A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Stabilizing a Femur Osteotomy with a Plate Fixation in Ambystoma mexicanum
In This Article
Summary
A protocol for femoral osteotomy surgery with the use of internal plate fixation in mature axolotls is presented. The procedure can be used to perform comparative studies on limb regeneration and fracture healing in aquatic amphibians.
Abstract
The axolotl (Ambystoma mexicanum) is a promising model organism for regenerative medicine due to its remarkable ability to regenerate lost or damaged organs, including limbs, brain, heart, tail, and others. Studies on axolotl shed light on cellular and molecular pathways ruling progenitor activation and tissue restoration after injury. This knowledge can be applied to facilitate the healing of regeneration-incompetent injuries, such as bone non-union. In the current protocol, the femur osteotomy stabilization using an internal plate fixation system is described. The procedure was adapted for use in aquatic animals (axolotl, Ambystoma mexicanum). ≥20 cm snout-to-tail tip axolotls with fully ossified, mouse-size comparable femurs were used, and special attention was paid to the plate positioning and fixation, as well as to the postoperative care. This surgical technique allows for standardized and stabilized bone fixation and could be useful for direct comparison to axolotl limb regeneration and analogous studies of bone healing across amphibians and mammals.
Introduction
The axolotl (Ambystoma mexicanum) is an important model for organ regeneration, including the tail, spinal cord, brain, heart, gills, and limbs1,2,3,4,5. Detailed studies of axolotl limb regeneration uncovered mechanisms of cell dedifferentiation and the formation of a stem cell pool, blastema, at the amputation site. Due to the ability of the blastema cells to reconstruct all missing limb parts, including a patterned skeleton6,7, the axolotl appears to be an attractive model organism for bone healing studies. Recently, several studies focused more on bone biology in axolotls, describing skeletal morphology, cellular composition, and ossification dynamics.
It was found in mammals that the bone healing process in long bones occurs via endochondral ossification and consists of several stages: hematoma, granulation tissue, and soft callus formation, callus ossification into hard callus and woven bone, and bone remodeling8. A recent study has shown that similar stages can be observed in axolotl bone healing9.
Until now, axolotl fractures were studied in a non-stabilized system, whereby bone is simply cut with iridectomy scissors. The large fractures were created in the zeugopod, where osteotomy is performed on one of the bones, whereas the other serves as support10,11. In contrast, fractures are routinely studied in mammals, including rats and mice, using reliable fixation systems, such as intramedullary pin and bone-aligning plates, to control fracture size and ensure bone alignment.
Thus, the method aims to ensure stabilized and uniform fixation of the axolotl femur prior to osteotomy. In order to make axolotl studies more comparable to mammals, including mice and humans, intramedullary pin12, external plate fixator13,14, and internal bone aligning plate15,16,17 fixation were considered. The latter was shown to ensure proper bone fixation and allow for creating a gap of a certain size by using one or two cuts with a Gigly saw of a specific diameter. As axolotls represent the aquatic larvae of Ambystoma mexicanum, the external fixator might have caused post-surgical complications due to the open wound and contact with water. As axolotls do not develop secondary ossification centers even until very late in their development (20 years old18), and thus the standard intramedullary nail used in mice might not be prevented from puncturing the epiphyses, a decision was made to apply an internal plate fixation method to large axolotls. In large axolotls, the femur size and degree of ossification resemble that of an adult mouse, thus allowing for mid-diaphyseal osteotomy with titanium plate fixation1.
The fracture gap size largely determines the healing dynamics and outcome. For example, in a mouse, a 0.25 mm stabilized fractures heal mostly through intramembranous ossification due to their small size and rigid stabilization; a 0.7 mm fracture heals by endochondral ossification, with the formation of a cartilaginous callus around the fracture; large defects, such as 3.5 mm critical-sized defects do not heal completely and thus are used to model bone fracture non-union16. In this study, the plate fixation protocol of the axolotl femur prior to the osteotomy using the example of a 0.7 mm fracture gap was established with the ultimate goal of comparing axolotl bone healing to that of the mouse9.
After osteotomy, the fractures underwent the process of endochondral ossification, albeit slower than in mice, possibly due to the aquatic lifestyle of axolotls and slower cell division rates. In the method presented here, the 0.7 mm gap osteotomy with rigid plate fixation is shown; however, other gap sizes and semi-flexible fixators, as well as plates of different materials, are potentially possible. Overall, the method presented here can be used for standardized bone fixation and will be helpful for studies comparing axolotl limb regeneration to bone healing or studying bone healing in axolotls under different conditions to ensure standardized fracture fixation.
Protocol
The following procedure was performed with approval from the Magistrate of Vienna (GZ: MA 58-65248-2021-26). 5-8 years old, ≥ 20 cm snout-to-tail tip (snout to the tip of the tail) long axolotls (Ambystoma mexicanum) were used for fracture surgery and amputations. Both males and females were used for the surgeries. Axolotls were bred in the Research Institute of Molecular Pathology facility. Pain and risk of infections were managed with proper analgesics and antibiotics to ensure a successful outcome. The reagents and equipment used for the study are listed in the Table of Materials.
1. Animal preparation
- Bath the animal in 0.03% benzocaine solution for about 15-20 min until full sedation is reached and there is no reflexive movement upon limb touching with tweezers.
- Place the animal with the ventral side down on wet paper towels soaked in 0.03% benzocaine solution and cover it with benzocaine-soaked paper towels. The skin of aquatic animals, such as axolotls, is sensitive to drying, and thus, it is essential to cover the body surface to prevent dehydration and ensure cutaneous (skin) respiration.
- Stretch out the hindlimb to be operated using ring forceps. Do not apply disinfection reagents, such as ethanol, since axolotl skin is sensitive to chemicals and easily irritated. Instead, use 0.7x PBS (A-PBS) with 50 U/mL penicillin and 20 µg/mL streptomycin for cleaning the limb and later on for bone irrigation upon sawing.
NOTE: The infection is generally not a concern for the surgeries performed on axolotls. However, due to the aquatic nature of these animals and the sutures placed on the skin surface, we recommend the use of antibiotics to prevent any contamination of the surgical site.
2. Surgery
NOTE: Sterilize all surgical tools. Common sterilization methods such as heat sterilization, autoclaving, and washing in 70% ethanol, followed by thorough removal of the alcohol remnants, are suitable for this purpose. If operating on multiple animals, sterilize tools in between using a hot bead sterilizer or 70% ethanol.
- Make a lateral longitudinal incision (1.5-2 cm) with a scalpel above the femur bone spanning the whole thigh in the upper hind limb. In order to do this, palpate the bone before cutting the skin.
- Carefully displace the muscles and nerves from the surgery site without cutting. Use bowed forceps to do it efficiently.
- Gently put bowed forceps under the femur to expose it for the surgery.
- Place a rigid 7.75 mm 4-hole fixator plate along with the femur diaphysis, avoiding touching the joints, and secure it in the aligned position with forceps.
- Use four 2 mm titanium screws to attach the bone to the plate.
NOTE: The screws used in this protocol have a complex design and consist of 4 parts: the main part (will be screwed into the bone), screw head (allows removal of the screws and plate using the square box wrench), narrower neck (used as a breaking point once the screw is tightened in the bone) and a screw handle (used for attaching to the screwdriver and the saw-guiding device). - The order of screw attachment is important. Start with the inner screws first, and then the two outer screws to ensure the plate is aligned with the bone axis. Use a manual drill to create the first hole in the bone for easy insertion of the screw, followed by 1st screw placing. Drill in the middle of the bone circumference to avoid thinner bone on one side, which may result in spontaneous bone fracture. Use irrigation with 0.7x PBS + 1% Pen/Strep during drilling. Do not break off the handle of the 1st (optional: 1st and 2nd) screw(s).
- Apply the saw-guiding device onto the 1st (optional: 1st and 2nd ) screw(s) and align it with the bone and plate.
NOTE: In this protocol, a plate, screws, a saw-guiding device, and a saw are provided by the same manufacturer and optimized to fit each other. The saw-guiding device can come in different sizes to be compatible with different plates and saw sizes. - Use the saw-guiding device to drill and insert the rest of the screws. Ensure alignment of the plate with the bone. Break off the handles of the screws.
- Place a piece of plastic film (6-7 mm by 4-5 cm), sterilized by wiping with 70% ethanol and then autoclaved or heat sterilized (140 °C for 4 hours), under the femur to prevent soft tissue damage during the osteotomy process.
NOTE: For this purpose, a piece of plastic film, cut off a bag for heat sterilization can be used. - Place Gigly wire saw between bone and protection film.
- Cut bone using the 0.66 mm Gigly wire saw, creating a single 0.7 mm cut in the femur. Use constant irrigation with 0.7x PBS + 1% Pen/Strep during sawing to minimize tissue damage and friction.
- Remove the saw, and saw guide and use a screwdriver to break off the screw handles from the screws.
- Remove the protection film, and irrigate the surgery site with 0.7x PBS + 1% Pen/Strep.
- Cover the top of the plate and screws with sterile bone bee wax to protect skin and muscles from irritation by the edges of the screws.
- Place muscles and skin on top of the bone bee wax.
- Close the incision site with a 7.0 synthetic (polypropylene/polyethylene) suture using simple interrupted stitches. Synthetic suture is used to minimize contamination with water-born bacteria and fungi.
3. Postoperative management
- To reawaken the animal, place it in a tank with fresh artificial pond water, supplemented with 50 U/mL penicillin, 20 µg/mL streptomycin, and analgetic Butorphanol (0.5 mg/L water).
- Observe the animal to start moving gills, making steps, and swimming - usually within 1 h post-surgery.
- Keep the animal for 3 days in artificial pond water with 50 U/mL penicillin and 20 µg/mL streptomycin before returning it to the holding tank. To ensure analgesia, add Butorphanol (0.5 mg/L water).
- Ensure that the sutures remain in place and proper wound healing occurs.
Results
The surgical procedure described here (Figure 1) lasts between 20 min and 30 min and requires a surgeon and an assistant. Optionally, use a binocular dissection microscope or magnifying glass system.
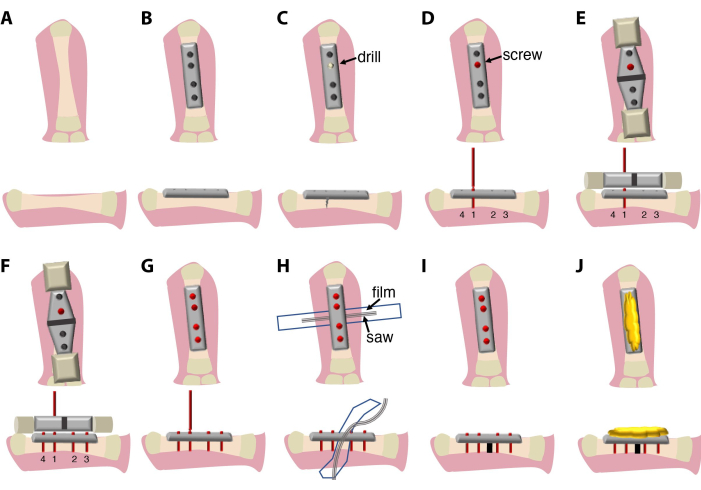
Figure 1: Schematics of the surgical procedure and the experimental setup. (A
Discussion
The currently described method of femur plate fixation and osteotomy allows for its application in aquatic animals, such as Ambystoma mexicanum (axolotl). This surgical method was recently used to compare fracture healing and limb regeneration in axolotls to fracture healing in mice9. As in mice, a 4-hole fixator plate can be attached to the bone with self-breaking screws, and a Gigly saw can be used to create a fracture of uniform size15. Plate fixation facilitate...
Disclosures
The authors declare no competing interests.
Acknowledgements
The authors would like to thank Sabine Stumpp for excellent technical support and Lidia Grösser for assistance in the surgeries. This research was funded by the Austrian Science Fund [Hertha Firnberg Fellowship number T-1219], ERC [Advanced Grant, 742046 RegGeneMems], DFG [CRC 1444].
Materials
| Name | Company | Catalog Number | Comments |
| 0.66 mm Gigly wire saw | RISystem | RIS.590.120 | |
| 7.0 Optilene suture | Braun | C3090538 | |
| Benzocaine | Sigma-Aldrich | E1501 | dilute to 0.03% prior to using |
| Butorphanol (Butomidor 10 mg/mL) | Richter Pharma AG | - | dilute to 0.5 mg/L prior to using |
| Drill bit 0.30 mm | RISystem | RIS.590.200 | |
| Dumont #5 Forceps - Standard/Inox | Fine Science Tools | 11251-20 | |
| Hand drill | RISystem | RIS.390.130 | better to have at least 3 pieces |
| Micro CT data analyzer | Bruker, Billerica, MA, USA | SkyScan NRecon software | |
| Micro CT specimen scanner | Bruker, Billerica, MA, USA | SkyScan 1172 | |
| Moria MC31b Iris forceps - smooth, curved, 10 cm | Fine Science Tools | 11373-12FST | 2 pieces |
| MouseFix Drill-&Saw guide 1.75 mm, rigid | RISystem | RIS.301.102 | |
| MouseFix plate 4 hole, rigid | RISystem | RIS.401.110 | |
| MouseFix screw, L =2.00 mm | RISystem | RIS.401.100 | need 4 per bone |
| Narrow Pattern Forceps | VWR | FSCI11002-12 | |
| penicillin/streptomycin | Gibco | 15140-122 | |
| Ring forceps | Fine Science Tools | 11103-09 | |
| scalpel #15 | B Braun, Thermo Fischer Scientific | 5518032 | |
| Square box wrench 0.50 mm | RISystem | RIS.590.111 | |
| Sterile bone wax, 2.5 g | Ethicon, Johnson & Johnson | W810 | |
| Student Fine Scissors - Straight/11.5cm | Fine Science Tools | 91460-11 |
References
- Amamoto, R., et al. Adult axolotls can regenerate original neuronal diversity in response to brain injury. Elife. 5, 13998 (2016).
- Butler, E. G., Ward, M. B. Reconstitution of the spinal cord following ablation in urodele larvae. J Exp Zool. 160 (1), 47-65 (1965).
- Echeverri, K., Tanaka, E. M. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science. 298 (5600), 1993-1996 (2002).
- Vargas-Gonzalez, A., Prado-Zayago, E., Leon-Olea, M., Guarner-Lans, V., Cano-Martinez, A. Myocardial regeneration in Ambystoma mexicanum after surgical injury. Arch Cardiol Mex. 75 (3), S321-S329 (2005).
- Vieira, W. A., Wells, K. M., McCusker, C. D. Advancements to the axolotl model for regeneration and aging. Gerontology. 66 (3), 212-222 (2020).
- Song, F., Li, B., Stocum, D. L. Amphibians as research models for regenerative medicine. Organogenesis. 6 (3), 141-150 (2010).
- McCusker, C., Bryant, S. V., Gardiner, D. M. The axolotl limb blastema: Cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration (Oxf). 2 (2), 54-71 (2015).
- Einhorn, T. A., Gerstenfeld, L. C. Fracture healing: Mechanisms and interventions). Nat Rev Rheumatol. 11 (1), 45-54 (2015).
- Polikarpova, A., et al. The specialist in regeneration-the Axolotl-a suitable model to study bone healing. NPJ Regen Med. 7 (1), 35 (2022).
- Chen, X., et al. The axolotl fibula as a model for the induction of regeneration across large segment defects in long bones of the extremities. PLoS One. 10 (6), e0130819 (2015).
- Cosden-Decker, R. S., Bickett, M. M., Lattermann, C., MacLeod, J. N. Structural and functional analysis of intra-articular interzone tissue in axolotl salamanders. Osteoarthritis Cartilage. 20 (11), 1347-1356 (2012).
- Williams, J. N., Li, Y., Valiya Kambrath, A., Sankar, U. The generation of closed femoral fractures in mice: A model to study bone healing. J Vis Exp. (138), e58122 (2018).
- Cheung, K. M., et al. An externally fixed femoral fracture model for mice. J Orthop Res. 21 (4), 685-690 (2003).
- Jiang, S., Knapstein, P., Donat, A., Tsitsilonis, S., Keller, J. An optimized protocol for a standardized, femoral osteotomy model to study fracture healing in mice. STAR Protoc. 2 (3), 100798 (2021).
- Matthys, R., Perren, S. M. Internal fixator for use in the mouse. Injury. 40, S103-S109 (2009).
- Manassero, M., et al. Establishment of a segmental femoral critical-size defect model in mice stabilized by plate osteosynthesis. J Vis Exp. (116), e52940 (2016).
- Gunderson, Z. J., Campbell, Z. R., McKinley, T. O., Natoli, R. M., Kacena, M. A. A comprehensive review of mouse diaphyseal femur fracture models. Injury. 51 (7), 1439-1447 (2020).
- Riquelme-Guzman, C., et al. Postembryonic development and aging of the appendicular skeleton in Ambystoma mexicanum. Dev Dyn. 251 (6), 1015-1034 (2022).
- Gentz, E. J., et al. Medicine and surgery of amphibians. ILAR J. 48 (3), 255-259 (2007).
- Lang, A., et al. Collagen I-based scaffolds negatively impact fracture healing in a mouse-osteotomy-model although used routinely in research and clinical application. Acta Biomater. 86, 171-184 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved