Method Article
High-Resolution C. elegans Imaging Across All Larval Stages
In This Article
Summary
This protocol describes microfluidic-based C. elegans time-lapse imaging across the entire post-embryonic development.
Abstract
Caenorhabditis elegans has become one of the most widely studied and best-understood animal models in biology. Three features are key to C. elegans' success as a model organism: its invariant cell lineage, transparency, and genetic tractability. These render it ideal for a diverse range of microscopy-based studies directly in vivo. Live C. elegans larvae and adults often need to be immobilized during image acquisition. Traditional immobilization methods adversely affect animal development, especially in time-lapse imaging applications. Here, a detailed setup and operation protocol for a novel microfluidic imaging method is introduced, which addresses the limitations associated with traditional agar-pad-based immobilization and other microfluidic strategies. This approach enables simultaneous live imaging across various larval stages while preserving worm orientation and identity over time. To achieve this, a microfluidic trap channel array is employed, with its geometry precisely designed to maintain a stable worm orientation while accommodating growth and molting. Immobilization is facilitated by an active hydraulic valve that applies pressure to secure worms against the cover glass solely during image acquisition. This design allows high-resolution imaging with minimal effects on worm viability or developmental timing.
Introduction
C. elegans imaging can be carried out in several ways depending on the application, desired throughput, and resolution. When a high resolution is needed, the standard method utilizes agar pads1,2,3, a simple construction of glass slide, agarose, and cover glass. Animals on these slides are immobilized by the pressure exerted by a cover glass. Immobilization can further be improved by the addition of anesthetizing agents, e.g., levamisole or sodium azide1,2, such that animals on agar pads are perfectly still and can be imaged at high resolution, including various super-resolution methods2. Unfortunately, agar pads are known to affect developmental progression, with the combination of high applied pressures and anesthetizing agents retarding development or causing complete developmental arrest4,5.
Besides these standard methods, several microfluidic methodologies have emerged in recent years, each with its advantages and disadvantages or specialized use cases6,7,8,9,10,11,12,13,14,15,16,17,18,19. For instance, Gritti et al.20 introduced a method in which worms are confined within large chambers. Embryos placed in these chambers develop and move freely while remaining within the designated region of interest on a microscope. While generally effective, this approach is limited to the use of bright fluorescent markers. More recently, Keil et al.21 proposed a modified version of this method, incorporating an on-chip hydraulic valve to restrain animals during image acquisition, thereby addressing some limitations of the original approach. However, since both methods utilize larger chambers than those used for trapped worms, the animals continue to move and rotate throughout the experiment. As a result, tracking developmental processes becomes challenging and requires extensive image post-processing to correct for animal motion.
To address these limitations, the approach introduced by Berger et al.18 (Figure 1), where animals are not held in a large chamber but housed in a trap channel designed to fit animals within a shorter developmental window (up to 2.5 larval stages) is adapted. Unlike existing microfluidic methods, this approach preserves animal orientation and identity throughout an experiment and allows the acquisition of high-resolution images of multiple worms in parallel and across multiple larval stages within a single device.
The animals are trapped using a specialized channel geometry where the channel height is close to the thickness of an animal at the beginning of an experiment, thus preventing rotation. Channel widths and lengths are selected so that animals can move and grow over time, facilitating molting and the transition from one larval stage to the next. Parallelization is then achieved simply by placing multiple trap channels next to each other, such that multiple animals are visible in a single field of view (FOV, Figure 1A'), and up to 41 animals can be imaged in a single device unit (Figure 1A). Animals on the chip are continually supplied a highly concentrated bacterial food suspension via a low-height channel, which at the same time helps confine animals at the end of the trap channel array (height step, Figure 1A, red). Similar to the approach taken by Keil et al.21, a large hydraulic, on-chip valve is implemented, which can be inflated during image acquisition, pressing the animals against the coverglass, thus improving animal immobilization (Figure 1B). Limiting hydraulic valve actuation to only the image acquisition period minimizes any adverse effect on animal development, ensuring reliable development across all larval stages. Unlike the methods described by Gritti et al.20 and Keil et al.21, animals cannot be imaged across the entire post-embryonic development within the same device. Instead, five distinct devices are introduced, termed L1, L1-4, L2-A, L3-A, and L4-A, covering the whole L1 stage and early L2 stage, the mid-late L1 stage up to the mid-L4 stage, the mid-late L2 up to young adulthood, the mid-late L3 up to young adulthood, and the late L4 up to young adulthood, respectively (Figure 1C), which allow uninterrupted imaging of large parts of the post-embryonic development. This approach is taken due to the large increase in animal size from L1 to adulthood, such that a channel suitable for L1 animals would be much too small for adults, resulting in developmental arrest, and a channel fitting adults would be much too large for L1s, resulting in excessive movement, rotation and animals escaping the trap channels.
Using this approach, reliable developmental progression for a variety of tissues is achieved18, e.g., the post-embryonic development of the hypodermis (L1 to L4 stage), induction of the vulval cell fates (L2 and L3 stage), vulval morphogenesis (L3 to adulthood19) and anchor cell invasion. Since their introduction, these long-term imaging devices have been used to study a variety of processes22,23, with the platform even being combined with techniques such as AiryScan super-resolution microscopy24, enabling numerous discoveries previously impossible.
In the following, the operation aspects of these long-term imaging devices are introduced in a step-by-step protocol, highlighting the expected results and some potential challenges when setting up the system.
Protocol
The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Device fabrication
NOTE: All devices were fabricated using the protocol below, however, devices are directly available from the authors. A separate wafer is fabricated for each device type, with each device wafer fabricated from two layers of SU8 of different heights (i.e., food layer and trap layer). The valve wafer is fabricated on a separate wafer, with a single height of SU-8, omitting steps 1.3-1.6. The silanes are toxic and release corrosive vapors. This step, therefore, needs to be performed in a fume hood. For details on the fabrication procedure, refer to Berger et al.18.
- Clean wafers using air plasma.
- Spin coat the first SU8 layer, then perform a soft bake at 95 °C.
- Expose the first layer, followed by a post-bake at 95 °C and development.
- Perform plasma cleaning again after wafer development and drying.
- Spin coat the second SU8 layer, followed by a soft bake at 95 °C (if necessary, conduct an initial bake at 65 °C).
- Align the second mask to the existing features using alignment markers on either side of the wafer, then expose the second layer.
- Post-bake and develop the wafer, then perform a hard bake at 200 °C.
- Treat the wafer with chlorotrimethyl silane for at least 2 h.
- Prepare a batch of 20 g PDMS pre-polymer (Part A) and thoroughly mix it with the 1 g of the cross-linker (ratio 20:1, Part B) (see Table of Materials).
- Degas for 10 min or until all bubbles disappear.
- Remove 2 mL of the PDMS mixture and reserve for step 1.13.
- Add another 3 g of cross-linker to the remaining PDMS mixture, and thoroughly mix (final ratio approx. 5:1).
- Using the batch of 20:1 PDMS, spin coat the device wafer at 750 rpm for 30 s.
- Place the valve wafer in an aluminum dish and cast the 5:1 PDMS onto it. Degas the device wafer for approximately 10 min. After degassing, remove any remaining bubbles using a pipette.
- Bake both the valve and device layers at 70 °C for approximately 15/20 min respectively.
- Assess the curing of the device and valve layers by gently probing them with a pipette tip or tweezers. The valve layer should be solid, while the device layer should remain slightly tacky.
- Detach the valve layer and trim it to the required size using a scalpel or razor blade.
- Punch an access hole into the valve channel (gauge 20).
- Position the device wafer under a stereomicroscope equipped with top illumination.
- Carefully place the PDMS piece containing the valve layer onto the device wafer.
- Align the valve and device features precisely, ensuring the removal of all trapped air between the layers.
- Transfer the assembled device to an oven and bake at 70 °C overnight.
- Detach the device from the wafer.
- Punch all remaining access holes (gauge 20).
- Bond the PDMS to a cover glass using air plasma, then place the bonded device in an oven at 70 °C for a few hours.
2. Worm preparation
NOTE: This section describes worm preparation by bleaching, which allows the generation of large numbers (hundreds) of animals. Other synchronization methods, such as manual picking, are possible but are typically more time-consuming and labor-intensive. All worms used in the protocol are maintained according to standard protocols at 20 °C and on NGM plates seeded with E. coli OP50.
- Starting from plates with many gravid animals.
- Wash animals off the plate using M9 buffer.
- Add 5% NaClO and 5 M of NaOH to the worm suspension (200 µL/100 µL for every 1 mL worm suspension).
- Gently shake the bleaching mix until the animals begin to break apart (~10 min).
- Centrifuge bleaching mix at room temperature, 1300 x g for 1 min.
- Remove the supernatant using a pipette and add an equal amount of fresh M9 buffer.
- Again, centrifuge at room temperature, 1300 x g for 1 min.
- Remove the supernatant and transfer the pelleted worms to a 15 mL tube with 5 mL of fresh M9 buffer.
- Shake overnight at 20 °C.
- Pass the worm suspension through a 10 µm cell strainer.
- Centrifuge the worm suspension at 1300 × g for 1 min at room temperature.
- Discard the supernatant and resuspend the worms in an equal volume of M9 buffer.
- Centrifuge the worm mixture again at 1300 × g for 1 min at room temperature.
- Remove the supernatant and transfer the worms to NGM plates. If L1 larvae are needed, use them immediately.
- Once the worms reach the desired developmental stage, wash them off the plate using fresh S-Basal buffer filtered through a 0.2 µm filter.
- Allow the worms to sediment by gravity or pellet them by centrifugation at 750 × g for 1 min at room temperature.
- Discard the supernatant and add an equal volume of fresh S-Basal buffer.
- Repeat the sedimentation or centrifugation steps (steps 2.16 and 2.17)
- Remove the supernatant and add an equal volume of fresh S-Basal buffer.
- Perform the sedimentation or centrifugation steps one final time.
- Remove most of the supernatant.
- Keep the worms in the tube until the experiment begins.
3. Bacteria preparation
NOTE: Different bacteria strains (e.g., OP50, NA22, HT114, etc.) can be used on the chip. Adjust the proportion of density gradient medium added to the food mix to compensate for differences in density. All buffers are passed through a 0.2 µm filter to remove particulates.
- Prepare 40 mL of LBroth, either in 2 x 20 mL in centrifuge tubes, sterile Erlenmeyer flasks, etc.
- Inoculate LB with bacteria picked from a stock plate.
- Grow the bacteria while shaking overnight at 37 °C (~16 h) (OD600 = 1.9).
- Centrifuge bacteria at 3000 x g for 10 min.
- Remove the supernatant and add 2 mL of fresh S-Basal buffer to each tube. Combine all tubes.
- Mechanically resuspend the bacteria using a pipette.
- Centrifuge the bacteria at 3000 x g for 5 min.
- Remove the supernatant and add 2 mL of fresh S-Basal buffer again.
- Mechanically resuspend the bacteria using a pipette.
- Centrifuge the bacteria at 3000 x g for 5 min.
- Remove as much of the supernatant as possible and add 1 mL of fresh S-Basal buffer before mechanically resuspending all bacteria.
- Combine 0.60 mL of density gradient medium, 0.38 mL of S-Basal + 1% by weight Pluronic F-127 (a non-ionic triblock copolymer, represented here as PF) with 1 mL of OP50 bacteria suspension, or 0.65 mL of density gradient medium, 0.33 mL of S-Basal + 1% by weight PF with 1 mL of NA22 or HT114 bacteria suspension.
- Thoroughly mix the food preparation using a vortex mixer.
- Filter the food through a 5 µm or 10 µm cell strainer. A smaller strainer is especially advised when using smaller devices, e.g., L1 devices.
NOTE: Food can be kept at room temperature for up to 2 days.
4. Chip preparation
NOTE: Refer to Figure 2 for a schematic overview of the device, tubing, and connections. All buffers are passed through a 0.2 µm filter to remove dust. The steel pin mentioned in the following steps refers to a short, hollow tube that is used to interface the larger OD tubing (1/16") to the small diameter holes fabricated in the PDMS device. These steel pins are simply inserted into the inner diameter of the tubing and then pushed into the PDMS material, effectively connecting the two.
- Begin by filling a syringe with deionized (DI) water.
- Attach a 23 G needle and a long piece of 1/16" tubing, with a hollow steel pin (bent at a 90° angle) at the end. The tubing should be long enough to extend from the solenoid to the microscope stage.
- Fill the tubing with DI water from the syringe and connect it to the valve inlet by inserting the steel pin into the punched hole (Figure 2A,J).
- Remove the syringe and needle, then attach the tubing to the off-chip solenoid (Figure 2A,J).
- Using the imaging software, turn on the solenoid and pressurize the device for several minutes to expel all air from the valve. Verify completion by visually checking the air-water interface on the chip, which should appear dark and disappear into the PDMS material (Figure 2B).
- Turn off the solenoid (Figure 2C).
- Fill a 1 mL syringe with the filtered bacteria solution (~0.5 mL of food is sufficient).
- Attach a 30 G needle and a long piece of 1/32" tubing to the needle.
- Press the plunger to fill both the needle and attached tubing, ensuring no air remains in the syringe or tubing.
- Insert the 1/32" tubing directly into the food inlet of the microfluidic device, using tweezers to prevent damage to the tubing (SMD tweezers are recommended) (Figure 2D,K).
- Place the syringe on the syringe pump (Figure 2D).
- Press the syringe plunger using the thumbscrew at the back to fill the device with liquid. A drop of liquid should appear at each open connection (Figure 2D, arrow).
- Block both the worm inlet and outlet with a sealed steel pin (a steel pin with a short piece of 1/16" tubing attached and sealed by burning) (Figure 2E,L).
- Apply additional pressure using the thumbscrew to remove any remaining air in the device (Figure 2E, arrow).
- Remove the blocked pin at the outlet and attach the waste container (made from a cryovial or microcentrifuge tube, with two steel pins stuck through the lid and secured with glue). The waste container is connected using a short piece of 1/16" tubing, with one end attached to the container and the other end connected to a hollow steel pin (bent 90°) (Figure 2F,G,M).
- Push the syringe to ensure the waste container is properly connected and that no blockages exist in the system. For this, push a small amount of liquid through the system (turn the thumbscrew on the syringe pump) until liquid flows through the waste tubing (Figure 2G, arrow).
- Remove the second blocked steel pin.
- Push the syringe until a small drop of liquid appears at the worm inlet (Figure 2H,N).
- Attach a longer piece of 1/16" tubing (15-20 cm) to a 1 mL syringe filled with S-Basal buffer, using a 23 G needle (Figure 2H,N).
- Attach a straight 23 G steel pin to the other end of the tubing.
- Fill the needle and tubing with buffer from the syringe, ensuring no air remains in the syringe or tubing.
- Insert the steel pin at the end of the tubing into the tube containing the worms.
- Push a small amount of liquid through the tubing, ensuring no air is left.
- Pull the worms into the tubing, but do not pull them into the syringe.
- Push the syringe connected to the worms until a small drop of liquid appears on the steel pin (Figure 2H).
- Insert the steel pin into the worm inlet (Figure 2I).
NOTE: Device preparation can be performed prior to the final worm preparation and left on the microscope until the worms are ready.
5. Worm loading and imaging
- Place the device onto a microscope at low magnification (5x or 10x) or on a dissection microscope.
- Identify the device unit currently in use and find the worm inlet using brightfield illumination.
- Position the device such that the inlet is visible on one side of the field of view and the back of the trap channel inlet is visible on the other side.
- Gently push on the worm syringe's plunger. The liquid will noticeably flow from the inlet through the channel array towards the outlet. The presence of bacteria and the difference in refractive index due to the addition of the density gradient medium make the liquid flow apparent.
- Ensure that the worms appear from the inlet and flow toward the back of the channel array.
- Gently push the animals toward the channel array. Once an animal faces the channel, push it into the channel, and repeat for additional animals.
NOTE: Animals need to be oriented with their heads toward the outlet to have easy access to food. They can be oriented by pushing and pulling on the plunger and by their swimming. - Once sufficient animals have been trapped, place the syringe, still attached to the worm inlet, on the microscope stage, where it will remain throughout the experiment. Briefly detaching the worm tubing from the syringe may be necessary to release any pressure buildup, then carefully reattach it to the blunt needle.
- If loading was performed on a dissection microscope, transfer the device to the imaging microscope. The tubing can remain attached to the chip, and the syringe is connected to the pump.
- Switch on the syringe pump and run it at the preset rate of 1 µL/h for 0.5 µL, increase the rate by 100 µL/h for 0.5 µL, and reduce it back to 1 µL/h. This cycle is automatically repeated for the entire experiment. For information on how to program the pump, see the user manual of the equipment.
- Place the device onto the microscope stage and ensure it is firmly held (suggestions for mounting mechanisms can be found in Figure 3A).
- If loading was not done on the imaging microscope, identify the device unit of interest at low magnification.
- Switch to the desired imaging magnification.
NOTE: The devices are compatible with all objective types. - Identify the animals and regions of interest within the trap channel array and set up the desired imaging conditions.
- Image at the desired imaging conditions, with the on-chip valve, actuated through the solenoid 10 s before image acquisition, so the animals are held in place.
NOTE: Actuating the on-chip valve will elongate and flatten the animals, possibly requiring adjustments to the imaging region of interest (ROI).
6. Setup preparation
NOTE: This section refers to the initial preparation of the device control mechanism and does not need to be repeated before each experiment. Refer to Figure 3B,C. Because many solenoids require a higher voltage, the solenoid will most likely not be connected directly to the microcontroller but via a relay or MOSFET (see Figure 3C).
- Identify a pressurized air source (e.g., wall supply installation or gas cylinder).
- Connect the pressure source to a suitable pressure regulator (0-2 bar pressure range) using 6 mm tubing (wall source) or directly attach a suitable regulator to the gas cylinder.
- Connect the pressure regulator to the solenoid valve input (6 mm tubing, see Figure 3B).
- Place the solenoid as close to the microscope stage as possible (~0.5-1 m from the stage is suggested, though longer distances are possible if needed).
- Connect the solenoid valve electronically to the selected controller. Different options are available, such as controlling it using a separate microcontroller board (e.g., Arduino) or an existing DAq card.
7. Cleanup and storage
- Once the experiment is completed, remove the device from the microscope.
- Remove the immersion liquid. Immersion oil can be removed with organic solvents (e.g., acetone, methanol, etc.).
- Place the device on a flat surface and disconnect all tubing except the valve tubing. To safely disconnect each tubing, press down on the PDMS close to it and gently pull. Do not lift and bend the device, as this may cause the cover glass to break.
- Clean the PDMS surface with 70% ethanol.
- Store the device at room temperature with the valve tubing attached.
NOTE: The device can be reused until all units have been used, likely without needing to refill the valve tubing. However, the water in the valve tubing will evaporate over an extended period and may eventually need to be refilled.
Results
Device design and dimensions
Five distinct devices were designed to accommodate all four larval stages. These devices are designated as follows: L1, for L1 larvae loaded immediately after hatching and imaged until the L2 stage; L1-L4, capable of holding larvae from the mid-to-late L1 stage through early-to-mid L4; L2-A, suitable for animals from approximately the mid-L2 stage to young adulthood; L3-A, for animals from approximately the mid-L3 stage to young adulthood; and L4-A, which accommodates animals from approximately the late L4 stage to young adulthood.
All devices follow the same essential layout, consisting of a worm inlet, 1 (or 2 in case of media exchange) bacterial food inlet(s), and a common waste outlet. In between the inlet and outlet portion, an array of 41 parallel trap channels is placed, with the inlet and outlet side effectively separated by the lower height food supply channel (Figure 1A,A′). Channel dimensions were chosen to be significantly longer and wider than the worms loaded at the beginning of an experiment, with a length/width of 400/15 µm for L1 devices, 575/22 µm for L1-L4 devices, 800/27 µm for L2-A devices, 900/30 µm for L3-A devices and 1000/65 µm for L4-A devices (Supplementary File 1, Supplementary File 2, Supplementary File 3, Supplementary File 4, and, Supplementary File 5).
The channel length was selected to match the size the animals reached by the end of the experiment, while the width was chosen so that worms at the start of the experiment occupied approximately two-thirds of the channel width. This design allows sufficient space for growth and movement during molting while preventing worms from turning or rotating. Unlike width, channel height was set close to the thickness of the worms at the beginning of the experiment: 8 µm for early L1 larvae, 12 µm for mid/late L1 larvae, 15 µm for mid-L2 larvae, 17.5 µm for mid-L3 larvae, and 22.5 µm for late L4 larvae, corresponding to the L1, L1-L4, L2-A, L3-A, and L4-A devices, respectively. The reduced channel height, combined with a carefully selected width (maintaining a height-to-width ratio of 0.5-0.6), ensures that worms remain in a fixed orientation. Notably, with a sufficiently small channel height, all worms are consistently positioned in the desired lateral orientation.
The food supply channel was fabricated at a height of 3 µm for L1, and 5 µm for all other devices, ensuring that bacteria can easily reach the animals without the animals escaping from the trap channels. All devices use the same food supply layer (Supplementary File 6) and the same valve layer (Supplementary File 7). Note the valve layer is fabricated on a separate wafer at a height of 20 µm and only placed on the device layer during the PDMS device fabrication (step 1.21).
These stage-specific geometric constraints, essential for preventing worms from rotating or reversing head to tail, necessitate the use of three distinct device types to accommodate all larval stages. If L1 larvae were placed in a channel sized for young adult animals, they would not remain stable and would quickly exit the trap channel.
C. elegans preparation
Unless stated otherwise, worms were maintained according to standard protocols25 on NGM plates seeded with E. coli OP50 at 20 °C. The worm preparation section explicitly describes the preparation of a synchronized worm population used in an imaging experiment. Synchronization was carried out according to standard protocols using sodium hypochlorite bleach26, modified with an additional filtration step to remove debris and unhatched animals after overnight starvation. The aim is to obtain many synchronized L1 larvae, which are then seeded on NGM plates or loaded directly into the imaging device. Other synchronization methods, such as manual picking or controlled egg laying, are equally suited. However, they are more laborious in generating sufficient animals (200 per condition is recommended).
Once worms have reached the desired stage, they can be gently washed off the NGM plate using clean S-Basal (ideally filtered through a 0.2 µm filter). The aim is to collect as many animals as possible without collecting bacteria or debris from the plate. Animals are collected in a small centrifuge tube and washed 2-3 times with fresh S-Basal buffer, finally removing as much liquid as possible. Animals are then directly taken from the concentrated volume. If animal concentration is too low, loading will be slower, requiring a larger volume of liquid to flow through the device and increasing the risk of blocking trap channels with debris.
Bacterial food preparation
Bacteria growth is carried out following standard protocols, with the initial bacteria concentration (OD600 = 1.9) increased approximately 40-fold in S-Basal buffer (filtered through a 0.2 µm filter) and mixed with the density gradient medium and S-Basal buffer containing PF (filtered through a 0.2 µm filter). The expected result of this process step is the preparation of a very dense bacteria suspension and the prevention of bacteria sedimentation during the imaging experiment. The high density is necessary to feed the animals on-chip at the low flow rates employed in media delivery (1 µL/h). The purpose of the density gradient medium is to prevent bacteria precipitation during the experiment, and the purpose of PF is to prevent bacteria from sticking to the channel walls. Both the density gradient medium and PF are non-toxic and do not affect animal viability.
If a different bacteria type is used in an experiment, the necessary concentration of the density gradient medium will need to be determined. This can be achieved by mixing bacteria suspension with varying amounts of the medium followed by centrifugation. If the density gradient concentration is too low, bacteria will sediment; if the concentration is too high, bacteria will float. Once a suitable concentration has been found, validating the result by leaving the bacteria mixture at room temperature for approximately 48 h to indicate how the food will behave throughout the experiment is recommended. After the experiment, checking the syringe through which the bacteria are supplied and ensuring bacteria do not sediment or float is likewise recommended. The difference in volume resulting from adding different amounts of Optiprep can be compensated by adding more or less S-Basal + 1% by weight PF. Similarly, additional media or compounds (e.g., auxin) can be added to the suspension, and the extra volume is subtracted from the volume of S-Basal + 1% by weight PF.
Setup preparation
Set up the pressure supply system as close as possible to the microscope, such that it can be operated conveniently. All tubing runs, especially the 1/16" tubing connecting to the chip, should remain short (less than 0.5 m for the 1/16" OD tubing) (Figure 2 and Figure 3B). As indicated in the above section, computer control of the on-chip valve can be achieved in a variety of ways. It should be possible in any microscope software (tested in Micro-Manager, Metamorph, NIS, and ZenBlue), either by connecting a dedicated microcontroller board (e.g., Arduino) or by connecting the system to an existing data acquisition (DAq) card, using a digital output. The selected board output should then be configured as a shutter, which turns on 10 s before image acquisition or is actuated manually during device setup. As mentioned previously, connecting the solenoid directly to the microcontroller is not recommended; instead, connecting via a MOSFET or relay switch so that the solenoid and the microcontroller remain electronically isolated (Figure 3C).
Similar to the pressure supply tubing, keep all other tubing runs to the chip (1/16" OD and 1/32" OD) as short as possible. Keep the worm and waste tubings (1/16" OD) below 2.5 cm and 20 cm, respectively. The bacteria supply tubing (1/32" OD) must be long enough to connect from the syringe pump to the device once placed onto the microscope stage. Nonetheless, shortening this as much as possible and placing the syringe pump close to the microscope stage is highly recommended, e.g., next to the stage, using a shelf or on top of an incubator enclosure. Any length of tubing added to the system will increase the backpressure felt when loading animals into the device and increase the total pressure on trapped animals.
Device operation
All tubing can be prepared and connected to the respective blunt needle, steel pin, and syringe at this stage (beginning of protocol step 4). As mentioned above, keep the tubing length as short as possible (Figure 2J-N). Importantly, care must be taken that no air bubbles remain in the syringes or tubing, as these will interfere with animal loading and may result in animals escaping from the trap channel during the experiment. Air bubbles can be removed from the syringe in multiple different ways, e.g., by filling the syringe, inverting it, and gently shaking it such that the air bubbles rise to the top of the syringe (flicking the syringe is not recommended, as that can result in additional air bubbles being trapped). Alternatively, the syringe can be filled by sticking it into the buffer and rapidly moving the plunger up and down to remove most of the bubbles quickly.
Once the syringe is filled and bubble-free, the blunt needle and tubing are attached and filled with buffer. Special care must also be taken when filling the syringe, as air can easily be trapped in the plastic part of the blunt needle. If air is trapped, replace the needle. Alternatively, prefilling the needle with liquid, e.g., using a pipette, can alleviate the issue.
With all components in place, the tubing can be attached to the device, starting with the tubing connecting the solenoid and on-chip valve (Figure 2A-C,J). This tubing is initially filled with deionized water, and the system is pressurized to remove all air from the dead-end valve channel. PDMS is gas permeable; thus, the elevated pressure will push all air from the channel into the PDMS, leaving the valve channel filled with water. This process can be followed on a microscope, ensuring the entire valve channel is filled. After filling the valve, the bacteria supply can be connected, and the device can be filled and pressurized by blocking all open inlets/outlets (Figure 2D,E,K,L). Air is displaced, and the process can be monitored on a microscope. Once all air is displaced, the bacteria should fill the device uniformly. The blocking pins are removed, and the waste collection tube is connected (Figure 2F,G,M). At this point, a small amount of liquid should flow through the device into the waste collection tube, ensuring that all connections to the tube are open. This can be visually confirmed before connecting the tubing to the collection tube. If no liquid flows to the waste collection tube, it is likely blocked. This would result in pressure buildup over the experiment, negatively affecting the trapped animals. If the tube or its connections are blocked, remove it and clean all connections using a needle or flush them with a water-filled syringe.
Finally, the worm tubing can be connected (Figure 2H,N). Worms are picked up from the centrifuge tube simply by sucking them into the tubing attached to the S-Basal-filled syringe. The animals should only be pulled into the tubing, not the syringe. The tubing can then be connected to the device. Before that, a small amount of liquid is pushed out from the device and the worm tubing, and the two droplets are connected such that no air is pushed into the device during loading.
Worm loading
With all connections made, animals can be loaded by pushing and pulling on the worm syringe's plunger. Animals will be flushed from the inlet towards the trap channel array; however, they will naturally swim against the flow they experience. Animals, therefore, need to be reoriented with their head facing the trap channel. Correct animal orientation is accomplished by pushing and pulling on the plunger and giving the animals time to rotate into the desired orientation. This can almost be achieved on a single-worm basis but will require some practice. Once animals are correctly oriented, they can be pushed into the trap channel and slide to the end of the channel, where a height step stops them (Figure 1A,B). If animals cannot enter the channel, they are too big for the selected device and need to be imaged in a larger device. If animals can rotate or turn around when placed into the trap channel, they are too young, and a smaller device needs to be used, or the animals need to be left to grow for a few more hours.
Importantly, animals in different channels will not affect each other. Therefore, animals loaded incorrectly will not affect those loaded correctly, however, they will not have sufficient access to food and will therefore grow slower or arrest development.
If animals are loaded at the right age, minimal motion along the longitudinal channel axis should occur. However, animals are free to wiggle (Figure 1B) and begin feeding within a couple of minutes. If animals show significant movement along the longitudinal channel axis, this is likely caused by pressure buildup in the device or by an air bubble lodged somewhere in the tubing, blunt needle, or syringe. Pressure buildup within the device can be released by gently detaching the worm tubing from the blunt needle and gently reattaching it. If an air bubble has remained lodged within the system despite the abovementioned steps and precautions, the entire worm syringe may need to be removed and replaced with a new one without air bubbles. Air bubbles in the system function as springs that are compressed during loading and slowly released after loading animals. The flow created by the air bubbles expanding can result in system instabilities, pushing the animals out of the trap channels.
Image acquisition and development on-chip
Finally, once animals are loaded, image acquisition can start. In principle, the devices are compatible with most imaging methods, brightfield, epifluorescence18,19,22,23 (Figure 4), spinning disk confocal19, and even super-resolution modalities (AiryScan24), as all imaging is performed through a 170 µm thick cover glass. Consequently, the microfluidic device shows little to no effect on the achievable image quality on-chip. However, appropriate imaging conditions that do not affect animal viability must be selected regardless of the imaging modality. High excitation intensities will readily result in fluorophore bleaching and developmental arrest due to the high energy and heat imparted on the sample. Short exposure times (~10 ms) and low excitation intensities (less than 10% of a typical fluorescence LED source) are recommended, yielding a usable image at a contrast setting of min = 0 and max = 1000 (when using a 16-bit camera) (Figure 4).
Representative data obtained using the L1, L1-4, and L2-A devices are shown in Figure 4. Showing development of the C. elegans epithelial cells from hatching/overnight starvation to the mid-L2 larval stage (Figure 4A, Supplementary Movie 1), induction of the 1°-fated vulva precursor cells and their subsequent divisions from late L1 to the early L4 larval stage (Figure 4B, Supplementary Movie 2) and finally formation of the C. elegans vulva from the early L3 larval stage up to eversion at the transition to adulthood (Figure 4C, Supplementary Movie 3).
Each application is accompanied by a quantification of different developmental timing metrics. Images in Figure 4A,B are shown without any applied post-processing, i.e., no deconvolution or image registration was applied. Images in Figure 4C, on the other hand, were deconvolved27 and registered, highlighting the improvements in image quality possible when using long-term imaging devices.
First, identifying the onset of each seam cell division for animals shown in Figure 4A (see Supplementary Movie 1 for a full-time course), indicating the consistent and timely division of all cells across 12 animals (Figure 4D). Using the seam cells, the general developmental timing across all four larval stages was also assessed, and found that all seam cell divisions were completed after 12.0 h ± 1.7 h (mean ± SD) in L1 (n = 22), 10.2 h ± 1.0 h in L2 (n = 27), 10.9 h ± 1.4 h in L3 (n = 27), and 14.6 h ± 2.2 h in L4 animals (n = 19). These data are consistent with literature values2,28 as well as values measured in animals grown on plate, yielded median developmental times for L1 15 h (n = 21), L2 10 h (n = 15), L3 12 h (n = 21) and L4 12.5 h (n = 61) (Adapted from Berger et al.18). The slight delays observed are likely a consequence of image acquisition and resulting phototoxicity or the increased confinement once animals have grown and will a trap channel.
Second, we followed animal growth across the L2 and L3 stages, quantifying gonad length as an excellent indicator of developmental progression (Figure 4E, see Supplementary Movie 2 for a full-time course). As with seam cell division, the precise gonad length could be measured thanks to the straight animal orientation, and consistent gonad growth across all animals imaged was found (n = 19). The first VPC division in these experiments was observed 14.26 h ± 2.47 h after the experiment began (~30 h after seeding), with the second occurring 1.46 h ± 0.13 h later (n = 19). These data are in good agreement with literature values established on NGM plates29,30. Euling and Ambros30 found the first division to occur after approximately 29 h and the second approximately 3 h later.
Finally, the time by which specific stages in vulval development are reached was quantified (Figure 4F, see Supplementary Movie 3 for the full-time course), specifically assessing the time needed to progress from invagination (L4.0, t1) to the mid-L4 stage (L4.5, t4) according to the sub-stages defined by Mok et al.31. The average progression time between t1-t2 was 3.8 h ± 1.2 h, between t2-t3 was 3.5 h ± 1.2 h, and between t3-t4 was 2.8 h ± 1.6 h (n = 7), indicating an approximately linear developmental progression. Similar to the developmental timing assay, these times, particularly t1-t2, appear slightly delayed compared to reported literature values (t1-t2: 0.7 h, t2-t3: 3 h, t3-t4: 2.3 h31), possibly due to the narrow device dimensions slowing molting. Nevertheless, developmental timing remains highly consistent among animals, and all successfully progress to adulthood.
Compensating animal motion and microscope drift
Animal motion throughout a single Z-stack is typically minimal when the on-chip valve is actuated. However, animals are not expected to remain completely still. The residual jitter observed in a single stack can be compensated by increasing the pressure to actuate the on-chip valve, decreasing the used exposure time, or increasing the selected Z-step to accelerate stack acquisition. Stack acquisition time can further be reduced by using a piezo Z-drive, resulting in significantly faster Z-motion compared to a conventional microscope focus drive, or by utilizing multi-band filters (e.g., GFP/mCherry) such that separate colors can be acquired without the need for time-consuming filter changes. For reference, in the provided examples (Figure 4 and Supplementary Movie 1, Supplementary Movie 2, and Supplementary Movie 3), the stack acquisition time was typically between 3 and 8 seconds. Residual motion can also be removed through various image registration methods if necessary.
Animals on-chip are expected to grow steadily. Thus, features of interest will inevitably shift along the longitudinal channel axis (Figure 4A,B, Supplementary Movie 1 and Supplementary Movie 2). Therefore, setting the imaging FOV such that the feature of interest can grow into the FOV and is not lost over the course of an experiment is recommended (Figure 1B and Figure 4A,B). Alternatively, animals can be imaged in multiple adjacent FOVs, covering the entire animal body. However, this may impart additional phototoxicity and stress. As with Z-motion, various registration procedures can compensate for animal motion in the channel over time. If animals show erratic movement along the longitudinal axis, more extensive than what can be attributed to growth, this is likely to be caused by an air bubble.
Each long-term imaging device contains six individual imaging units, each of which can be used for a separate experiment. Each unit can house up to 41 animals, and the trap channels are spaced such that multiple animals can be imaged in a single FOV. Additionally, multiple FOVs can be imaged within a single unit or device. As with all microscope samples, movement across the sample surface may introduce focus drift due to loss of immersion media. This drift can be compensated by using a lower-viscosity immersion oil or through various autofocus modalities. However, some hardware autofocus systems may not function when used with a PDMS microfluidic device, as these use the transition from glass to an aqueous medium as a reference point (sample side of the cover glass). Much of the FOV is filled with silicone within the device, which has a higher refractive index than water; while this does not affect image quality, it may negatively affect the autofocus. When imaging a single FOV, the focus drift observed within the device is minimal (less than 2 µm). If excessive focus drift is observed, this may result from the thermal instability of the microscope system or environment, excessive XY motion acquiring multiple FOVs, or improper mounting of the device on the microscope (see Figure 3A for suggested mounting mechanisms).
Similarly, excessive XY movement and improper mounting may also result in XY drift. Since the device and microscope objective are connected via the immersion liquid, fast movements in X, Y, or Z may cause forces to be applied to the device. If the devices are correctly mounted and no external forces act on them, they are exceptionally stable with minimal focus drift and no XY drift.
Finally, if specific system parameters are inappropriate, the PDMS microfluidic devices may adversely affect animal development. Viability is affected by excessive valve pressure, high phototoxicity resulting from high excitation intensities, long exposure times, or short time intervals between stack acquisitions. Especially when first using this system, assessing animal viability using a bright fluorescent marker or brightfield imaging is highly recommended, ensuring that the imaging condition does not affect animal growth. Once animals develop reliably, imaging parameters can be varied as needed.
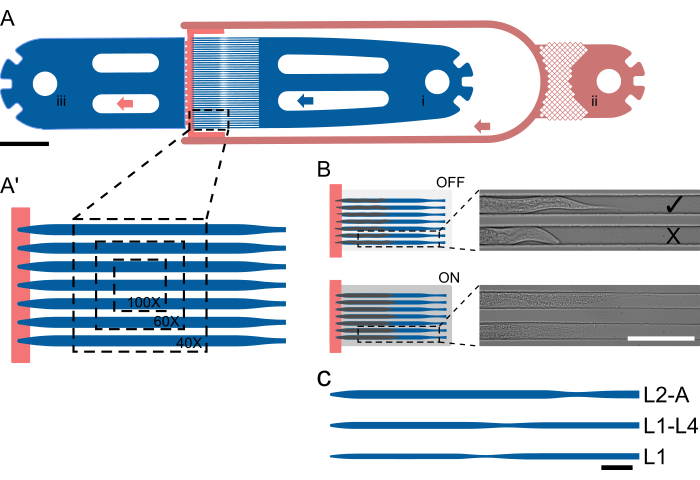
Figure 1: Device layout and schematic operation. (A) Device overview showing a single unit with the worm inlet (i), the bacterial food inlet (ii), and the general outlet (iii). (A') Magnified view of the trap channel in an L2-A device, highlighting the number of worms visible in a single FOV at different magnifications (40x-100x). (B) Functional principle of the on-chip valve. When OFF, the channel height is selected so that animals can comfortably fit in the trap channel. When ON, the channel height decreases, pressing the animal onto the coverglass, thereby reversibly immobilizing the trapped animals. Images show two trapped animals on-chip with acquired with the on-chip valve OFF and with the valve ON, with the animals visibly straightening and elongating under pressure. The upper animal loaded correctly and facing toward the food source ( ), and the lower facing away (X). (C-C") Schematic device setup. (C) Available device sizes. L1 is suitable for animals from the L1 to mid-L2 stage, L1-4 for animals from the mid-L2 to mid-L4 larval stage, and L2-A for animals from the mid-L2 larval stage to adulthood. Scale bars are (A) 1000 µm, (B) 100 µm, and (C) 50 µm. The figure is partially adapted from Berger et al.18. Please click here to view a larger version of this figure.
), and the lower facing away (X). (C-C") Schematic device setup. (C) Available device sizes. L1 is suitable for animals from the L1 to mid-L2 stage, L1-4 for animals from the mid-L2 to mid-L4 larval stage, and L2-A for animals from the mid-L2 larval stage to adulthood. Scale bars are (A) 1000 µm, (B) 100 µm, and (C) 50 µm. The figure is partially adapted from Berger et al.18. Please click here to view a larger version of this figure.
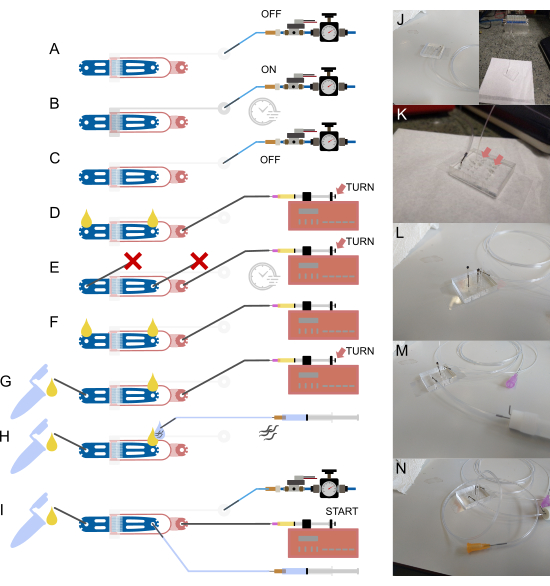
Figure 2: Device setup and connections. (A-I) Schematic overview of the setup process. (A) Connection of 1/16" water-filled tubing to the valve inlet using a hollow steel pin (gray), with the other end attached to the pressure supply system. (B) Filling of the on-chip hydraulic valve via the off-chip solenoid and pressurizing the device for a few minutes. (C) Deactivation of pressure before proceeding to the next steps. (D) Connection of a syringe containing bacterial food to the food inlet using 1/32" tubing and a 30 G blunt needle, followed by filling the device until liquid appears at both open connections. (E) Sealing of the worm inlet and waste outlet with steel pins blocked by a piece of 1/16" tubing ( ) and pressurizing the device using the food syringe and pump (via thumbscrew adjustment), maintaining pressure for a few minutes. (F) Removal of plugs from (E), ensuring liquid droplets form at each open connection. (G) Connection of the waste container via a short piece of 1/16" tubing and a hollow steel pin. (H) Attachment of a buffer-filled syringe to the device using a hollow steel pin and a 23 G blunt needle. Worms are drawn into the tubing before connecting it to the device, ensuring no air is introduced by aligning liquid droplets at the inlet and tubing end. (I) Final setup with all device connections established. (J) Image of the device with valve tubing connected to the inlet and solenoid (background), corresponding to (A-C). (K) Magnified view of the device with food supply tubing attached, showing two liquid droplets (arrows) on the worm inlet and waste outlet, corresponding to (D). (L) Magnified view of the device with food supply tubing attached and the worm inlet and waste outlet blocked, corresponding to (E). (M) Magnified view of the device with waste tubing and container attached, corresponding to (G). (N) Final assembly with all tubing connections in place, corresponding to (I). Partially adapted from Berger et al.18. Please click here to view a larger version of this figure.
) and pressurizing the device using the food syringe and pump (via thumbscrew adjustment), maintaining pressure for a few minutes. (F) Removal of plugs from (E), ensuring liquid droplets form at each open connection. (G) Connection of the waste container via a short piece of 1/16" tubing and a hollow steel pin. (H) Attachment of a buffer-filled syringe to the device using a hollow steel pin and a 23 G blunt needle. Worms are drawn into the tubing before connecting it to the device, ensuring no air is introduced by aligning liquid droplets at the inlet and tubing end. (I) Final setup with all device connections established. (J) Image of the device with valve tubing connected to the inlet and solenoid (background), corresponding to (A-C). (K) Magnified view of the device with food supply tubing attached, showing two liquid droplets (arrows) on the worm inlet and waste outlet, corresponding to (D). (L) Magnified view of the device with food supply tubing attached and the worm inlet and waste outlet blocked, corresponding to (E). (M) Magnified view of the device with waste tubing and container attached, corresponding to (G). (N) Final assembly with all tubing connections in place, corresponding to (I). Partially adapted from Berger et al.18. Please click here to view a larger version of this figure.
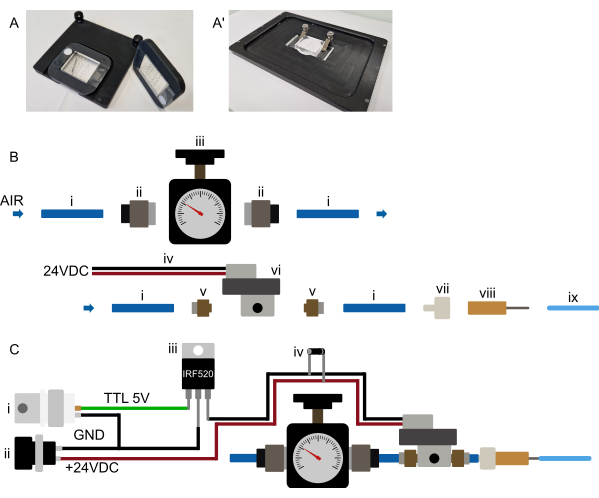
Figure 3: Hardware setup. (A-A') Suggested device mounting. (A) Possible mount for an upright microscope. Devices are attached to a rectangular, 3D-printed frame with a large central cutout. Two small strips of double-sided tape are initially placed on the cover glass, on either side of the PDMS portion of the device. The 3D-printed frame is then placed over the device, such that the PDMS portion sticks through the cutout and the frame is firmly pressed onto the double-sided tape, permanently attaching the device to the frame. The frame, therefore, holds the device in a secure manner and is itself held on the microscope stage using magnets embedded in the frame and stage insert. This ensures stable device mounting and prevents collision of the microscope objective with any mounting hardware. All tubing is routed to the front of the device. (A') Possible mounting for an inverted microscope. A set of clamps tightly holds the device. Ensuring the clamps press the device onto the stage insert to prevent any XY movement during operation. (B) Pressure system parts view. (i) 6 mm OD polyurethane tubing connected to a pressure source. (ii) G1/8-to-6 mm push-in adapter connecting the tubing (i) to the pressure regulator (iii). The tubing is then connected to a solenoid (vi) through an M5-to-6 mm push-in adapter (v), and finally, the tubing is connected to a luer-lock-to-barb connector (vii) and a blunt 23 G needle (viii) to the 1/16" OD tubing connected to the microfluidic device. (C) Schematic overview of the electronic connections and the assembled pressure system. (i) The TTL trigger (+3.3-5 V) generated by an Arduino, a DAq-card, or any other microcontroller, along with a suitable power supply (ii, 24 VDC) is connected to a MOSFET (iii) and the solenoid's electronic connections as shown in the schematic, with a flyback diode (iv) connected across the solenoids power connections. Once a TTL signal is sent from the microcontroller to the MOSFET's gate, +24 VDC is applied to the normally closed solenoid, and the on-chip hydraulic valve is inflated. Once the TTL signal is removed, the pressure is released, and the on-chip hydraulic valve deflated. Please click here to view a larger version of this figure.
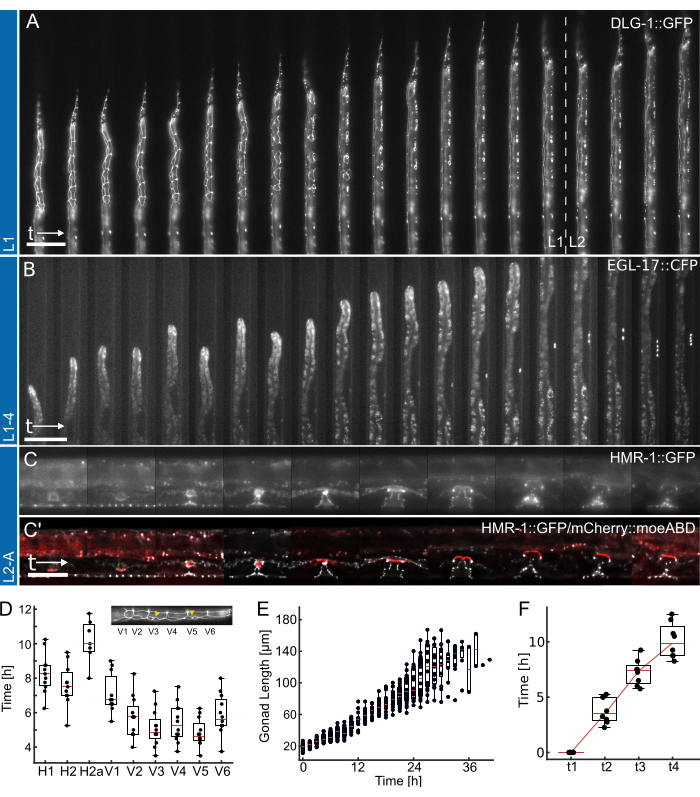
Figure 4: Representative results. (A) Animal showing an epithelial junction maker (ML2615, dlg-1(mc103[dlg-1::gfp])) growing from overnight starvation to early/mid-L2 larval stage, using the L1 device. Visible are the dividing seam cells, as well as the P-cells, which migrate and divide toward the late L1 larval stage. (B) Animal growing from the late L1 larval stage up to the early L4 stage, using the L1-4 device. Visible is a marker for the 1°-fated vulva precursor cell (VPC) (AH1187, arIs92[egl-17::cfp]), with a single cell visible early during the experiment, followed by two rounds of visible cell divisions in the L3 larval stage. (C) Animals growing from the late L2 larval stage up to early adulthood, using the L2-A device. Visible is an epithelial junction maker (AH5786, hmr-1(cp21[hmr-1::gfp + LoxP])), highlighting the developing vulval tissue forming distinct toroidal structures, and a combined image with a marker for the uterine anchor cell (AC) (AH5786, qyIs50[Pcdh-3>mCherry::moeABD, unc-119(+)]) which invades the vulval tissue toward the end of the L3 larval stage, connecting the tissues. For all experiments (A-C), the FOV is initially set up so that only part of the animals is visible. As the experiment progresses, the animal will fill more of the channel with the features shifting along the channel axis as a consequence of animal growth. (D) Time at which individual seam cell divisions start dividing during the L1 larval stage (n = 12). Insert highlights seam cell identities (H1-V6) along the anterior-posterior body axis. (E) Shows gonad growth with time for animals in Figure 4B (n = 19). (F) Shows the time required to reach 4 distinct developmental time points in vulval morphogenesis (t1-t4) indicating consistent transition for all animals imaged (n = 7). Scale bars are (A,B) 50 µm and (C) 25 µm. See Supplementary Movie 1, Supplementary Movie 2, and Supplementary Movie 3 for the full-time course. The box plots show the median values (red lines) with upper and lower quartiles and bars indicating the extremes of the distribution, excluding outliers. The figure is adapted from Berger et al.18. Please click here to view a larger version of this figure.
Supplementary Movie 1: Single C. elegans larva developing from overnight starvation up to the early L2 stage. Displayed are maximum intensity projected epifluorescence images (total height of the projection is 1.5µm), in otherwise unprocessed format. Visible are all seam cell division and fusion events occurring in the specified stage (top). Features are outlined using the DLG-1::GFP (epithelial junction) marker at 15-min intervals for a total of 25 h. The movie is reused from Berger et al.18. Please click here to download this Movie.
Supplementary Movie 2: Single larva developing from late L1 stage up to early L4 stage, expressing an EGL-17::CFP marker (1°-fated vulval precursor cell (VPC)). In early L2 (~400 min), EGL-17::CFP fluorescence manifests in P6.p, continually increasing in intensity. During L3, P6.p undergoes two consecutive rounds of division, forming the 1-cell, 2-cell, and 4-cell stages of vulva development. Images are shown at 30-min intervals for a total of 34 h. The movie is reused from Berger et al.18. Please click here to download this Movie.
Supplementary Movie 3: Top view: Z-projection of the developing vulva from the L3 stage up to the L4/adult transition. Bottom view: X-projection of the same vulva over time. Both views show the initial division of the VPCs, followed by invagination, formation, and enlargement of the toroids, followed by collapse during eversion. Epifluorescence images are displayed after cropping, deconvolution, registration, and projection. Features are outlined through the HMR-1::GFP marker (hypodermis and VPCs) and the mCherry::moeABD marker (outlining the AC). The movie is reused from Berger et al.18. Please click here to download this Movie.
Supplementary File 1: CAD file for the L1 device. Please click here to download this File.
Supplementary File 2: CAD file for the L1-4 device. Please click here to download this File.
Supplementary File 3: CAD file for the L2-A device. Please click here to download this File.
Supplementary File 4: CAD file for the L3-A device. Please click here to download this File.
Supplementary File 5: CAD file for the L4-A device. Please click here to download this File.
Supplementary File 6: CAD file for the food distribution structure. Please click here to download this File.
Supplementary File 7: CAD file for the valve layer. Please click here to download this File.
Discussion
This article has described the operation of a unique microfluidic long-term imaging platform, which is suitable for high-resolution imaging and tracking of various developmental processes in C. elegans during all four larval stages into early adulthood18. The ability to follow developmental processes over time in vivo, using this imaging method and others, has become indispensable in many fields of C. elegans research and has allowed previously inaccessible questions to be answered23,24,29.
In contrast to traditional imaging methods using agar pads and previously published microfluidic-based long-term imaging strategies, this approach preserves worm identity and orientation throughout the experiment, enabling the tracking of complex developmental processes over time. Regardless of developmental stage, animals are confined to one of 41 parallel trap channels, each connected to an on-chip food supply and covered by a large hydraulic valve. Immobilization of all trapped worms occurs through the inflation of the hydraulic valve, pressing the worms onto the cover glass only during image acquisition. High-throughput imaging is possible due to the narrowly spaced trap channel array, with multiple animals visible within a single field of view. The long-term imaging devices are compatible with most microscope setups, requiring minimal additional hardware (less than $1000) and no modifications to existing hardware. The operation of the devices is intentionally simple, so all users should quickly be able to learn it by following the above protocol and outlined results.
Briefly, animals can be imaged on-chip across up to 2.5 larval stages (Figure 1C). Animals are initially synchronized and either directly loaded on-chip (L1 device) or seeded onto the NGM plate until the desired developmental stage (L1-L4 and L2-A devices). Animals on-chip are fed using a highly concentrated bacteria suspension and immobilized using an on-chip hydraulic valve actuated by the imaging software (Figure 1B). Animals are maneuvered into the trap channels using a syringe, carefully orienting animals with the head toward the food supply and finally trapping animals at the end of the trap channel (Figure 1A and Figure 2). Care must be taken so that no air is trapped in the syringes, blunt needles, and tubing, as air bubbles will readily disturb the animal's position. Devices are compatible with most imaging modalities18,19,24, as the PDMS material is highly transparent, non-fluorescent, and sealed against a thin cover glass (Figure 4, Supplementary Movie 1, Supplementary Movie 2, and Supplementary Movie 3). However, care must be taken when selecting imaging conditions, as long exposure times and high excitation intensities will readily affect animal viability and result in photobleaching.
In addition to the presented version of this imaging method, several modifications to the protocol are possible and already available. The most straightforward modification is replacing the standard bacteria strains, e.g., with RNAi-expressing bacteria18 or bacteria with different nutritional values32. Bacterial strains can readily be exchanged by adjusting the Optiprep content to account for differences in density. Similarly, any number of compounds can be added to the bacterial food, e.g., auxin, nutrients, or small molecule drug compounds, and their effect is observed directly in vivo, without any need for any changes to the protocol. In particular, a media exchange device was developed for auxin-induced protein degradation or any application that needs timed or reversible exposure by adding a food inlet to the device layout without changing the geometry or operation. The additional inlet allows simple switching between two different conditions, e.g., auxin and no auxin, attached to the device in the same manner as shown here for a single food source, and the resulting changes are observed directly. Device variants were also developed where the hydraulic valve is not shared across all units on a device but separated for each unit, such that multiple conditions can be imaged within the same device and experiment individually, reducing the potentially harmful effects of long valve actuation and acquisition times. Finally, beyond the original device variants described in Berger et al.18, two new device variants were developed, L3-A and L4-A (introduced here), allowing imaging from the mid-L3 or mid-L4 stage until adulthood. The purpose of these modifications is to delay the start time of an experiment to a later larval stage, such that larger animals can be loaded in applications where the earlier developmental stages are not of interest.
Disclosures
The authors declare no competing or financial interests.
Acknowledgements
We wish to thank the members of the Hajnal laboratory for critical discussion and comments on the manuscript. We are also grateful to the C. elegans Genetics Center CGC, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Furthermore, we wish to acknowledge the members of the Galli lab (Hubrecht Institute), the Conradt lab (University College London), and the van den Heuvel lab (Utrecht University) for valuable input on the presented protocol. This work was supported by grants from the Swiss National Science Foundation no. 31003A-166580 to AH, the Swiss Cancer League no. 4377-02-2018 to AH, and funding by ETH Zürich to AdM.
Materials
| Name | Company | Catalog Number | Comments |
| 3/2 solenoid valve | Distrelec | 154-22-898 and 154-22-899 | Alternative to above. Solenoid valve connecting the pressure regulator to the on-chip valve. |
| Arduino | Distrelec | 301-01-956 | Optional microcontroller depending on the selected controller and interface. |
| Bacto-Tryptone | Thermo Fisher Scientific | 211705 | For L Broth production. |
| Bacto-Yeast | Thermo Fisher Scientific | 212750 | For L Broth production. |
| Blunt needle 23G | Gonano Dosiertechnik | GGA723050 | Blunt needle connecting the 1/16" tubing to a syringe. |
| Blunt needle 30G | Gonano Dosiertechnik | IG-TE730050 | Blunt needle connecting the 1/32" tubing to a syringe. |
| C. elegans Construct 1 | NA | NA | AH5786 LGI: hmr-1(cp21[hmr-1::gfp + LoxP]) Marston, D.J. et al. MRCK-1 drives apical constriction in C. elegans by linking developmental patterning to force generation. Curr. Biol. 26 (16), 2079-2089. 2016. |
| C. elegans Construct 2 | NA | NA | AH1187 LGII: arIs92[egl-17::cfp] Yoo, A.S., Bais, C., Greenwald, I. Crosstalk between the EGFR and LIN-12/Notch pathways in C. elegans vulval development. Science. 303 (5658), 663-666. 2004. |
| C. elegans Construct 3 | NA | NA | AH5786 LGV: qyIs50[Pcdh-3>mCherry::moeABD, unc-119(+)] Ziel, J.W., Hagedorn, E.J., Audhya, A., Sherwood, D.R. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol., 11 (2), 183-189. 2009. |
| C. elegans Construct 4 | NA | NA | ML2615 LGX: dlg-1(mc103[dlg-1::gfp]) Vuong-Brender, T.T.K., Suman, S.K., Labouesse, M. The apical ECM preserves embryonic integrity and distributes mechanical stress during morphogenesis. Development. 144 (23), 4336-4349. 2017. |
| Cable | Distrelec | 143-46-644 | Needed if selecting the solenoid from distrelec. Cable connecting the solenoid to the MOSFET. |
| Chlorotrimethyl silane | Sigma Aldrich | 386529-25ML | Silane for wafer passivation. |
| Cholesterol | Sigma Aldrich | C8667 | For S-Basal production. |
| Flyback Diode | Distrelec | 110-52-628 | Flyback diode protecting the microcontroller. |
| Hollow steel pin | Gonano Dosiertechnik | GGA7R23050 | 23G hollow steel pin connecting the 1/16" tubing to the device. |
| L Broth | NA | NA | Prepared in house following standard recipe. 10 g Bacto-tryptone, 5 g Bacto-yeast, 5 g NaCl, H2O to 1 litre, pH to 7.0 using 1 M NaOH |
| Long-term Imaging Device L1 | NA | NA | Long-term imaging device for L1 to early/mid L2 larvae. Made inhouse, available on request. Also available as media exchange variant. |
| Long-term Imaging Device L1-4 | NA | NA | Long-term imaging device for mid L1 to mid L4 larvae. Made inhouse, available on request. Also available as media exchange variant. |
| Long-term Imaging Device L2-A | NA | NA | Long-term imaging device for mid L2 to adulthood (eversion). Made inhouse, available on request. Also available as media exchange variant. |
| Long-term Imaging Device L3-A | NA | NA | Long-term imaging device for mid L3 to adulthood (eversion). Made inhouse, available on request. Also available as media exchange variant. |
| Long-term Imaging Device L4-A | NA | NA | Long-term imaging device for mid L4 to adulthood (eversion). Made inhouse, available on request. Also available as media exchange variant. |
| M9 Buffer | NA | NA | Prepared in house following standard recipe. |
| MOSFET | Distrelec | 303-41-391 | Metal-oxide-semiconductor field-effect transistor, used to switch the 24VDC required for the solenoid. |
| NGM plates | NA | NA | Prepared in house following standard recipe. |
| Nylon Luer-Fitting | Fisher Scientific | 11776048 | Adapter from OD 6mm tubing to 23G blunt needle. |
| Optiprep | Sigma Aldrich | D1556 | Density matching liquid. |
| PDMS | Ameba | Elastosil RT601A/B | PDMS elastomer kit. |
| Pluronic F-127 | Sigma Aldrich | P2443 | Non-ionic surfactant. |
| Potassium dihydrogen phosphate | Sigma Aldrich | 1.37039 | For S-Basal production. |
| Potassium hydrogen phosphate | Sigma Aldrich | 1.05099 | For S-Basal production. |
| Power Supply 24VDC | Distrelec | 301-63-304 | 24VDC power supply. Select a power supply suitable for the solenoid selected. |
| Precision pressure regulator | Distrelec | 154-23-578 | Alternativ to above. Pressure regulator. |
| Pressure Gauge G1/8 | Distrelec | 154-23-643 | Pressure gauge attaches to the pressure regulator. |
| PU Tubing OD 6mm | Distrelec | 154-23-866 | Tubing connecting the pressure source and regulator, as well as regulator and solenoid valve. |
| Push-in fitting G1/8 to 6mm | Distrelec | 301-69-330 | Alternativ to above. 2x Push-in fitting attaches to the pressure regulator and connects to the 6mm tubing. |
| Push-in fitting M5 to 6 mm | Distrelec | 301-69-459 | 2x Push-in fitting attaches to the solenoid and the 6mm tubing. |
| S-Basal Buffer | NA | NA | Prepared in house following standard recipe. 5.85 g NaCl, 1 g K2HPO4, 6 g KH2PO4, 1 ml cholesterol (5 mg/ml in ethanol), H2O to 1 litre. Sterilize by autoclaving. |
| Sodium Chloride | Sigma Aldrich | S9625 | For L Broth and S-Basal production. |
| Sodium hydroxite (5M) | Sigma Aldrich | 567530 | Sodium hydroxide used when bleaching C. elegans (prepare solution at 5M concentration) |
| Sodium hypochlorite (5%) | Sigma Aldrich | 1.05614 | Sodium hypochlorite used when bleaching C. elegans (prepare solution at 5% concentration) |
| Strainer 10 µm | pluriSelect | 43-10010-40 | Alternative for bacteria food preparation, especially if smaller devices are used. |
| Strainer 5 µm | pluriSelect | 43-10005-40 | Strainer used to filter L1 larvae after overnight starvation and bacteria after completed food preparation. |
| SU-8 | Gersteltec | GM1050 | SU-8 for height from 1-10 µm. |
| SU-8 | Gersteltec | GM1060 | SU-8 for height from 5-30 µm. |
| SU-9 | Gersteltec | GM1070 | SU-8 for height from 15-200 µm. |
| Syringe 1 mL | Fisher Scientific | 11338763 | 1mL syringe. If using a different type ensure that the syringe body and plunger are stiff, such that they won't be deformed by the syringe pump. |
| Syringe Pump | WPI | Al1000-220 | Syringe pump (https://www.wpiinc.com/var-2300-aladdin-single-syringe-pump.html). |
| Tube 15 mL | Fisher Scientific | 50-809-220 | Tube used for worm bleaching and bacteria preparation. |
| Tube 2 mL | Fisher Scientific | NC1186931 | Tube used for worm preparation and bacteria preparation. |
| Tube 50 mL | Fisher Scientific | 50-465-232 | Tube used for bacteria preparation and growth. |
| Tweezer SMD | Brütsch Rüegg Werkzeuge | 448980 | Tweezers used to plug the 1/32" tubing into the device. |
| Tygon tubing 1/16" | Fisher Scientific | 642002 | Flexible 1/16" OD tubing connecting most components to the device. |
| Tygon tubing 1/32" | Fisher Scientific | 641900 | Flexible 1/32" OD tubing connecting the bacteria supply to the device. |
| UV KUB-3 | Kloe | NA | Mask aligner and UV exposure system. |
| Wafer | Siegert Wafer | 4P0/>1/525±25/SSP/TTV<10 | Silicon Wafer. |
| Waste Container | NA | NA | Prepared in house using a 2mL tube or a cryo vial and hollow steel pins. |
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved