Method Article
Preservation of Porcine Biomodels for the Study of Comparative Human Anatomy
In This Article
Summary
This article presents a protocol for preserving porcine biomodels. The proposed method involves utilizing a modified Chilean solution with a reduced formalin concentration. The preservation process consists of administering the solution through both repletion and immersion of the specimen, followed by deformolization and tissue softening using glycerin.
Abstract
The teaching of human anatomy in medical education faces challenges, necessitating effective alternatives for students to practically explore anatomical complexity. Porcine biomodels, with their anatomical similarities to humans, offer a promising solution. This article presents a detailed protocol for preserving porcine biomodels, addressing the need for accessible and efficient methods in comparative anatomy studies. This protocol proposes the use of a modified Chilean solution for biomodel preservation, employing formalinization techniques through repletion and immersion. Subsequently, deformalization is achieved using the modified Chilean solution without formaldehyde, followed by a glycerin softening process. Given the scarcity of literature on preservation techniques and the absence of a standardized procedure or test to evaluate tissue conservation, we suggest assessing tissue quality based on measures of resistance and elasticity. Our findings indicate a qualitatively high level of tissue preservation in our specimens. Furthermore, these biomodels are currently utilized successfully for morphology studies and in teaching human comparative anatomy to medical students.
Introduction
The teaching of anatomy in medical schools often encounters obstacles, such as difficulties in accessing human bodies for dissection and the lack of suitable facilities. These limitations underscore the need for effective alternatives that enable students to explore anatomical complexity practically and realistically. In this context, porcine biomodels have emerged as a promising alternative due to their anatomical similarities to humans, offering an accessible and efficient means of learning and teaching anatomy1.
Anatomical preservation techniques aim to maintain the integrity of biological tissues while minimizing damage. These techniques have been employed for educational, academic, and research purposes in the study of both human and veterinary anatomy. Numerous substances have been tested to preserve bodies, organs, and tissues in their natural state for as long as possible2. However, preserving and conserving anatomical specimens remains challenging, particularly for those intended for morphological studies, where high tissue preservation is required3.
Even though traditionally used solutions are widely available, limitations in day-to-day practice are evident. Formalin, one of the most common substances used, has several documented disadvantages for tissue preservation. These include its irritating odor, high toxicity, associated risks of cancer and mutagenicity for handlers, and the organoleptic changes it induces in tissues, such as stiffness and discoloration. These changes can alter the perception of the tissues' physiological properties when studied after fixation, potentially hindering precise and accurate morphological studies4. Studies have shown that fixation with high concentrations of formalin results in a greater degree of tissue stiffness5. Alternatives, such as the Thiel embalming technique, have demonstrated promising results with better conservation of original coloration and pliability of tissues6. However, this technique is more costly compared to other solutions7. These challenges present an opportunity to design and test new, affordable preservation techniques that still enable high-quality anatomy teaching.
The aim of this protocol is to describe the methodology employed at the Anatomy Laboratory of Universidad Icesi in Cali, Colombia, for the preservation of porcine biomodels used as educational tools for the comparative study of human anatomy.
Protocol
The technique described was developed in full compliance with the guidelines of the Institutional Ethics Committee for the Care and Use of Animals in Experimentation (CIECUAE) of Universidad Icesi, in accordance with Law 84 of 1989 and Rector's Resolution No. 847 (July 9, 2012). This ensures both scientific integrity and the welfare of the animals used, minimizing their suffering. Male Landrace pigs, 3 months old and weighing 15-20 kg, were utilized for this study. The reagents and equipment required are listed in the Table of Materials.
1. Porcine biomodel
- Sacrifice of the animal
- Conduct a general veterinary evaluation and quarantine the selected pig specimen for 24 h. If the animal does not present any symptoms during this period, proceed with euthanasia.
- Anesthesia
- Administer anesthesia intramuscularly using Ketamine + Xylazine + Atropine at doses of 10 mg/kg, 0.5 mg/kg, and 0.04 mg/kg, respectively (following institutionally approved protocols).
NOTE: Ensure deep anesthesia. Assess the depth of anesthesia through physical examination. Deep anesthesia is indicated by a dilated and centered pupil, absence of the palpebral reflex, depression of the corneal reflex, and decreased respiratory rate, heart rate, and blood pressure.
- Administer anesthesia intramuscularly using Ketamine + Xylazine + Atropine at doses of 10 mg/kg, 0.5 mg/kg, and 0.04 mg/kg, respectively (following institutionally approved protocols).
- Euthanasia
- Administer 5 mL of Sodium Pentobarbital/Diphenylhydantoin (390/50 mg/mL) intravenously. After administering the euthanasia agent, auscultate the animal's physiological parameters for the Suis Scrofa species: HR, 60-90; FR, 8-18; and T° 37-39°C8.
- Monitor the heart rate until it gradually decreases and stops completely. Observe immediate changes such as sphincter relaxation, cyanotic membranes, and absence of response to body stimuli. Consider euthanasia complete after 5 min of cardiac auscultation without detecting a heartbeat.
- Storage and transportation
- Transport the porcine biomodel from the procedure room to the anatomy laboratory within half an hour of euthanasia to prevent decomposition and microbial proliferation, thereby optimizing the conservation of the biomodel.
2. Preservative solution based on Chilean solution
- Preparation of the modified Chilean solution
- Use a modified Chilean solution containing Ethanol, Glycerin, Formaldehyde, Benzalkonium Chloride, coffee aromatic essence, and distilled water. Ensure the solution has a pH of 7.0.
- Prepare the preservative solution in a 50 L tank. First, add 10.6 L of distilled water to the tank. Next, add 7.6 L of 96% ethanol, 5 L of glycerin, 0.5 L of 50% benzalkonium chloride, and 0.1 L of coffee aromatic essence.
NOTE: Refer to Table 1 for the required concentrations and volumes of each component needed to prepare 25 L of the modified Chilean solution.
3. Administration of the modified Chilean preservative solution
NOTE: Once the preservative solution is prepared, administer it through repletion and immersion.
- Administration by repletion of the modified Chilean preservative solution
NOTE: Use biosafety clothing for this procedure: a reusable half mask with A1P2 filters, biosafety goggles, a long-sleeved disposable surgical gown, a surgical cap, leggings, and nitrile gloves.- Surgical approach
- Make an incision along the linea alba (using a scalpel handle 4, blade 22) from the xiphoid process to the pubic region.
- Identify the subcutaneous cellular tissue and the muscle fibers of the rectus abdominis muscle.
NOTE: At the moment of the incision, identify the scant fatty tissue adhered to the skin, known as subcutaneous cellular tissue, and the muscle fibers of the rectus abdominis muscle located deeper9. - Carefully cut to access the peritoneum, ensuring its opening to the abdominal cavity without injuring the liver and intestines, which will be exposed immediately.
- Retract the intestinal loops to the right side of the abdominal cavity. Identify the parietal peritoneum overlying the left kidney and renal vessels. Use these vascular structures to locate the abdominal aorta.
- Find the emergence of the left renal pedicle. Remove the peritoneum covering the aorta 5 cm above the renal artery.
- Upon exposing the left renal pedicle, mobilize the intestinal loops and incise the peritoneum covering the hilum. Dissect anteroposteriorly to identify first the renal vein, which is larger and located anteriorly.
- Locate the renal artery posteriorly, following its trajectory to its origin in the abdominal aorta. Finally, identify the renal pelvis, the most posterior structure of the hilum9.
- Carefully dissect the posterior part of the aorta. Use 0/0 silk to ligate the aorta with a knot above the level of the renal artery and another knot 5 cm above.
- Clamp the aorta immediately with two Kelly clamps at both ends of the isolated aortic segment.
- Incise the aortic wall with the tip of Metzembaum scissors, making a cut of approximately 0.3 cm in the anterior wall of the aorta, taking care not to perforate it.
- Knot the distal end while removing the Kelly clamp to insert a 3.2 mm blunt L-shaped needle into the arterial lumen. Remove the proximal Kelly clamp and start the perfusion of the modified Chilean preserving solution.
- Aortic perfusion of the modified Chilean preserving solution
- Proceed with the perfusion of the modified Chilean preserving solution.
NOTE: Administer 0.25 L/kg with a pressure of 10 pounds using a perfusion pump. - End of perfusion
- Once the perfusion of the preserving solution is complete, remove the perfusion cannula and knot the insertion site with 0/0 silk to prevent reflux of the preserving solution. Close the abdomen by suturing the incision at the linea alba with 0/0 silk.
NOTE: To evaluate if adequate perfusion was achieved, check the acute indicators of saturation: extension of the upper and lower extremities, cervical and thoracic plethora, and runoff of the solution through the nostrils. The approximate perfusion time is 2 h.
- Once the perfusion of the preserving solution is complete, remove the perfusion cannula and knot the insertion site with 0/0 silk to prevent reflux of the preserving solution. Close the abdomen by suturing the incision at the linea alba with 0/0 silk.
- Surgical approach
- Administration by immersion
NOTE: Use biosafety clothing for this procedure: a reusable half mask with A1P2 filters, biosafety goggles, a long-sleeved disposable surgical gown, a surgical cap, leggings, and nitrile gloves.- Administration by immersion of the modified Chilean preserving solution
- Store the porcine specimen in a 300 L tank filled with the same preservative solution described in step 2.
NOTE: Ensure the biomodel is completely immersed using approximately 150 L of the solution. After immersion, store the biomodel at 20 °C for 6 months. Indicators of adequate preservation include the absence of decomposition signs (e.g., body edema, swelling, foul odor, lividity, and friability of the tissues).
- Store the porcine specimen in a 300 L tank filled with the same preservative solution described in step 2.
- Administration by immersion of the modified Chilean preserving solution
4. Deformolization solution based on Chilean solution
- Preparation of the deformolization solution
- Use a modified Chilean solution containing Ethanol, Glycerin, Benzalkonium Chloride, coffee aromatic essence, and distilled water. Ensure the solution has a pH of 7.0.
- Prepare the deformolization solution in a 50 L tank. First, add 11.1 L of distilled water, then 7.8 L of 96% ethanol, 5 L of glycerin, 1 L of 50% benzalkonium chloride, and 0.1 L of coffee aromatic essence.
NOTE: Refer to Table 2 for the required concentrations and volumes of each component needed to prepare 25 L of the formaldehyde-free modified Chilean solution.
5. Deformolization
NOTE: Use biosafety clothing for this procedure: a reusable half mask with A1P2 filters, biosafety goggles, a long-sleeved disposable surgical gown, a surgical cap, leggings, and nitrile gloves.
- Administration by immersion of the formaldehyde-free modified Chilean preserving solution
- At the end of the 6-month immersion preservation period, transfer the biomodel to a 300 L tank filled with formaldehyde-free modified Chilean preserving solution. Store the biomodel for 4 weeks.
NOTE: Ensure the biomodel is completely immersed using approximately 150 L of the solution.
- At the end of the 6-month immersion preservation period, transfer the biomodel to a 300 L tank filled with formaldehyde-free modified Chilean preserving solution. Store the biomodel for 4 weeks.
6. Glycerin softening
- Immediately after the deformolization process, immerse the biomodel in solutions with increasing concentrations of glycerin diluted in water: 50%, 70%, and 90% glycerin. Maintain the biomodel in each concentration for one week.
Results
The goal of this protocol is to present an effective and viable technique that allows for the preservation of porcine biomodels for comparative human anatomy teaching. There are currently no standardized methods or tests to evaluate model preservation. Therefore, the overall preservation of the model was assessed using indicators of solution repletion and signs of decomposition after the protocol was completed on the specimens. Furthermore, to objectively assess the biomodels' viability for teaching and research, a comparison of tissue characteristics of selected anatomic structures, such as resistance and elasticity, was planned before and after preservation. The selected anatomic structures were the vagus nerve, the aortic artery, and the cava vein. These structures were chosen because nerves, arteries, and veins are fundamental in gross anatomy teaching and learning. Consequently, 4 vagus nerves, 4 aortic arteries, and 3 cava veins were isolated from fresh, unpreserved specimens.
Accordingly, using the presented protocol, the bodies of 12 porcine specimens were preserved. The preserved porcine biomodels were evaluated for adequacy of preservation using indicators of repletion, such as the extension of the forelimbs, blood, and preservative solution outflowing through the nostrils (Figure 1), edema, and an increase in the thoracic and cervical perimeter (Figure 2 and Figure 3), as well as the appearance of a whitish mottled pattern in the liver (Figure 4) and paling of the intestinal loops (Figure 5). All of the biomodels exhibited successful indicators of repletion. After completing the protocol, none of the biomodels showed changes associated with decomposition, such as distension of the intestinal loops, foul odor, or tissue friability.
After inspection of the biomodels, it was evident that this technique successfully preserved the tissues for manipulation and dissection. Examples include muscles and their aponeuroses (Figure 6), the peritoneum (Figure 7), and the stomach and bowels (Figure 8). This was assessed subjectively, as all these organs maintained high flexibility during manipulation. The liver's consistency, although firm, permitted the identification of the intrahepatic ducts and their associated vascularization after dissection (Figure 9 and Figure 10).
However, results were not always as promising. Prior to the development of the protocol, fungal colonization of the biomodels was a frequent problem. This was possibly attributable to the use of a non-standardized technique for repletion and administration by immersion of the preservative solution. Figure 6 shows a biomodel that was affected by colonization.
Additionally, to objectively assess the preservation of the tissues, specific characteristics of interest were measured in both unpreserved and preserved structures. Four vagus nerves, four aortic arteries, and three cava veins were isolated from preserved biomodels. The characteristics measured were the tissue's resistance (quantified as the newtons required to tear the structure) and the tissue's flexibility (measured using Young's modulus)10,11. Results are presented as mean ± standard deviation (SD) in Table 3.
The surgical extraction of these structures and the evaluation of their elasticity and resistance were conducted to provide a quantitative approximation of the preservation degree of the entire biomodel and how well it retained its original mechanical properties. The selection of these specific anatomic structures was based on the fact that vascular and nervous structures are most frequently manipulated by teachers and students during dissection, thus posing a greater risk of rupture.
To evaluate the mechanical properties of animal biological tissues, a method was designed that involved suspending the sample between two clamps, with one end connected to a force transducer and the other end subjected to an increasing load through a constant flow of water. The force exerted was calculated considering the density of the water and gravity, while the elongation of the sample was measured with increasing load. These measurements facilitated the determination of Young's modulus, representing tissue stiffness, as well as the identification of the maximum resistance to the application of a specific force, reflecting the tissue's ability to withstand loads before rupture. This method provides an accurate assessment of the elastic and strength properties of biological tissues, which is fundamental to understanding their mechanical behavior.
The preserved vagus nerves demonstrated a significant increase in resistance and elasticity compared to the unpreserved specimens. Contrary to expectations, the preserved aortic artery exhibited slightly lower resistance but a moderate increase in elasticity compared to unpreserved specimens. Preservation of the cava vein resulted in a notable increase in both resistance and elasticity. These findings indicate that the preservation process has a discernible impact on the mechanical properties of the evaluated tissues, providing valuable insights for the application of these preserved tissues in anatomical studies.
The morphology research team that developed this protocol possesses extensive experience in the preservation of porcine biomodels. A qualitative improvement in preservation results has been observed in terms of the organoleptic properties of the tissues due to modifications and optimization of conventional techniques previously used, such as preservation with 10% formalin. The preserved biomodels are currently used successfully at Universidad Icesi for morphology studies and human comparative anatomy teaching for medical students. The model with the longest lifespan in the laboratory has reached 7 years at room temperature without requiring new immersions or additional processes. Teaching experience has shown that, despite high levels of manipulation by students, the biomodels maintain the integrity of anatomical structures for up to 4 years after preservation.

Figure 1: Blood and preservative solution outflow through the nostrils. (A) Outflow before perfusion. (B) Outflow during perfusion. Please click here to view a larger version of this figure.

Figure 2: Thoracic perimeter before and after preservative solution perfusion. (A) Thoracic perimeter before perfusion. (B) Thoracic perimeter after perfusion. An increase of 4 cm in the thoracic perimeter is observed. Please click here to view a larger version of this figure.
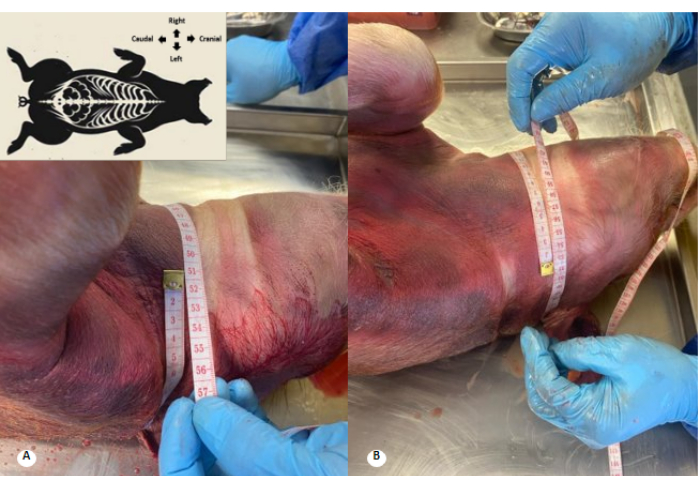
Figure 3: Cervical perimeter before and after preservative solution perfusion. (A) Cervical perimeter before perfusion. (B) Cervical perimeter after perfusion. An increase of 1 cm in the cervical perimeter and effacement of the cervical folds are observed. Please click here to view a larger version of this figure.
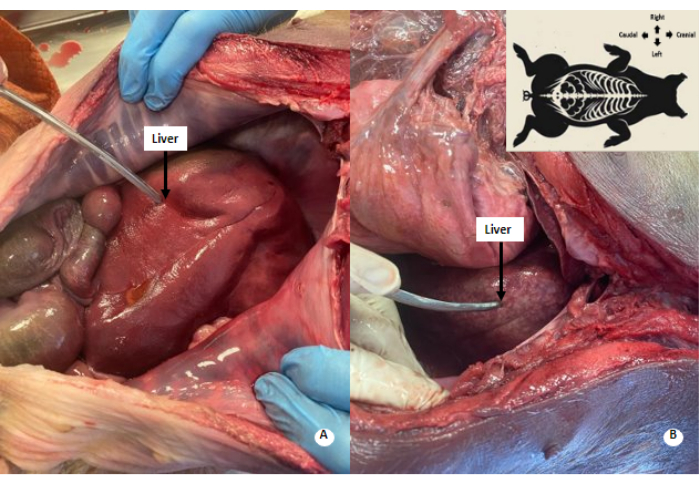
Figure 4: Whitish mottled pattern in the liver. (A) Liver before perfusion. (B) Liver after perfusion. A whitish-mottled pattern is observed in the perfused liver. Please click here to view a larger version of this figure.

Figure 5: Paleness of the intestinal loops. (A) Small intestine before perfusion. (B) Small intestine after perfusion. Paleness of the intestinal loops is observed. Please click here to view a larger version of this figure.

Figure 6: Biomodel colonized by fungus. Ventral traction of the inferior cava vein shows fungal colonization. Please click here to view a larger version of this figure.

Figure 7: Transversus abdominis muscle and its aponeurosis. (1) Transversus abdominis muscle. (2) Aponeurosis of the transversus abdominis muscle. Please click here to view a larger version of this figure.

Figure 8: Parietal peritoneum of the preserved porcine biomodel. (1) Parietal peritoneum. (2) Left lobe of the sectioned liver. (3) Stomach. Please click here to view a larger version of this figure.
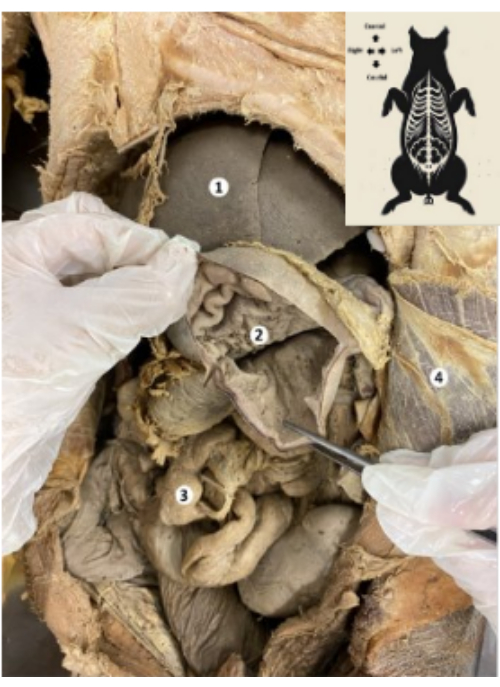
Figure 9: Stomach of the preserved porcine biomodel. (1) Liver. (2) Gastric mucosa. (3) Bowels. (4) Transverse abdominis muscle and parietal peritoneum. Please click here to view a larger version of this figure.
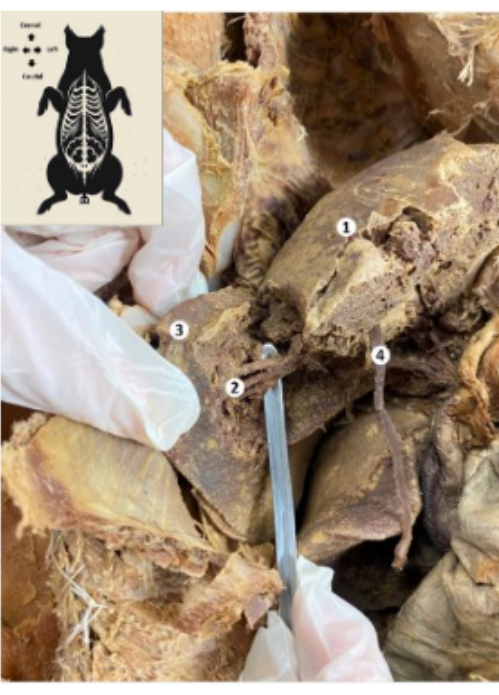
Figure 10: Liver of the preserved porcine biomodel. (1) Diaphragmatic face of the liver. (2) Lower posterior segment of the liver (hepatic artery, bile duct, lower posterior branch of the portal vein). (3) Right lobe of the liver. (4) Liver round ligament. Please click here to view a larger version of this figure.
| Reactive | Final concentration | Quantity | Final volume |
| Ethanol 96% | 30% | 7.8 L | 25 L |
| Glycerin | 20% | 5L | |
| Formaldehyde 40% | 2% | 0.5 L | |
| Benzalkonium Chloride 50% | 2% | 1 L | |
| Aromatic essence (Coffee) | 0.40% | 0.1 L | |
| Distilled water | NA | 10.6 L |
Table 1: Preservative solution based on a modified Chilean solution.
| Reactive | Final concentration | Quantity | Final volume |
| Ethanol 96% | 30% | 7.8 L | 25 L |
| Glycerin | 20% | 5L | |
| Benzalkonium Chloride 50% | 2% | 1 L | |
| Aromatic essence (Coffee) | 0.40% | 0.1 L | |
| Distilled water | NA | 11.1 L |
Table 2: Formaldehyde-free preservative solution based on a modified Chilean solution.
| Tissue | Unpreserved | Preserved | ||
| Newton ± SD* | Young´s Modulus ± SD* | Newton ± SD* | Young’s Modulus ± SD* | |
| Vagus nerve | 4.92 ± 0.98 | 879.5 ± 427.02 | 8.59 ± 0.83 | 1392.21 ± 729.6 |
| Aortic artery | 9.33 ± 1.5 | 325.89 ± 176.15 | 8.48 ± 1.26 | 417.16 ± 379.95 |
| Cava vein | 5.73 ± 2.3 | 145.5 ± 40.89 | 8.92 ± 3.97 | 264.69 ± 188.72 |
Table 3: Tissue characteristics of unpreserved and preserved specimens of anatomic structures. *SD: Standard deviation.
Discussion
Traditionally, anatomical studies have relied on human cadavers; however, challenges in acquiring these specimens have led to the exploration of alternative methods. Porcine biomodels have proven to be valuable tools for studying human anatomy, offering anatomical similarities that facilitate learning and its extrapolation to medical applications in humans12,13,14,15. Although cadaveric preservation techniques have traditionally been described qualitatively, measuring the strength and elasticity of preserved tissues using Young's modulus could provide quantitative evidence of the degree of preservation. The search for innovative approaches in biological tissue preservation responds to the need to facilitate the exploration of anatomical complexity in realistic models. Therefore, the protocol for preserving porcine biomodels developed and used in the Anatomy Laboratory of Universidad Icesi is presented as a viable alternative for tissue preservation and subsequent anatomical study.
Based on experience, approaching the specimen circulation through the abdominal aorta in porcine biomodels is a more effective alternative compared to femoral arterial cannulation, which is commonly performed in human cadavers16. The fragile nature of porcine femoral arterial walls has prompted a preference for the abdominal approach, as these walls are susceptible to rupture from the pressure exerted by the perfusion pump. Securing a viable entry point is crucial, as the repletion step in the protocol is critical for the adequate preservation of the entire specimen. Sub-optimal repletion poses a high risk of decomposition. Therefore, successful repletion signs must be evaluated before continuing with the protocol. These signs include the extension of the upper and lower extremities, cervical and thoracic plethora, and runoff of the solution through the nostrils due to the perfusion of the preserving solution. A common challenge that may arise during the execution of this protocol is tearing of the aortic artery during the infusion of the preserving solution. In such instances, it is advisable to ligate the artery and proceed with the dissection of a superior segment to facilitate secondary cannulation and resume the repletion step. Additionally, if signs of decomposition become apparent at any point during the protocol, the entire specimen must be discarded.
The preservative solution used in this protocol includes variations compared to the conventional Chilean solution, such as the absence of sodium chloride, an increase in ethanol concentration from 24% to 30%, an increase in glycerin concentration from 16% to 20%, a decrease in formaldehyde concentration from 3.7% to 2%, and the replacement of eucalyptus essence with coffee essence17.
The deformolization process is another crucial step in the protocol, as proper deformolization reduces personnel exposure to formaldehyde, including teachers, laboratorians, and students. Additionally, the softening process with glycerin is essential for achieving a texture similar to that of fresh tissues. Conventional preservation with 10% formaldehyde results in stiff tissues that lose elasticity and become more prone to tearing during handling. However, using the modified Chilean solution with a reduced formaldehyde concentration of 2%, followed by deformolization and softening with glycerin, mitigates this issue.
Following this protocol, a high degree of flexibility was achieved upon manipulation, while solid organs, such as the liver, exhibited a consistency that, though firm, allowed for dissection and identification of their structures. Nevertheless, further quantitative studies are necessary to compare the preservation quality of tissues across different preservation techniques.
Several potential limitations should be considered when conducting this protocol. Firstly, meticulous attention must be given to the size of the specimen, with a recommended range of 15-20 kg. Larger animals may present challenges in executing the preservation protocol and managing the disposition of the biomodel. Secondly, the designated laboratory should be equipped with an appropriate vapor extraction system and a waste trap in the sewage system. This precaution aims to mitigate the exposure of technical personnel to organic vapors and minimize environmental contamination resulting from residues produced during the preservation process. Thirdly, the potential presence of anatomical variants in the biomodels must be acknowledged. Additionally, while porcine specimens share anatomical similarities with humans, they also exhibit differences. Therefore, careful consideration of such variations is warranted in comparative anatomical studies.
The evaluation of the organoleptic properties of tissues has traditionally been approached qualitatively, focusing on characteristics such as odor, color, stiffness, brittleness, and ease of handling18. However, there is a notable paucity of studies aiming to determine these parameters objectively. One example is the work of HP Theeuwes, which compared the mobility of upper limb joints in cadavers preserved with 11% formalin, fresh tissue, and a modified method that immobilized the joints to measure the force required for flexion5. In this context, the research team is developing methods to objectively evaluate the mechanical properties of tissues, seeking to overcome the limitations of existing qualitative evaluations.
In terms of cadaver preservation, although formalin is the most commonly used agent, several alternative substances have also been used successfully. These substances include glutaraldehyde, monoethylene glycol, diethylene glycol, ethanol-glycerin solutions, and saturated salts. The diversity of preservation methods reflects the ongoing quest to optimize tissue preservation for medical research, educational, or exhibition purposes3,19. These advances highlight the importance of research in developing more effective preservation techniques to maintain the structural and functional properties of human tissues optimally for extended periods.
For future applications, the observed high level of tissue preservation with this protocol holds significant promise for advancing anatomical research and education. The potential of this method extends to advanced anatomical studies, providing realistic models for surgical training and contributing to the development of immersive medical simulations. Additionally, the protocol's adaptability opens avenues for exploration in diverse anatomical specimens, thereby expanding its utility in various medical and scientific contexts.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
Gratitude is extended to the Department of Basic Medical Sciences and the Research Office of Universidad Icesi for their support in this research.
Materials
| Name | Company | Catalog Number | Comments |
| Benzalkonium Chloride | Protécnica Ingeniería | PROQUAT BC 50/80 | CAS Number. 68424-85-1 |
| Ethanol | Not applicable | Not applicable | Ethanol 96% |
| Formaldehyde | Albor químicos | Not applicable | Formaldehyde |
| Glycerin | Not applicable | Not applicable | Glycerin |
| Injection pump | Disánchez | Special Injector YA-02 | Injection pump |
| Kelly forceps | Not applicable | Not applicable | Kelly forceps |
| Metzembaum scissors | Not applicable | Not applicable | Metzembaum scissors |
| Needle | Disánchez | L canule | L canule |
| Scalpel | Not applicable | Not applicable | Scalpel handle 4/ Scalpel blade 22 |
| Suture Silk | Not applicable | Not applicable | Suture Silk 0/0 |
References
- Gonzalez, L. M., Moeser, A. J., Blikslager, A. T. Porcine models of digestive disease: the future of large animal translational research. Transl Res. 166 (1), 12-27 (2015).
- Guerra, J. A. B. Historia de la preservación de cadáveres humanos. Morfolia. 1, 3(2009).
- Balta, J. Y., Cronin, M., Cryan, J. F., O'Mahony, S. M. Human preservation techniques in anatomy: A 21st century medical education perspective. Clin Anat. 28 (6), 725-734 (2015).
- Bernardini, L., Barbosa, E., Charão, M. F., Brucker, N. Formaldehyde toxicity reports from in vitro and in vivo studies: A review and updated data. Drug Chem Toxicol. 45 (3), 972-984 (2022).
- Theeuwes, H. P., van Riel, M., Lange, J. F., Kleinrensink, G. J. A new model for training on human specimens in surgical-anatomical skills labs. Anat Physiol Biochem Int J. 3 (1), 0013-0017 (2017).
- Rakuša, M., Šaherl, L. K. Thiel embalming method used for anatomy dissection as an educational tool in teaching human anatomy, in research, and in training in comparison of different methods for long term preservation. Folia Morphol. 82 (3), 449-456 (2023).
- Hammer, N., et al. Comparison of modified Thiel embalming and ethanol-glycerin fixation in an anatomy environment: Potentials and limitations of two complementary techniques. Anat Sci Educ. 8 (1), 74-85 (2015).
- Jackson, P. G. G., Cockcroft, P. D., Elmhurst, S. Clinical examination of farm animals: Wiley Online Library. , (2002).
- Delaney, C. P. Netter's surgical anatomy and approaches. E-Book: Netter's Surgical Anatomy and Approaches E-Book. , Elsevier Health Sciences. (2020).
- Agache, P. G., Monneur, C., Leveque, J. L., De Rigal, J. Mechanical properties and Young's modulus of human skin in vivo. Arch Dermatol Res. 269, 221-232 (1980).
- McKee, C. T., Last, J. A., Russell, P., Murphy, C. J. Indentation versus tensile measurements of Young's modulus for soft biological tissues. Tissue Eng Part B Rev. 17 (3), 155-164 (2011).
- Echarte, O. Z. Evaluación del nuevo dispositivo Surgicric para cricotiroidotomía de emergencia en un modelo porcino. Rev Electr AnestesiaR. 8 (12), 2(2016).
- Vargas, D., et al. Caracterización de las arterias coronarias en corazón de porcino como modelo anatómico didáctico en estudiantes del área de la salud. Morfolia. 12 (1), 56-74 (2020).
- Fernández-Trujillo, L., et al. El biomodelo porcino en la investigación médica traslacional: del biomodelo al humano en trasplante pulmonar. Biomédica. 39 (2), 300-313 (2019).
- Villate, M. A. M., Méndez, J. D. B., Echeverry, J. E. P. Anatomía quirúrgica del ojo: Revisión anatómica del ojo humano y comparación con el ojo porcino. Morfolia. 8 (3), 21-44 (2016).
- Kocbek, L., Rakuša, M. Thiel's embalming method: Review of the literature and our institute's experience. Acta Med Biotechnol. 10 (2), 34-42 (2017).
- Guerrero Guzmán, C. C., et al. Restoration and conservation of anatomic pieces. Anat Cell Biol. 52 (3), 255-261 (2019).
- Balta, J. Y., Lamb, C., Soames, R. W. A pilot study comparing the use of Thiel- and formalin-embalmed cadavers in the teaching of human anatomy. Anat Sci Educ. 8 (1), 86-91 (2015).
- Hayashi, S., et al. History and future of human cadaver preservation for surgical training: From formalin to saturated salt solution method. Anat Sci Int. 91, 1-7 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved