A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Bioluminescence Resonance Energy Transfer (BRET)-Based Assay for Measuring Interactions of CRAF with 14-3-3 Proteins in Live Cells
In This Article
Summary
This protocol describes a BRET-based assay for measuring the interactions of the CRAF kinase with 14-3-3 proteins in live cells. The protocol outlines steps for preparing the cells, reading BRET emissions, and data analysis. An example result with identification of appropriate controls and troubleshooting for assay optimization is also presented.
Abstract
CRAF is a primary effector of RAS GTPases and plays a critical role in the tumorigenesis of several KRAS-driven cancers. In addition, CRAF is a hotspot for germline mutations, which are shown to cause the developmental RASopathy, Noonan syndrome. All RAF kinases contain multiple phosphorylation-dependent binding sites for 14-3-3 regulatory proteins. The differential binding of 14-3-3 to these sites plays essential roles in the formation of active RAF dimers at the plasma membrane under signaling conditions and in maintaining RAF autoinhibition under quiescent conditions. Understanding how these interactions are regulated and how they can be modulated is critical for identifying new therapeutic approaches that target RAF function. Here, I describe a bioluminescence resonance energy transfer (BRET)-based assay for measuring the interactions of CRAF with 14-3-3 proteins in live cells. Specifically, this assay measures the interactions of CRAF fused to a Nano luciferase donor and 14-3-3 fused to a Halo tag acceptor, where the interaction of RAF and 14-3-3 results in donor-to-acceptor energy transfer and the generation of the BRET signal. The protocol further shows that this signal can be disrupted by mutations shown to prevent 14-3-3 binding to each of its high-affinity RAF docking sites. This protocol describes the procedures for seeding, transfecting, and replating the cells, along with detailed instructions for reading BRET emissions, performing data analysis, and confirming protein expression levels. In addition, example assay results, along with optimization and troubleshooting steps, are provided.
Introduction
RAF kinases (ARAF, BRAF, and CRAF) are the direct effectors of RAS GTPases and the initiating members of the pro-proliferative/pro-survival RAF-MEK-ERK kinase cascade. Recent studies have shown that CRAF expression plays a key role in the tumorigenesis of several KRAS-driven cancers, including non-small cell lung cancer and pancreatic ductal adenocarcinoma1,2,3,4,5. Moreover, germline CRAF mutations cause a particularly severe form of the RASopathy, Noonan syndrome6,7. Understanding CRAF regulation is critical for developing successful therapeutic approaches that target its function in cells.
All RAF kinases can be divided into two functional domains, a C-terminal catalytic (CAT) domain and an N-terminal regulatory (REG) domain, that controls its activity (Figure 1A)8. The REG domain encompasses the RAS binding domain (RBD), the cysteine-rich domain (CRD), and a serine/threonine-rich region (S/T-rich). Notably, the S/T-rich region contains the N' site, which binds to 14-3-3 in a phosphorylation-dependent manner (S259 in CRAF; Figure 1A)8. The CAT domain encompasses the kinase domain, along with a second high-affinity 14-3-3 docking site, referred to as the C' site (S621 in CRAF; Figure 1A)8. The differential binding of dimeric 14-3-3 proteins to the N' and C' sites, along with the CRD, plays critical roles in both RAF activation and inhibition9,10,11,12,13. Under normal signaling conditions, RAF activation is initiated by its recruitment to the plasma membrane by RAS, allowing it to form active dimers, of which the BRAF-CRAF heterodimer is the predominant active form14,15. Biochemical assays with BRAF and CRAF, along with cryogenic electron microscopy (Cryo-EM) structures of dimeric BRAF, indicate that a 14-3-3 dimer stabilizes active RAF dimers by binding simultaneously to the C' site of both RAF protomers (Figure 1B)9,13,16,17. Conversely, studies have shown that under quiescent conditions, RAF adopts a cytosolic, autoinhibited confirmation, where the REG domain binds to the CAT domain and inhibits its activity12,18,19,20. This closed state is stabilized by a 14-3-3 dimer bound to the CRD and N' site in the REG domain and to the C' site in the CAT domain (Figure 1B)10,13,21. In BRAF, this model is supported by recent Cryo-EM structures of autoinhibited BRAF monomers and by our previous biochemical studies10,12,13,21,22. However, while 14-3-3 is shown to play an inhibitory role in CRAF regulation23, a BRAF-like autoinhibited state may play a lesser role in CRAF regulation12; therefore further studies are required to clarify the mechanisms by which 14-3-3 proteins regulate CRAF activity. The 14-3-3-mediated regulation of RAF kinases requires a plethora of RAF phosphorylation and de-phosphorylation events, the binding to various regulatory proteins, and interactions with the plasma membrane8. Therefore, it is critical that 14-3-3-RAF interactions are measured under physiologically relevant conditions and in the presence of an intact lipid bilayer.
To address this issue, NanoBRET (from here on referred to as N-BRET; see Table of Materials for kit details) technology was utilized to develop a proximity-based assay for measuring the interactions of CRAF with 14-3-3 proteins in live cells (Figure 1C). This BRET-based system measures the interactions of two proteins of interest (POI), where one protein is tagged with a nanoluciferase (Nano) donor and the other with a Halo tag, for labeling with the Halo618 energy acceptor ligand22,24. Interaction of the proteins of interest results in donor to acceptor energy transfer, which in turn generates the BRET signal (Figure 1C). The extremely bright Nano donor protein (emission (em) 460 nm) and the Halo618 ligand (em 618 nm) provide greater spectral separation and sensitivity over conventional BRET, making it an ideal platform for studying weaker interactions and detecting subtle changes in binding24. Indeed, we previously developed a N-BRET-based assay for measuring the autoinhibitory interactions of the RAF REG and CAT domains, which was essential for the characterization of a panel of RASopathy mutations in the BRAF CRD and demonstrated the critical importance of this domain for maintaining autoinhibition and preventing constitutive BRAF activation12.
The assay described here measures the interactions of CRAF, fused to an N-terminal Nano tag (Nano-CRAF), and the zeta isoform of 14-3-3 fused to C-terminal Halo tag (14-3-3ζ-Halo; Figure 1C). We show that the interactions of Nano-CRAF with 14-3-3ζ-Halo generates a robust BRET signal, which can in turn be disrupted by mutations which prevent 14-3-3 binding to the N' site (S259A) and/or the C' site (S621A). The following protocol provides detailed steps for performing, optimizing, and troubleshooting this assay.
Protocol
NOTE: This assay is performed in 293FT cells. A well characterized and readily transfectable epithelial line derived from human embryonic kidney cells. A single confluent 10 cm culture dish of these cells typically provides enough cells for seeding 20 wells of 6-well tissue culture plates. Steps 1-3 must be performed using sterile technique in a biological safety cabinet.
1. Cell seeding (Day 1)
NOTE: In this step, the cells are detached from the tissue culture dish(s), counted, and seeded in 6-well tissue culture plates for transfection in step 2 (Figure 2).
- Aspirate the media from the cells in the 10 cm dish. Wash cells with 5 mL of phosphate-buffered saline (PBS) and aspirate.
- Add 1 mL of trypsin-ethylenediaminetetraacetic acid (EDTA) and incubate for 3-5 min at 37 °C to detach cells from the dish.
- Add 9 mL of complete Dulbecco's modified eagle medium (DMEM) to the cells to neutralize trypsin and pipette up and down repeatedly to generate a homogenous single cell suspension.
- Immediately transfer 20 µL of cells to a 1.7 mL tube and mix with 20 µL of trypan blue stain. Count cells using either an automated cell counter (Table of Materials) or hemocytometer.
- Dilute cells to 2 x 105 cells/mL in complete DMEM media and add 2 mL to each well of a 6-well tissue culture plate (4 x 105 cells/well). Incubate cells at 37 °C and 10% CO2 overnight.
NOTE: It is recommended to use 293FT cells that have been passaged less than 20x. We have previously found that using cells with greater passage numbers can result in reduced BRET ratios and increased well-to-well signal variability.
2. Cell transfection (Day 2)
NOTE: Here, the cells are transfected with the pCMV5-NanoLuc-CRAF and pCMV5-14-3-3ζ-Halo expression constructs, along with pCDNA3.1 empty vector (Figure 2).
- Prior to transfection, dilute the N-BRET plasmids to 5 ng/µL and pCDNA3.1 to 100 ng/µL, and number a set of sterile 1.7 mL tubes.
- Add 100 µL of transfection media (see Table of Materials for details) to each tube, along with 5 ng of pCMV5-NanoLuc-CRAF, 10 ng of pCMV5-14-3-3ζ and 200 ng of PCDNA3.1.
- Add 2 µL of transfection reagent (see Table of Materials for details) and vortex gently to mix. Pulse spin the tubes briefly in a microcentrifuge to ensure that all liquid is collected at the bottom of the tubes and then incubate at around 25 °C for 15 min.
- Add transfection complexes dropwise to cells and incubate at 37 °C/10% CO2 for 16-20 h to allow the Halo- and Nano- tagged proteins to be expressed.
NOTE: The addition of an empty vector (pCDNA3.1) carrier DNA is essential for achieving high transfection efficiency of the N-BRET expression constructs. Failure to add empty vector results in reduced expression levels of the 14-3-3ζ-Halo and Nano-CRAF proteins and in turn results in weak and inconsistent BRET ratios, as discussed previously22.
3. Cell replating (Day 3)
NOTE: In this step, the cells are transferred to a 384-well plate and either Halo 618 ligand (+ligand; Table of Materials) or DMSO (+vehicle) is added for reading the BRET emissions in step 4. The remaining cells are transferred to fresh 6-well culture plates for western blot analysis in Step 5 (Figure 2).
- Collect the following materials and prepare the work area as described below.
- Using a 37 °C water bath, pre-warm trypsin-EDTA, along with both serum-free Assay Media and 10% FBS-supplemented Assay Media (see Table of Materials for details).
- Based on the number of samples to be measured, pre-label three sets of sterile 1.7 mL tubes (Set 1-3), and one set of sterile 15 mL conical bottom tubes. Equip a swing-bucket centrifuge with 15 mL tube inserts and pre-cool to 4 °C.
- Place the following items in the tissue culture hood: reagents reservoirs, 384-well tissue culture plates, 6-well tissue culture plates, a multichannel pipette and tips, cell counting slides/chambers and trypan blue stain (Table of Materials), along with 1.7 mL tube Sets 1-3.
- Harvest and count the cells as described below.
- Aspirate the media from the 6-well plates and add 250 µL of trypsin-EDTA to the cells. Incubate the 6-well plates at 37 °C until the cells begin to detach (3-5 min).
- Add 1 mL of 10% FBS-supplemented assay media to each well to neutralize the trypsin and pipette up and down vigorously to generate a single-cell suspension.
- Transfer 1 mL of the cell suspension to the pre-labeled 15 mL tubes. Add another 1 mL of 10% FBS-supplemented assay media to each of the 15 mL tubes.
- Invert the tubes 5x to mix and immediately transfer 20 µL of cell suspension to tube Set 1.
- Centrifuge in the pre-cooled swing bucket centrifuge for 5 min at ~250 x g. During the centrifugation step, mix 20 µL of trypan blue cell stain with the cells in tube set 1 and then count the cells using either an automated cell counter or hemocytometer. A yield of 6-8 x 105 cells/mL is typical.
- Remove the 15 mL tubes from the centrifuge and aspirate the media from the cell pellets. Resuspend the cell pellets to 2 x 105 cells/mL in serum-free assay media and pipette vigorously to generate a single cell suspension.
NOTE: The use of serum-free Assay Media is used to quiescence normal cell signaling pathways.
- Replate cells in 384-well and 6-well plates as described below.
- Invert the 15 mL tubes several times to ensure a homogenous cell suspension and transfer 500 µL to 1.7 mL tubes set 2 and set 3.
- Add 0.5 µL of DMSO (+vehicle) to set 2 and 0.5 µL of Halo 618 ligand (+ligand) to tube set 3 and pipette to mix.
- Transfer the +vehicle and +ligand cell suspensions to separate wells of reagent reservoirs. Using the multichannel pipette (Table of Materials), transfer 40 µL of the +vehicle cell suspension from the reagent reservoirs to quadruplicate wells of the 384-well culture plate. Repeat this step for the +ligand cell suspensions.
- Transfer the remaining cells into fresh 6-well culture plates. Incubate the 384-well and 6-well plates overnight at 37 °C and 5% CO2.
4. Reading BRET emissions (Day 4)
NOTE: In this step the nanoluciferase substrate (see Table of Materials for details) is added to the cells in the 384-well culture plate and the N-BRET acceptor (618 nm) and donor (460 nm) emissions are read (Figure 2). The corrected BRET ratios are then calculated.
- Pre-warm serum-free assay media in a 37 °C water bath and thaw the nanoluciferase substrate at 25°C. Dilute the nanoluciferase substrate 1:100 in serum-free assay media and transfer to a reagent reservoir. Prepare enough of this mixture for adding 10 µL to each well of the 384-well plate, plus 10%-15% extra volume.
- Using a multichannel pipette (Table of Materials), transfer 10 µL of the substrate mixture to each of the wells that contain cells in the 384-well culture plate. Gently rotate the plate for 1 min, either manually or using an orbital shaker.
- Insert the 384-well plate into the multimode plate reader and record the 460 nm and 618 nm emissions for all wells that contain cells.
NOTE: For the multimode plate reader used for this step (see Table of Materials for details), a read height of 6.5 mm, with a 0.1 s read time measurement was used, however further optimization may be required if using other plate readers. - Calculate the raw BRET ratios for +vehicle and +ligand in milliBRET units (mBU) individually, using the following equation:
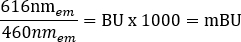
- Calculate the corrected BRET ratio by subtracting the +vehicle BRET ratio from that of the +Ligand (Supplementary Table 1).
NOTE: When comparing the results of multiple experiments, the corrected BRET ratios can be pooled and normalized to the average of the Wildtype (WT) N-BRET pair, consisting of Nano-CRAFWT and 14-3-3ζWT-Halo (Supplementary Table 1).
5. Confirmation of protein expression levels (Days 4 and 5)
NOTE: In this step the cells in the 6-well plates are lysed and the protein expression levels of the Nano-CRAF and 14-3-3ζ-Halo proteins are determined by western blot analysis using antibodies specific to the Halo and Nano tags. (Figure 2).
- Add protease and phosphatase inhibitors to NP40 lysis buffer (Table of Materials), allowing 200 µL of lysis buffer per sample.
- Aspirate media from 6-well plates that contain cells. Wash the cells once with 1 mL of cold PBS and aspirate.
- Add 125 µL of NP40 lysis buffer to each well and incubate 6-well plates at 4 °C on a rocking platform for 15 min to lyse the cells.
- Transfer lysates to 1.7 mL tubes on ice and centrifuge at 20,000 x g for 10 min at 4 °C. Place the cleared lysates back on ice and determine protein concentration using commercially available assays, such Bradford or Bicinchoninic acid (BCA) assays.
- Normalize protein concentration across all samples by transferring an appropriate volume of lysate and NP40 lysis buffer to fresh 1.7 mL tubes to a total volume of 100 µL.
- Boil 5x protein sample buffer (Table of Materials) for 1 min and add 25 µL to each sample. Boil samples for 5-6 min and then pulse centrifuge the tubes briefly to ensure all of the sample collects in the base of the tube.
- Load 25 µL of sample into each well of duplicate protein gels (Gel 1 and Gel 2) and then transfer proteins to nitrocellulose or PVDF membranes using standard western blotting procedures, as previously described22.
- Block membranes in 3% BSA-PBS at 25 °C for 30 min and then incubate membranes overnight with Halo antibody (1:1,000 dilution; Gel 1) and either nanoluciferase or CRAF antibody (1:500 dilution; Gel 2) in tris-buffered saline, supplemented with 0.2% Tween-20 (TBST; Table of Materials).
- Wash membranes 1x for 5 min in 10 mL of TBST and then incubate at room temperature for 1 h with anti-mouse HRP secondary antibody, diluted 1:10,000 in TBST.
- Wash membranes 3x on a rocking platform in 10 mL of TBST at 25 °C for 5 min each. Remove TBST and visualize protein bands using ECL reagents and either an X-ray film processor or other appropriate imaging system.
Results
When performed as described in this protocol (Figure 2), the interaction of Nano-CRAFWT and 14-3-3ζ-Halo should produce corrected BRET ratios of 50-60 mBU (Figure 3A; Supplementary Table 1). CRAF contains two phosphorylation-dependent 14-3-3 docking sites, the N' site and the C' site (Figure 1)8. Therefore, appropriate controls for reducing CRAF:14-3-3ζ binding in...
Discussion
Previous studies have shown that 14-3-3 proteins play critical roles in both the activation and inhibition of RAF kinases. Understanding how these binding events are regulated and the effects of modulating these interactions on RAF signaling and RAF-driven oncogenesis may uncover new therapeutic vulnerabilities that target CRAF function. However, the Raf activation cycle is supported by a plethora of associated proteins, post translational modifications, and changes in subcellular localization8, a...
Disclosures
Nothing to disclose.
Acknowledgements
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under project number ZIA BC 010329.
Materials
| Name | Company | Catalog Number | Comments |
| Antibodies | |||
| HaloTag® mouse monoclonal antibody | Promega | G9211 | Antibody for detecting HaloTag tagged proteins by immunoblot |
| NanoLuc® mouse monoclonal antibody | R&D Systems | MAB10026 | Antibody for detecting Nano-tagged proteins by immunoblot |
| CRAF mouse monoclonal antibody (E10) | Santa Crus Biotechnology | sc-7267 | Antibody directly detecting CRAF proteins by immunoblot |
| ECL anti-mouse HRP secondary antibody | Amersham | NA931-1ML | Secondary HRP conjugated mouse antibody (from sheep) |
| Reagents | |||
| X-tremeGENE™ 9 | Roche/Sigma | 6365809001 | |
| NanoBRET™ kit | Promega | N1661 | NanoBRET kit containing Halo 618 ligand and NanoGlo (nanoluciferase) substrate |
| DPBS, without Ca++ and Mg++ | Quality Biologicals | 114-057-101 | |
| Trypsin-EDTA (0.05%), phenol red | Life Technologies | 25300120 | |
| DMEM cell culture media | Life Technologies | 11995073 | High glucose, L-glutamine, phenol red, sodium pyruvate; without HEPES, suppliment media with 10% FBS, 2 mM L-glutamine and 100U penicillin-streptomycin |
| L-Glutamine (200 mM) | Life Technologies | 25030164 | |
| Penicillin-Streptomycin (10,000 U/mL) | Life Technologies | 15140163 | |
| Opti-MEM™ I reduced serum media | Gibco | 31985062 | For cell transfection |
| Opti-MEM reduced serum media, no phenol red | Gibco | 11058021 | For replating cells on Day 3. Supplement with 2 mM L-glutamine and 100U penicillin-streptomycin, along with 10% FBS (where indicated). |
| Invitrogen Trypan Blue Stain | Thermo Scientific | T10282 | |
| NP40 lysis buffer | N/A | N/A | 20 mM Tris (pH 8.0), 137mM NaCl, 10% glycerol, NP40 alternative (Milipore, Cat# 492016). Store at 4 degrees C.. Add the following protease and phosphatase immediately prior to use: 20 µM leupeptin, 0.5 mM sodium orthovanidate, 0.15 U/mL, 1mM PMSF. |
| 5x gel sample buffer | N/A | N/A | 240 mM Tris (pH 8.0), 9.5% SDS, 30% glycerol, 500mM DTT, 3mM bromophenol blue. Store at -20 degrees C. |
| Cell lines | |||
| 293FT cells (human) | Thermo Scientific | R70007 | |
| DNA vectors | |||
| pCMV5-Nano-CRAF WT and mutant | N/A | N/A | |
| pCMV5-14-3-3ζ-Halo | N/A | N/A | |
| Equipment | |||
| EnVision 2104 Multimode Plate Reader | PerkinElmer 2104 | 2104-0010 | 600LP NanoBRET & M460/50 nm NanoBRET emmisions filters, Luminescence 404 mirror, 6.5 mm measurement height and 0.1 s measurement time |
| Invitrogen Countess™ II Automated Cell Counter | Thermo Scientific | AMQAX1000 | |
| ThermoFisher E1-ClipTip™ Multichannel Pipettor | Thermo Scientific | 4672070 | |
| Software | |||
| GraphPad Prism (version 10.0.3) | GraphPad | www.graphpad.com | |
| Other | |||
| ThermoFisher ClipTip Multichannel pipette tips | Thermo Scientific | 94410153 | |
| Reagent Reservoir, 25 mL Divided, Sterile | Thomas Scientific | 1228K16 | |
| Perkin Elmer 384-well CulturPlate™ | PerkinElmer | 6007680 | White, polystyrene, tissue culture treated |
| Countess Cell Counting Chamber Slides | Thermo Scientific | C10228 |
References
- Blasco, R. B., et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 19, 652-663 (2011).
- Blasco, M. T., et al. Complete regression of advanced pancreatic ductal adenocarcinomas upon combined inhibition of EGFR and C-RAF. Cancer Cell. 35, 573-587 (2019).
- Karreth, F. A., Frese, K. K., DeNicola, G. M., Baccarini, M., Tuveson, D. A. C-Raf is required for the initiation of lung cancer by K-Ras(G12D). Cancer Discov. 1, 128-136 (2011).
- Lito, P., et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 25, 697-710 (2014).
- Sanclemente, M., et al. c-RAF ablation induces regression of advanced Kras/Trp53 mutant lung adenocarcinomas by a mechanism independent of MAPK signaling. Cancer Cell. 33, 217-228 (2018).
- Razzaque, M. A., et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 39, 1013-1017 (2007).
- Pandit, B., et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 39, 1007-1012 (2007).
- Terrell, E. M., Morrison, D. K. Ras-mediated activation of the Raf family kinases. Cold Spring Harb Perspect Med. 9 (1), 033746 (2019).
- Kondo, Y., et al. Cryo-EM structure of a dimeric B-Raf:14-3-3 complex reveals asymmetry in the active sites of B-Raf kinases. Science. 366, 109-115 (2019).
- Park, E., et al. Architecture of autoinhibited and active BRAF-MEK1-14-3-3 complexes. Nature. 575 (7783), 545-550 (2019).
- Tzivion, G., Luo, Z., Avruch, J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 394, 88-92 (1998).
- Spencer-Smith, R., et al. RASopathy mutations provide functional insight into the BRAF cysteine-rich domain and reveal the importance of autoinhibition in BRAF regulation. Mol Cell. 82, 4262-4276 (2022).
- Martinez Fiesco, J. A., Durrant, D. E., Morrison, D. K., Zhang, P. Structural insights into the BRAF monomer-to-dimer transition mediated by RAS binding. Nat Commun. 13, 486 (2022).
- Freeman, A. K., Ritt, D. A., Morrison, D. K. The importance of Raf dimerization in cell signaling. Small GTPases. 4, 180-185 (2013).
- Freeman, A. K., Ritt, D. A., Morrison, D. K. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Mol Cell. 49, 751-758 (2013).
- Rushworth, L. K., Hindley, A. D., O'Neill, E., Kolch, W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 26, 2262-2272 (2006).
- Garnett, M. J., Rana, S., Paterson, H., Barford, D., Marais, R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 20, 963-969 (2005).
- Tran, N. H., Wu, X., Frost, J. A. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 280, 16244-16253 (2005).
- Chong, H., Guan, K. L. Regulation of Raf through phosphorylation and N terminus-C terminus interaction. J Biol Chem. 278, 36269-36276 (2003).
- Cutler, R. E., Stephens, R. M., Saracino, M. R., Morrison, D. K. Autoregulation of the Raf-1 serine/threonine kinase. Proc Natl Acad Sci U S A. 95, 9214-9219 (1998).
- Park, E., et al. Cryo-EM structure of a RAS/RAF recruitment complex. Nat Commun. 14, 4580 (2023).
- Spencer-Smith, R., Morrison, D. K. Protocol for measuring BRAF autoinhibition in live cells using a proximity-based NanoBRET assay. STAR Protoc. 4, 102461 (2023).
- Clark, G. J., et al. 14-3-3 zeta negatively regulates raf-1 activity by interactions with the Raf-1 cysteine-rich domain. J Biol Chem. 272, 20990-20993 (1997).
- Machleidt, T., et al. NanoBRET--A novel BRET platform for the analysis of protein-protein interactions. ACS Chem Biol. 10, 1797-1804 (2015).
- Hekman, M., et al. Dynamic changes in C-Raf phosphorylation and 14-3-3 protein binding in response to growth factor stimulation: differential roles of 14-3-3 protein binding sites. J Biol Chem. 279, 14074-14086 (2004).
- Hatzivassiliou, G., et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 464, 431-435 (2010).
- Bondzi, C., Grant, S., Krystal, G. W. A novel assay for the measurement of Raf-1 kinase activity. Oncogene. 19, 5030-5033 (2000).
- Spencer-Smith, R., et al. Inhibition of RAS function through targeting an allosteric regulatory site. Nat Chem Biol. 13 (1), 62-68 (2016).
- Roy, S., et al. 14-3-3 facilitates Ras-dependent Raf-1 activation in vitro and in vivo. Mol Cell Biol. 18, 3947-3955 (1998).
- Durrant, D. E., et al. Development of a high-throughput NanoBRET screening platform to identify modulators of the RAS/RAF interaction. Mol Cancer Ther. 20, 1743-1754 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved