutter, E., Sulakvelidze, A. . Bacteriophages: Biology and Applications. , (2004).
- Bao, H., et al. Dysbiosis and intestinal inflammation caused by Salmonella Typhimurium in mice can be alleviated by preadministration of a lytic phage. Microbiological Research. 260, 127020 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved