Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Au-Interaction of Slp1 Polymers and Monolayer from Lysinibacillus sphaericus JG-B53 - QCM-D, ICP-MS and AFM as Tools for Biomolecule-metal Studies
W tym Artykule
Podsumowanie
To obtain basic information on the sorption and recycling of gold from aqueous systems the interaction of Au(III) and Au(0) nanoparticles on S-layer proteins were investigated. The sorption of protein polymers was investigated by ICP-MS and that of proteinaceous monolayers by QCM-D. Subsequent AFM enables the imaging of the nanostructures.
Streszczenie
In this publication the gold sorption behavior of surface layer (S-layer) proteins (Slp1) of Lysinibacillus sphaericus JG-B53 is described. These biomolecules arrange in paracrystalline two-dimensional arrays on surfaces, bind metals, and are thus interesting for several biotechnical applications, such as biosorptive materials for the removal or recovery of different elements from the environment and industrial processes. The deposition of Au(0) nanoparticles on S-layers, either by S-layer directed synthesis 1 or adsorption of nanoparticles, opens new possibilities for diverse sensory applications. Although numerous studies have described the biosorptive properties of S-layers 2-5, a deeper understanding of protein-protein and protein-metal interaction still remains challenging. In the following study, inductively coupled mass spectrometry (ICP-MS) was used for the detection of metal sorption by suspended S-layers. This was correlated to measurements of quartz crystal microbalance with dissipation monitoring (QCM-D), which allows the online detection of proteinaceous monolayer formation and metal deposition, and thus, a more detailed understanding on metal binding.
The ICP-MS results indicated that the binding of Au(III) to the suspended S-layer polymers is pH dependent. The maximum binding of Au(III) was obtained at pH 4.0. The QCM-D investigations enabled the detection of Au(III) sorption as well as the deposition of Au(0)-NPs in real-time during the in situ experiments. Further, this method allowed studying the influence of metal binding on the protein lattice stability of Slp1. Structural properties and protein layer stability could be visualized directly after QCM-D experiment using atomic force microscopy (AFM). In conclusion, the combination of these different methods provides a deeper understanding of metal binding by bacterial S-layer proteins in suspension or as monolayers on either bacterial cells or recrystallized surfaces.
Wprowadzenie
Due to the increasing use of gold for several applications like electronics, catalysts, biosensors, or medical instruments, the demand of this precious metal has grown over the last few years' time 6-9. Gold as well as many other precious and heavy metals are released into the environment via industrial effluents in dilute concentrations, through mining activities, and waste disposal 7,8,10,although most environmental contamination by heavy or precious metals is an on-going process mainly caused by technological activities. This leads to a significant interference of natural ecosystems and could potentially threaten human health 9. Knowing these negative outcomes promotes the search for new techniques to remove metals from contaminated ecosystems and improvements in recycling metals from industrial wastewater. Well-established physico-chemical methods like precipitation or ion exchange are not so effective, especially in highly diluted solutions 7,8,11. Biosorption, either with living or dead biomass, is an attractive alternative for wastewater treatment 10,12. The use of such biological materials can reduce the consumption of toxic chemicals. Many microorganisms have been described to accumulate or immobilize metals. For instance, cells of Lysinibacillus sphaericus (L. sphaericus) JG-A12 have shown high binding capacities for precious metals, e.g., Pd(II), Pt(II), Au (III), and other toxic metals like Pb(II) or U(VI) 4,13, cells of Bacillus megaterium for Cr(VI) 14, cells of Saccharomyces cerevisiae for Pt(II) and Pd(II) 15, and Chlorella vulgar for Au(III) and U(VI) 16,17. The binding of previous metals like Au(III), Pd(II), and Pt(II) has also been reported for Desulfovibrio desulfuricans18 and for L. sphaericus JG-B53 19,20. Nevertheless, not all microbes bind high amounts of metals and their application as sorptive material is limited 12,21. Furthermore, metal binding capacity depends on different parameters, e.g., cell composition, the used bio-component, or environmental and experimental conditions (pH, ionic strength, temperature etc.). The study of isolated cell wall fragments 22,23, like membrane lipids, peptidoglycan, proteins, or other components, helps to understand the metal binding processes of complex constructed whole cells 8,21.
The cell components focused on in this study are S-layer proteins. S-layer proteins are parts of the outer cell envelope of many bacteria and archaea, and they constitute about 15 - 20% of the total protein mass of these organisms. As the first interface to the environment, these cell compounds strongly influence the bacterial sorption properties 3. S-layer proteins with molecular weights ranging from forty to hundreds of kDa are produced within the cell, but are assembled outside where they are able to form layers on the lipid membranes or polymeric cell wall components. Once isolated, nearly all S-layer proteins have the intrinsic property to spontaneously self-assemble in suspension, at interfaces, or on surfaces forming planar or tube-like structures 3. The thickness of the protein monolayer depends on the bacteria and is within a range of 5 - 25 nm 24. In general, the formed S-layer protein structures can have an oblique (p1 or p2), square (p4), or hexagonal (p3 or p6) symmetry with lattice constants of 2.5 to 35 nm 3,24. The lattice formation seems to be in many cases dependent on divalent cations and mainly on Ca2+ 25,26, Raff, J. et al. S-layer based nanocomposites for industrial applications in Protein-based Engineered Nanostructures. (eds Tijana Z. Grove & Aitziber L. Cortajarena) (Springer, 2016 (submitted)). Nevertheless, the full reaction cascade of monomer folding, monomer-monomer interaction, the formation of a lattice, and the role of different metals, especially of divalent cations such as Ca2+ and Mg2+, are still not fully understood.
The gram-positive strain L. sphaericus JG-B53 (renamed from Bacillus sphaericus after new phylogenetic classification) 27 was isolated from the uranium mining waste pile "Haberland" (Johanngeorgenstadt, Saxony, Germany) 4,28,29. Its functional S-layer protein (Slp1) possesses a square lattice, a molecular weight of 116 kDa 30, and a thickness of ≈ 10 nm on living bacteria cells 31. In previous studies, the in vitro formation of a closed and stable protein layer with a thickness of approximately 10 nm was achieved in less than 10 min 19. The related strain L. sphaericus JG-A12, also an isolate from the "Haberland" pile, possesses high metal binding capacities and its isolated S-layer protein has shown a high chemical and mechanical stability and good sorption rates for precious metals like Au(III), Pt(II), and Pd(II) 4,32,33. This binding of precious metals is more or less specific for some metals and depends on the availability of functional groups on the outer and inner protein surface of the polymer and in its pores, ionic strength, and the pH value. Relevant functional groups for metal interaction by the proteins are COOH-, NH2-, OH-, PO4-, SO4-, and SO-. In principle, metal binding capacities open a wide spectrum of applications,Raff, J. et al. S-layer based nanocomposites for industrial applications in Protein-based Engineered Nanostructures. (eds Tijana Z. Grove & Aitziber L. Cortajarena) (Springer, 2016 (submitted)).e.g., as biosorptive components for removal or recovery of dissolved toxic or valuable metals, templates for synthesis or defined deposition of regularly structured metallic nanoparticles (NPs) for catalysis, and other bio-engineered materials like bio-sensory layers 3,5,18,33. Regularly arranged NP arrays like Au(0)-NPs could be used for major applications ranging from molecular electronics and biosensors, ultrahigh density storage devices, and catalysts for CO-oxidation 34-37. The development of such applications and smart design of these materials necessitates a deeper understanding of the underlying metal binding mechanisms.
A prerequisite for the development of such bio-based materials is the reliable implementation of an interface layer between the biomolecule and the technical surface 38,39. For example, polyelectrolytes assembled with the layer-by-layer (LbL) technique 40,41 have been used as an interface layer for recrystallization of S-layer proteins 39. Such an interface offers a relatively easy way to perform the protein coating in a reproducible and quantitative way. By performing different experiments with and without modification with adhesive promoters, it is possible to make statements regarding coating kinetics, layer stability, and interaction of metals with biomolecules 19,42,Raff, J. et al. S-layer based nanocomposites for industrial applications in Protein-based Engineered Nanostructures. (eds Tijana Z. Grove & Aitziber L. Cortajarena) (Springer, 2016 (submitted)). However, the complex mechanism of the protein adsorption and protein-surface interaction is not completely understood. Especially information on conformation, pattern orientation, and coating densities is still missing.
Quartz crystal microbalance with dissipation monitoring (QCM-D) technique has attracted attention in the recent years as a tool for studying protein adsorption, coating kinetics, and interaction processes on the nanometer scale 19,43-45. This technique allows for the detailed detection of mass adsorption in real-time, and can be used as an indicator for the protein self-assembling process and coupling of functional molecules on protein lattices 19,20,42,46-48. In addition, QCM-D measurements open the possibility to study metal interaction processes with the proteinaceous layer under natural biological conditions. In a recent study, the interaction of the S-layer protein with selected metals like Eu(III), Au(III), Pd(II),and Pt(II) has been studied with QCM-D 19,20. The adsorbed protein layer can serve as a simplified model of a cell wall of gram-positive bacteria. The study of this single component can contribute to a deeper understanding of metal interaction. However, solely QCM-D experiments do not allow statements regarding surface structures and influences of metals to protein. Other techniques are necessary to obtain such information. One possibility for imaging bio-nanostructures and obtaining information on structural properties is the atomic force microscopy (AFM).
The objective of the presented study was to investigate the sorption of gold (Au(III) and Au(0)-NPs) to S-layer proteins, in particular Slp1 of L. sphaericus JG-B53. Experiments were done with suspended proteins on batch scale in a pH range of 2.0 - 5.0 using ICP-MS and with immobilized S-layers using QCM-D. Additionally, the influence of metal salt solution on the lattice stability was investigated with subsequent AFM studies. The combination of these techniques contributes to a better understanding of in vitro metal interaction processes as a tool for learning more about binding events on whole bacterial cells regarding specific metal affinities. This knowledge is not only crucial for the development of applicable filter materials for the recovery of metals for environmental protection and the conservation of resources 49, but also for the development of arrays of highly ordered metallic NPs for various technical applications.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Microorganism and Cultivation Conditions
Note: All experiments were done under sterile conditions. L. sphaericus JG-B53 was obtained from a cryo-preserved culture 29,30.
- Transfer cryo-preserved culture (1.5 ml) under the clean bench to 300 ml sterile nutrient broth (NB) media (3 g/L meat extract, 5 g/L peptone, 10 g/L NaCl). Afterwards stir the solution for at least 6 hr at 30 °C to obtain the pre-culture for cultivation.
- Cultivate the bacteria under aerobic conditions in NB media at pH = 7.0, 30 °C in a 70 L scaled steam-in-place bioreactor. Therefore, fill the reactor with ≈ 57 L deionized water. Add and dissolve solid NB media directly in bioreactor (concentrations see above).
- Additionally add antifoam agent (30 µl/L NB-media) to the media to suppress foam formation during cultivation, then autoclave (122 °C, temperature holding time 30 min) the media inside the reactor facility.
- Cool down the media and perform complete oxygen saturation. Adjust the pH to 7.0 (using 1 M H2SO4 and 2 M NaOH) and start the automatic inoculation of the 300 ml pre-culture. Start the data recording of cultivation parameters at the inoculation point. Log online parameters e.g., dissolved oxygen level (dO2), acid and base addition, and pH-values within the cultivation.
- Monitor the bacterial growth online by the non-invasive turbidity measurements.
- Perform additional sampling after every hour of cultivation and determine further parameter such as bio dry weight (BDW) and offline optical density (OD). Therefore, collect 20 ml cultivation broth at each sampling point under sterile conditions.
- Determine offline OD by photometric measurements of the adsorption at 600 nm. Use sterile filtrated NB-medium as a blank value. After reaching adsorption >0.4 dilute the cell suspension following the linearity of the Lambert-Beer law.
- For determination of BDW centrifuge 1 to 5 ml of bacterial suspension (depending on cell density) at 5,000 x g for 5 min at RT. Dry the obtained cell pellet at 105 °C in a heating oven up to mass stability and measure the pellet mass.
- Take microscopic images with optical phase contrast research microscope in 400 and 1,000 fold magnification (phase contrast condenser 2 and 3 respectively) for checking of the bacterial growth and as a cross-contamination control.
- After reaching the exponential growth phase detected by online dO2 and online turbidity, harvest the biomass by flow-through centrifugation at 15,000 x g, 4 °C and wash the biomass twice with standard buffer (50 mM TRIS, 10 mM MgCl2, 3 mM NaN3, pH = 7.5).
Note: The obtained biomass pellet can be stored at -18 °C until further usage for isolation.
2. S-layer Protein Isolation and Purification
Note: Purify Slp1 polymers according to an adapted method as described previously2,19,30,32,50,51.
- Homogenize the washed and defrosted crude biomass obtained from cultivation in standard buffer (1:1 (w/v)) to remove flagella by using a disperser (level 3, 10 min) under ice bath cooling at 4 °C.
- Centrifuge the suspension (8,000 x g, 4 °C for 20 min) and wash the obtained pellet twice with standard buffer (1:1 (w/v)). After washing and centrifugation (8,000 x g, 4 °C for 20 min), resuspend the pellet in standard buffer (1:1 (w/v)), add DNase II and RNase (0.4 units/g biomass) to the suspension and disintegrate the cells at 1,000 bar with a high-pressure homogenizer. Afterwards centrifuge the suspension at 27,500 x g, 4 °C for 1 hr.
Note: Control cell suspension with the research microscope. Rupture is completed when less than 2 - 3 intact cells are visible in the view field of the microscope in 400 fold magnification. - Wash the pellet twice with standard buffer (1:1 (w/v)) and perform the centrifugation again. Afterwards resuspend the pellet in standard buffer (2:1 (w/v)) mixed with 1% Triton X-100 and incubate it for 20 min under successive shaking (100 rpm) to solubilize lipid deposits.
- Centrifuge the solution (27,500 x g, 4 °C for 1 hr) and wash the obtained pellet three times with standard buffer (1:1 (w/v)).
- Incubate the pellet obtained after additional centrifugation (27,500 x g, 4 °C for 1 hr) for 6 hr in standard buffer (1:1 (w/v)) mixed with 0.2 g/L lysozyme, to hydrolyze linkages in peptidoglycan 50. Additionally add DNase II and RNase (each 0.4 units/g biomass) to the suspension.
- After centrifugation (45,500 x g, 4 °C, 1 hr), resuspend the upper white protein phase with a low volume of the centrifugation supernatant (<30 ml) containing protein subunits.
- Solubilize the white suspension by mixing 1:1 with 6 M guanidine hydrochloride (6 M GuHCl, 50 mM TRIS, pH = 7.2). The solution becomes bright.
- Perform sterile filtration (0.2 µm) of the GuHCl treated solution followed by an additional high-speed centrifugation (45,500 x g, 4 °C for 1 hr).
- Transfer the supernatant to dialysis membrane tubes (MWCO 50,000 Dalton) and dialyzed it against recrystallization buffer (1.5 mM TRIS, 10 mM CaCl2, pH = 8.0) for 48 hr.
- Transfer the white recrystallized protein polymer solution into tubes and centrifuge at 45,500 x g, 4 °C for 1 hr. Resuspend the pellet in a low volume of ultrapure water (<30 ml).
- Afterwards, transfer the suspension into dialysis membrane tubes and perform a dialysis against ultrapure water for 24 hr to remove buffer content.
Note: Several changes of buffer or ultrapure water during dialysis are indispensable. - Lyophilize the purified Slp1 in a freeze dryer.
3. Characterization and Quantification of Slp1 for Experiments
Note: Slp1 concentration for sorption and coating experiments were quantified by UV-VIS spectrophotometry.
- Pipette 2 µl of dissolved Slp1 sample directly onto the lower measurement pedestal of the photometer. Determine protein concentration at adsorption maximum at a wavelength of 280 nm, characteristic for proteins. Use the extinction coefficient of 0.61 to determine Slp1 concentration. Use Slp1 free solution for reference measurements.
- Dilute the protein with the buffer (for sorption experiments in batch mode use 0.9% NaCl, pH = 6.0 and for QCM-D experiments use recrystallization buffer, pH = 8.0) to desired concentration for experiments (1 g/L and 0.2 g/L respectively).
- Analyze Slp1 quality and molecular weight by the standard bioanalytical method sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE) described by Laemmli, U. K. 52.
- Perform SDS-PAGE before using Slp1 within experiments and e.g., after Au(0)-NP incubation using 10% polyacrylamide separation gels.
- For SDS-samples mix ≈10 µl of the cultivation or protein sample with sample buffer (1.97 g TRIS, 5 mg bromophenol blue, 5.8 ml glycerin, 1 g SDS, 2.5 ml β-mercaptoethanol, fill with ultrapure water to 50 ml) in a ratio of 1:1 (v/v) and pipette the mixture after 4 min incubation at 95 °C into the gel pockets.
- Run SDS-PAGE 30 min at a voltage of 60 V until the samples pass the collection gel and change voltage to 120 V once passing the separation gel.
- Remove the gels from the gel system, rinse with ultrapure water and place for 1 hr into fixation solution (10% acidic acid, 50% absolute ethanol). Afterwards, rinse the gels with ultrapure water.
- Stain gels by using an adapted unspecific colloidal Coomassie brilliant blue method 53,54. After destaining72,73, take SDS-PAGE images by the gel documentation system according to manufacturer's protocol.
4. Sorption Experiments in Batch-mode and Metal Quantification
- For batch sorption experiments prepare Au(III) stock solution from HAuCl4 ∙ 3 H2O, dilute the metal salt and mix it with the Slp1/ NaCl solution to an initial metal concentration of 1 mM and final Slp1 concentration of 1 g/L. Perform experiments in triplets with an additional negative control without Slp1. Use a total volume of 5 ml for sorption experiments.
- Shake the suspension continuously at RT at different pre-adjusted pH values between 2.0 to 5.0 for 24 hr (adjust pH with low concentrated HCl and NaOH solution).
- After sorption, centrifuge the samples at 15,000 x g, 4 °C for 20 min) to separate Slp1 from supernatant.
- Transfer the supernatant into ultrafiltration tubes (MWCO 50,000 Da) and centrifuge this at 15,000 x g, 4 °C for 20 min to remove dissolved protein monomers.
- Determine the metal concentration in the resulting filtrate by ICP-MS 19,20 and use the results for back-calculation of sorbed metal by the Slp1 dry mass. Measuring principles, opportunities of the method and components of the used ICP-MS were described in literature 55.
- Prepare samples and references for ICP-MS measurements using 1% HNO3 as matrix and rhodium as an internal standard (1 mg/ml).
5. Synthesis of Au-NP and Determination of Particle Size
Note: Citrate stabilized Au(0)-NP were synthesized according to an adapted method described previously by Mühlpfordt, H. et al. (1982) to obtain spherical particles with a diameter of 10 - 15 nm 56,57.
- Prepare a stabilized 25 mM HAuCl4 ∙ 3 H2O stock for the NP formation.
- Dilute 250 µl of this stock solution in 19.75 ml ultrapure water and incubate these at 61 °C for 15 min under successive shaking.
- Prepare 5 ml of a second stock solution (12 mM tannic acid, 7 mM sodium citrate di-hydrate, 0.05 mM K2CO3) and incubate the 2nd solution separately at 61 °C for 15 min.
- Add under constant stirring the 2nd stock solution to solution one. Stir the reaction mixture for at least 10 min at 61 °C. Afterwards cool down the solution and use it for NP coating on Slp1 lattice within QCM-D experiments.
Note: The resulting Au(0)-NP were characterized with UV-VIS spectroscopy at the absorbance maximum of 520 nm, typically used for the detection of formed Au(0)-NPs 58. The solution can be stored at 4 °C. - Analyze the size of the formed Au(0)-NP by photon correlation spectroscopy (PCS) which is also known as dynamic light scattering.
- For determination of NP size, transfer 1.5 ml of synthesized Au(0)-NP solution into cuvettes under dust-free conditions in a laminar flow box and analyze it with a size and zeta potential particle sizer. A detailed description of PCS and sample preparation is given in Schurtenberger, P. et al. (1993) 59 and Jain, R. et al. (2015) 60.
6. QCM-D Experiments - Slp1 Coating on Surfaces and Au-NP Adsorption onto Slp1 Lattice
Note: Measurements were carried out with a QCM-D equipped with up to four flow modules. All QCM-D experiments were performed with a constant flow rate of 125 µl/min at 25 °C. Slp1 coating and metal/NP incubation were done on SiO2 piezoelectric AT-cut quartz sensors (Ø 14 mm) with a fundamental frequency of ≈ 5 MHz. Rinsing steps and addition of solution are marked in the figures of the representative results part. The QCM-D experiments could be described as a step by step way beginning with cleaning and surface modification of the used sensors followed by Slp1 recrystallization and later on the metal and metal NP interaction.
- Cleaning Procedures:
- Equip the fluid cells with sensor dummies. Pump at least 20 ml (each per module) of an alkaline liquid cleansing agent (2% cleanser in ultrapure water (v/v)) through the QCM-D and tube system. Afterwards pump the fivefold volume (each per module) of ultrapure water through the system (flow rate up to 300 µl/min). Perform the cleaning according to the manufacturer's protocol.
- Clean the SiO2 sensors outside the flow modules by incubation (at least 20 min) in 2% SDS solution and rinse the sensors afterwards several times with ultrapure water 61,62.
- Dry the crystals with filtered compressed air and place them in an ozone cleaning chamber for 20 min 63,64.
- Repeat the cleaning procedure twice to remove all organic contents.
- To remove bound metals from the sensor surface rinse the sensors with 1 M HNO3. Afterwards, perform all rinsing steps with ultrapure water.
- Sensor Surface Modification by Polyelectrolytes:
Note: Surface modification can be done either inside (flow through procedure) or outside the flow module (LbL technique). Within these experiments the following way to modify the surfaces was used.- Modify the sensors with 3 g/L of alternating PE layers of polyethylene imine (PEI, MW 25,000) and polystyrene sulfonate (PSS, MW 70,000) via dip coating using LbL technique 40,41 described previously for the special used system in the article by Suhr, M. et al. (2014) 19.
- Place the sensors inside the appropriate PE-solution in deep well plates and incubate these for 10 min at RT.
- Take the sensors out of PE-solution and rinse the sensors between every dip coating step intensively with ultrapure water.
Note: The new surface modification consists of at least three PE layer terminating with positively charged PEI. - After this external modification place the sensors inside the flow module and equilibrate the sensors by rinsing with ultrapure water before starting the experiments.
- Slp1 Monolayer Recrystallization:
- Dissolve Slp1 in 4 M urea for converting polymers into monomers.
- Centrifuge the monomerized proteins at 15,000 x g, 4 °C for 1 hr to remove bigger protein agglomerates.
- Mix the solubilized and centrifuged Slp1 supernatant with recrystallization buffer to a final protein concentration of 0.2 g/L.
Note: The calcium depending recrystallization of Slp1 (self-assembling) starts by addition of the recrystallization buffer. Therefore, pump the mixed solution with a flow rate of 125 µl/min to the sensors (placed inside the flow modules) immediately. The recrystallization is done after stable values of frequency and dissipation shifts were detected within QCM-D experiments. - After successful protein recrystallization on top of the PE-modified sensors inside the flow modules rinse the coated sensors with recrystallization buffer or ultrapure waterintensively with a flow rate of 125 µl/min until stable values of frequency and dissipation shifts were detected.
Note: The SiO2 surface modification with PE for later sorption experiments onto Slp1 monolayer and AFM studies is visualized in Figure 1.

Figure 1. Schematic Design of PE Surface Modification and Slp1 Monolayer Coating; This figure has been modified from Suhr, M. et al. (2015) 19 with permission from Springer. Please click here to view a larger version of this figure.
- Metal and Metal NP Interaction:
Note: The sorption with the Au metal salt solution (HAuCl4 ∙ 3 H2O) was carried out in concentrations of 1 mM or 5 mM at pH = 6.0 in 0.9% NaCl solutions. Au-NP adsorption was done with undiluted Au-NPs in 1.6 mM tri-sodium-citrate buffer at pH ≈ 5.0.- After successful Slp1 coating in the flow modules, rinse the obtained Slp1 layer intensively with 0.9% NaCl solution until stable values of frequency and dissipation shifts were detected.
- Pump the prepared metal solution (1 mM) and NP solution to the flow modules with a flow rate of 125 µl/min and track the mass adsorption to the Slp1 layer. Mass adsorption can be detected directly by tracking the frequency shifts referring to the Sauerbrey equation (Equation 1).
- After completing the metal and metal NP interaction, rinse the layer with metal/NP free buffer to remove weak bound or weak attached metals or nanoparticles.
Note: An illustration of the experimental setup is shown in Figure 2.

Figure 2. Schematic Design of QCM-D Setup using Flow Module QFM 401*66. Please click here to view a larger version of this figure.
- Data Recording and Evaluation:
- Record the shifts in frequency in Hz (Δfn) and dissipation (ΔDn) within the QCM-D experiments by using QCM-D specific software.
- Use for evaluation of the adsorbed mass sensitivity (Δm) the Sauerbrey equation/model (Equation 1) 65 66 that is valid for thin and rigid films coupled without friction to the sensor surface applied to the nth overtone. The term C (Sauerbrey constant) for the used 5 MHz AT cut quartz sensor is 17.7 ng ∙ Hz-1 ∙ cm-268. For rigid, evenly distributed, and sufficiently thin adsorbed layers use Equation 1 as a good approximation.
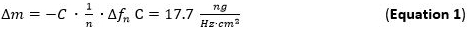
- Perform additional modeling according to the Kelvin-Voigt model valid for viscoelastic molecules 68-71 with the manufacturer specific software and compare the results with that of the Sauerbrey model.
- For calculation of the layer thickness and mass adsorption use as important modelling parameter a layer density of the adsorbed layer of 1.35 g ∙ cm-3 corresponding to values described previously for S-layer proteins 72-75. Use the same value for the calculation of metal interaction with the proteinaceous layer.
7. AFM Measurements
- Perform studies with fully capable AFM on an inverted optical microscope.
- Record AFM images in liquid using the recrystallization buffer or ultrapure water directly on the coated QCM-D sensors.
- Rinse the sensors with ultrapure water after QCM-D experiments and place them inside the AFM fluid cell. Therefore, use a closed fluid cell with a total volume of about 1.5 ml. Keep the temperature of the fluid cell constant at 30 °C.
- Use a cantilever with a resonance frequency of ≈ 25 kHz in water and a stiffness of <0.1 N/m. Adjust the scanning speed between 2.5 and 10 µm/sec.
- Take images in dynamic contact mode while the cantilever is excited by a piezo at its resonance frequency. Determine the distance of the cantilever to the surface by the oscillation damping 76.
Note: Height images are shown with z-scale while z-values represent the exact topography of the surface. Amplitude (Pseudo 3D) images are shown without z-scale because amplitude z-values depend on scanning parameters and bear limited information. Analysis of images was done using three different evaluation software's 77.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Cultivation of Microorganisms and Slp1 Characterization
The recorded data of bacterial growth indicates the end of the exponential growth phase at around 5 hr. Previous investigations have shown that Slp1 can be isolated from this point of harvest (4.36 g/L wet biomass (≈ 1.45 g/L (BDW)) with a maximum yield 19. Nevertheless, optimization of cultivation by using defined media components or fed-b...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In this work studied the binding of Au to S-layer proteins was investigated using a combination of different analytical methods. In particular, the binding of Au is very attractive not only for the recovery of Au from mining waters or process solutions, but also for the construction of materials, e.g., sensory surfaces. For studies of the Au interaction (Au(III) and Au(0)-NPs) with suspended and recrystallized monolayer of Slp1, the protein had to be isolated. Therefore, this study has shown the successful culti...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The present work was partially funded by the IGF-project "S-Sieve" (490 ZBG/1) funded by the BMWi and the BMBF-project "Aptasens" (BMBF/DLR 01RB0805A). Special thanks to Tobias J. Günther for his valuable help during AFM studies and to Erik V. Johnstone for reading the manuscript as a native English speaker. Further, the author of this paper would like to thank Aline Ritter and Sabrina Gurlit (from Institute for Resource Ecology for assistance in ICP-MS measurements), Manja Vogel, Nancy Unger, Karen E. Viacava and the group biotechnology of the Helmholtz-Institute Freiberg for Resource Technology.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| equiment and software | |||
| Bioreactor, Steam In Place 70L Pilot System | Applikon Biotechnology, Netherlands | Z6X | Including dO2, pH sensors of Applikon Biotechnology and BioXpert software V2 |
| Noninvasive Biomass Monitor BugEye 2100 | BugLab, Concord (CA), USA | Z9X | --- |
| Spectrometer Ultrospec 1000 | Amersham Pharmacia Biotech, Great Britain | 80-2109-10 | Company now GE Healthcare Life Sciences |
| MiniStar micro centrifuge | VWR, Germany | 521-2844 | For centrifugation of cultivation samples |
| Research system microscope BX-61 | Olympus Germany LLC, Germany | 037006 | Microscope in combination with imaging software |
| Cell^P (version 3.1) | Olympus Soft Imaging Solutions LLC, Münster, Germany | --- | together with microscope |
| Powerfuge Pilot Separation System Serie 9010-S | Carr Centritech, Florida, USA | 9010PLT | For biomasse harvesting |
| T18 basic Ultra Turrax | IKA Labortechnik, Germany | 431-2601 | For flagella removal and sample homogenization |
| Sorvall Evolution RC Superspeed Centrifuge | Thermo Fisher Scientific, USA | 728411 | Used within protein isolation |
| Mobile high shear fluid processor, M-110EH-30 Pilot | Microfluidics, Massachusetts, USA | M110EH30K | Used for cell rupture |
| Alpha 1-4 LSC Freeze dryer | Martin Christ Freeze dryers LLC, Osterode, Germany | 102041 | --- |
| UV-VIS spectrophotometry (NanoDrop 2000c) | Thermo Fisher Scientific, USA | 91-ND-2000C-L | For determination of protein concentration |
| Mini-PROTEAN vertical electrophoresis chamber | Bio-Rad Laboratories GmbH, Munich, Germany | 165-3322 | For SDS-PAGE |
| VersaDoc Imaging System 3000 | Bio-Rad Laboratories GmbH, Munich, Germany | 1708030 | Used for imaging of SDS-PAGE gels |
| ICP-MS Elan 9000 | PerkinElmer, Waltham (MA), USA | N8120536 | For determination of metal concentration |
| Zetasizer Nano ZS | Malvern Instruments, Worcestershire United Kingdom | ZEN3600 | For determination of nanoparticle size |
| Q-Sense E4 device | Q-Sense AB, Gothenburg, Sweden | QS-E4 | ordered via LOT quantum design (software included with E4 platform) |
| Q-Soft 401 (data recording) | Q-Sense AB, Gothenburg, Sweden | ||
| Q-Tools 3 (data evaluation and modelling) | Q-Sense AB, Gothenburg, Sweden | ||
| QCM-D flow modules QFM 401 | Q-Sense AB, Gothenburg, Sweden | QS-QFM401 | ordered via LOT quantum design |
| QSX 303 SiO2 piezoelectric AT-cut quartz sensors | Q-Sense AB, Gothenburg, Sweden | QS-QSX303 | ordered via LOT quantum design |
| Ozone cleaning chamber | Bioforce Nanoscience, Ames (IA), USA | QS-ESA006 | ordered via LOT quantum design |
| Atomic Force Microscope MFP-3D Bio AFM | Asylum Research, Santa Barbara (CA), USA | MFP-3DBio | AFM measurements and imaging software |
| Asylum Research AFM Software AR Version 120804+1223 | Asylum Research, Santa Barbara (CA), USA | --- | imaging software included in Cat. No. MFP-3DBio |
| Igor Version Pro 6.3.2.3 Software | WaveMetrics, Inc., USA | --- | imaging software included in Cat. No. MFP-3DBio |
| BioHeater | Asylum Research, Santa Barbara (CA), USA | Bioheater | Sample heater for AFM measurements |
| Biolever mini cantilever, BL-AC40TS-C2 | Olympus Germany LLC, Germany | BL-AC40TS-C2 | Prefered cantilever for AFM measurements |
| WSxM 5.0 Develop 6.5 (2013) | Nanotec Electronica S.L. , Spain | freeware | Software for AFM analysis |
| Name | Company | Catalog Number | Comments |
| Detergents and other equiment | |||
| acidic acid, 100 %, p.A. | CARL ROTH GmbH+CO.KG | 3738.5 | Danger, flammable and corrosive liquid and vapour. Causes severe skin burns and eye damage. |
| Antifoam 204 | Sigma-Aldrich Co. LLC. | A6426 | For foam suppression |
| bromophenol blue, sodium salt | Sigma-Aldrich Co. LLC. | B5525 | --- |
| Coomassie Brilliant Blue R (C45H44N3NaO7S2) | CARL ROTH GmbH+CO.KG | 3862.1 | --- |
| Deoxyribonuclease II from porcine spleen | Sigma-Aldrich Co. LLC. | D4138 | Typ IV , 2,000 - 6,000 Kunitz units/mg protein |
| Ethanol, 95% | VWR, Germany | 20827.467 | Danger, flammable |
| glycerine, p.A. | CARL ROTH GmbH+CO.KG | 3783.1 | --- |
| Guanidine hydrochloride (GuHCl) | CARL ROTH GmbH+CO.KG | 0037.1 | --- |
| Hellmanex III | Hellma GmbH & Co. KG | 9-307-011-4-507 | --- |
| Hydrochloric acid (HCl) (37%) | CARL ROTH GmbH+CO.KG | 4625.2 | Danger; Corrosive, used for pH adjustment |
| Lysozyme from chicken egg white | Sigma-Aldrich Co. LLC. | L6876 | Lyophilized powder, protein = 90 %, = 40,000 units/mg protein (Sigma) |
| Magnetic stirrer with heating, MR 3000K | Heidolph Instruments GmbH & Co.KG, Germany | 504.10100.00 | Standard stirrer within experiment |
| NB-Media DM180 | Mast Diagnostica GmbH | 121800 | --- |
| Nitric acid (HNO3) | CARL ROTH GmbH+CO.KG | HN50.1 | Danger; Oxidizing, Corrosing |
| PageRuler Unstained Protein Ladder | ThermoScientific-Pierce | 26614 | --- |
| Poly(sodium 4-styrenesulfonat) (PSS) | Sigma-Aldrich Co. LLC. | 243051 | Average Mw ~70,000 |
| Polyethylenimine (PEI), branched | Sigma-Aldrich Co. LLC. | 408727 | Warning; Harmful, Irritant, Dangerous for the environment; average Mw ~25,000 |
| Potassium carbonate anhydrous (K2CO3) | Sigma-Aldrich Co. LLC. | 60108 | Warning; Harmful |
| Ribonuclease A from bovine pancreas | Sigma-Aldrich Co. LLC. | R5503 | Type I-AS, 50 - 100 Kunitz units/mg protein |
| Sodium azide (NaN3) | Merck KGaA | 106688 | Danger; very toxic and Dangerous for the environment |
| Sodium chloride (NaCl) | CARL ROTH GmbH+CO.KG | 3957.2 | --- |
| Sodium dodecyl sulfate (SDS) | Sigma-Aldrich Co. LLC. | L-5750 | Danger; toxic |
| Sodium hydroxide (NaOH) | CARL ROTH GmbH+CO.KG | 6771.1 | Danger; Corrosive, used for pH regulation within cultivation and pH adjustment |
| Spectra/Por 6, Dialysis membrane, MWCO 50,000 | CARL ROTH GmbH+CO.KG | 1893.1 | --- |
| Sulfuric acid (H2SO4) | CARL ROTH GmbH+CO.KG | HN52.2 | Danger; Corrosive, used for pH regulation within cultivation |
| Tannic acid (C76H52O46) | Sigma-Aldrich Co. LLC. | 16201 | --- |
| TRIS HCl (C4H11NO3HCl) | CARL ROTH GmbH+CO.KG | 9090.2 | --- |
| Triton X-100 | CARL ROTH GmbH+CO.KG | 3051.3 | Warning; Harmful, Dangerous for the environment |
| VIVASPIN 500, 50,000 MWCO Ultrafiltration tubes | Sartorius AG | VS0132 | --- |
| β-mercaptoethanol | Sigma-Aldrich Co. LLC. | M6250 | Danger, toxic |
Odniesienia
- Merroun, M. L., Rossberg, A., Hennig, C., Scheinost, A. C., Selenska-Pobell, S. Spectroscopic characterization of gold nanoparticles formed by cells and S-layer protein of Bacillus sphaericus JG-A12. Mater. Sci. Eng. C. 27 (1), 188-192 (2007).
- Raff, J., Soltmann, U., Matys, S., Selenska-Pobell, S., Bottcher, H., Pompe, W. Biosorption of uranium and copper by biocers. Chem. Mat. 15 (1), 240-244 (2003).
- Sleytr, U. B., Schuster, B., Egelseer, E. M., Pum, D. S-Layers: Principles and Applications. FEMS Microbiol. Rev. , (2014).
- Pollmann, K., Raff, J., Merroun, M., Fahmy, K., Selenska-Pobell, S. Metal binding by bacteria from uranium mining waste piles and its technological applications. Biotechnol. Adv. 24 (1), 58-68 (2006).
- Raff, J., Selenska-Pobell, S. Toxic avengers. Nucl. Eng. Int. 51, 34-36 (2006).
- Tsuruta, T. Biosorption and recycling of gold using various microorganisms. J. Gen. Appl. Microbiol. 50 (4), 221-228 (2004).
- Sathishkumar, M., Mahadevan, A., Vijayaraghavan, K., Pavagadhi, S., Balasubramanian, R. Green Recovery of Gold through Biosorption, Biocrystallization, and Crystallization. Ind. Eng. Chem. Res. 49 (16), 7129-7135 (2010).
- Das, N. Recovery of precious metals through biosorption - A review. Hydrometallurgy. 103 (1-4), 180-189 (2010).
- Volesky, B. Biosorption and me. Water Res. 41 (18), 4017-4029 (2007).
- Vilar, V. J. P., Botelho, C. M. S., Boaventura, R. A. R. Environmental Friendly Technologies for Wastewater Treatment: Biosorption of Heavy Metals Using Low Cost Materials and Solar Photocatalysis. Security of Industrial Water Supply and Management.NATO Science for Peace and Security Series C-Environmental Security. Atimtay, T. A., Sikdar, S. K. , Springer. 159-173 (2010).
- Lovley, D. R., Lloyd, J. R. Microbes with a mettle for bioremediation. Nat. Biotechnol. 18 (6), 600-601 (2000).
- Schiewer, S., Volesky, B. Environmental Microbe-Metal Interactions. Lovely, D. R. , ASM Press. Washington. 329-362 (2000).
- Raff, J., Berger, S., Selenska-Pobell, S. Uranium binding by S-layer carrying isolates of the genus Bacillus. Annual Report 2006 Institute of Radiochemistry. , Forschungszentrum Rossendorf. Dresden. (2006).
- Srinath, T., Verma, T., Ramteke, P. W., Garg, S. K. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 48 (4), 427-435 (2002).
- Godlewska-Zylkiewicz, B. Biosorption of platinum and palladium for their separation/preconcentration prior to graphite furnace atomic absorption spectrometric determination. Spectroc. Acta Pt. B-Atom. Spectr. 58 (8), 1531-1540 (2003).
- Hosea, M., et al. Accumulation of elemental gold on the alga Chlorella-vulgaris. Inorg. Chim. A-Bioinor. 123 (3), 161-165 (1986).
- Vogel, M., et al. Biosorption of U(VI) by the green algae Chlorella vulgaris. in dependence of pH value and cell activity. Sci. Total Environ. 409 (2), 384-395 (2010).
- Creamer, N., Baxter-Plant, V., Henderson, J., Potter, M., Macaskie, L. Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnol Lett. 28 (18), 1475-1484 (2006).
- Suhr, M., et al. Investigation of metal sorption behavior of Slp1 from Lysinibacillus sphaericus. JG-B53 - A combined study using QCM-D, ICP-MS and AFM. Biometals. 27 (6), 1337-1349 (2014).
- Suhr, M. Isolierung und Charakterisierung von Zellwandkomponenten der gram-positiven Bakterienstämme Lysinibacillus sphaericus JG-A12 und JG-B53 und deren Wechselwirkungen mit ausgewählten relevanten Metallen und Metalloiden. , Technische Universität Dresden. (2015).
- Spain, A., Alm, E. Implications of Microbial Heavy Metal Tolerance in the Environment. Reviews in Undergraduate Research. 2, Rice University . Houston. 1-6 (2003).
- Ledin, M. Accumulation of metals by microorganisms - processes and importance for soil systems. Earth-Sci. Rev. 51 (1-4), 1-31 (2000).
- Maruyama, T., et al. Proteins and Protein-Rich Biomass as Environmentally Friendly Adsorbents Selective for Precious Metal Ions. Environ. Sci. Technol. 41 (4), 1359-1364 (2007).
- Sara, M., Sleytr, U. B. S-layer proteins. J. Bacteriol. 182 (4), 859-868 (2000).
- Baranova, E., et al. SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly. Nature. 487 (7405), 119-122 (2012).
- Teixeira, L. M., et al. Entropically Driven Self-Assembly of Lysinibacillus sphaericus S-Layer Proteins Analyzed Under Various Environmental Conditions. Macromol. Biosci. 10 (2), 147-155 (2010).
- Ahmed, I., Yokota, A., Yamazoe, A., Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 57 (5), 1117-1125 (2007).
- Panak, P., et al. Bacteria from uranium mining waste pile: interactions with U(VI). J. Alloy. Compd. 271, 262-266 (1998).
- Selenska-Pobell, S., Kampf, G., Flemming, K., Radeva, G., Satchanska, G. Bacterial diversity in soil samples from two uranium waste piles as determined by rep-APD, RISA and 16S rDNA retrieval. Antonie Van Leeuwenhoek. 79 (2), 149-161 (2001).
- Lederer, F. L., et al. Identification of multiple putative S-layer genes partly expressed by Lysinibacillus sphaericus JG-B53. Microbiology. 159 ( Pt 6), 1097-1108 (2013).
- Günther, T. J., Suhr, M., Raff, J., Pollmann, K. Immobilization of microorganisms for AFM studies in liquids. RSC Advances. 4, 51156-51164 (2014).
- Fahmy, K., et al. Secondary Structure and Pd(II) Coordination in S-Layer Proteins from Bacillus sphaericus. Studied by Infrared and X-Ray Absorption Spectroscopy. Biophys. J. 91 (3), 996-1007 (2006).
- Pollmann, K., Merroun, M., Raff, J., Hennig, C., Selenska-Pobell, S. Manufacturing and characterization of Pd nanoparticles formed on immobilized bacterial cells. Lett. Appl. Microbiol. 43 (1), 39-45 (2006).
- Corti, C., Holliday, R. Gold : science and applications. , CRC Press - Taylor&Francis Group. (2010).
- Daniel, M. C., Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104 (1), 293-346 (2004).
- Tang, J., et al. Fabrication of Highly Ordered Gold Nanoparticle Arrays Templated by Crystalline Lattices of Bacterial S-Layer Protein. Chem. Phys. Chem. 9 (16), 2317-2320 (2008).
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today. 36 (1), 153-166 (1997).
- Habibi, N., et al. Nanoengineered polymeric S-layers based capsules with targeting activity. Colloids and surfaces. B, Biointerfaces. 88 (1), 366-372 (2011).
- Toca-Herrera, J. L., et al. Recrystallization of Bacterial S-Layers on Flat Polyelectrolyte Surfaces and Hollow Polyelectrolyte Capsules. Small. 1 (3), 339-348 (2005).
- Decher, G., Lehr, B., Lowack, K., Lvov, Y., Schmitt, J. New nanocomposite films for biosensors - Layer-by-Layer adsorbed films of polyelectrolytes, proteins or DNA. Biosens. Bioelectron. 9 (9-10), 677-684 (1994).
- Decher, G., Schmitt, J. Fine-tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. Progress in Colloid & Polymer Science. 89 Trends in Colloid and Interface Science VI, Dr Dietrich Steinkopff Verlag. (1992).
- Günther, T. J. S-Layer als Technologieplattform - Selbstorganisierende Proteine zur Herstellung funktionaler Beschichtungen. , Technische Universität Dresden. (2015).
- Delcea, M., et al. Thermal stability, mechanical properties and water content of bacterial protein layers recrystallized on polyelectrolyte multilayers. Soft Matter. 4 (7), 1414-1421 (2008).
- Roach, P., Farrar, D., Perry, C. C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 127 (22), 8168-8173 (2005).
- Zeng, R., Zhang, Y., Tu, M., Zhou, C. R., et al. Protein Adsorption Behaviors on PLLA Surface Studied by Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D). Materials Science Forum. 610-613, 1219-1223 (2009).
- Bonroy, K., et al. Realization and Characterization of Porous Gold for Increased Protein Coverage on Acoustic Sensors. Anal. Chem. 76 (15), 4299-4306 (2004).
- Pum, D., Toca-Herrera, J. L., Sleytr, U. B. S-layer protein self-assembly. Int. J. Mol. Sci. 14 (2), 2484-2501 (2013).
- Weinert, U., et al. S-layer proteins as an immobilization matrix for aptamers on different sensor surfaces. Eng. Life Sci. , (2015).
- Umeda, H., et al. Recovery and Concentration of Precious Metals from Strong Acidic Wastewater. Mater. Trans. 52 (7), 1462-1470 (2011).
- Engelhardt, H., Saxton, W. O., Baumeister, W. 3-Dimensional structure of the tetragonal surface-layer of Sporosarcina-urea. J. Bacteriol. 168 (1), 309-317 (1986).
- Sprott, G. D., Koval, S. F., Schnaitman, C. A. Methods for general and molecular bacteriology. , American Society for Microbiology. 72-103 (1994).
- Laemmli, U. K. Cleavage of Structural Proteins during Assembly of Head Bacteriophage T4. Nature. 227 (5259), 680-685 (1970).
- Stoscheck, C. [6] Quantitation of protein. Methods in Enzymology. Deutscher, M. P. 182, Academic Press. 50-68 (1990).
- Sleytr, U. B., Messner, P., Pum, D. Analysis of Crystalline Bacterial Surface-Layers by Freeze-Etching Metal Shadowing, Negative Staining and Ultra-Thin Sectioning. Method Microbiol. 20, 29-60 (1988).
- PerkinElmer. ICP Mass Spectrometry - The 30-Min to ICP-MS. , PerkinElmer. USA. (2001).
- Mühlpfordt, H. The preparation of colloidal Gold Nanoparticles using tannic-acid as an additional reducing agent. Experientia. 38 (9), 1127-1128 (1982).
- Hayat, M. A. Colloidal Gold - Principles, Methods and Applications. , Academic Press. (1989).
- Amendola, V., Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. The Journal of Physical Chemistry C. 113 (11), 4277-4285 (2009).
- Schurtenberger, P., Newman, M. E. Characterization of biological and environmental particles using static and dynamic light scattering in Environmental Particles. Buffle, J., van Leeuwen, H. P. 2, Lewis Publishers. 37-115 (1993).
- Jain, R., et al. Extracellular Polymeric Substances Govern the Surface Charge of Biogenic Elemental Selenium Nanoparticles. Environmental Science & Technology. 49 (3), 1713-1720 (2015).
- Harewood, K., Wolff, J. S. Rapid electrophoretic procedure for detection of SDS-released oncorna-viral RNA using polyacrylamide-agarose gels. Anal. Biochem. 55 (2), 573-581 (1973).
- Penfold, J., Staples, E., Tucker, I., Thomas, R. K. Adsorption of mixed anionic and nonionic surfactants at the hydrophilic silicon surface. Langmuir. 18 (15), 5755-5760 (2002).
- Krozer, A., Rodahl, M. X-ray photoemission spectroscopy study of UV/ozone oxidation of Au under ultrahigh vacuum conditions. J. Vac. Sci. Technol. A-Vac. Surf. Films. 15 (3), 1704-1709 (1997).
- Vig, J. R. UV ozone cleaning of surfaces. J. Vac. Sci. Technol. 3 (3), 1027-1034 (1985).
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Zeitschrift Fur Physik. 155 (2), 206-222 (1959).
- Q-Sense - Biolin Scientific. Introduction and QCM-D Theory - Q-Sense Basic Training. , (2006).
- Edvardsson, M., Rodahl, M., Kasemo, B., Höök, F. A dual-frequency QCM-D setup operating at elevated oscillation amplitudes. Anal. Chem. 77 (15), 4918-4926 (2005).
- Hovgaard, M. B., Dong, M. D., Otzen, D. E., Besenbacher, F. Quartz crystal microbalance studies of multilayer glucagon fibrillation at the solid-liquid interface. Biophys. J. 93 (6), 2162-2169 (2007).
- Liu, S. X., Kim, J. T. Application of Kelvin-Voigt Model in Quantifying Whey Protein Adsorption on Polyethersulfone Using QCM-D. Jala. 14 (4), 213-220 (2009).
- Reviakine, I., Rossetti, F. F., Morozov, A. N., Textor, M. Investigating the properties of supported vesicular layers on titanium dioxide by quartz crystal microbalance with dissipation measurements. J. Chem. Phys. 122 (20), (2005).
- Voinova, M. V., Rodahl, M., Jonson, M., Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanics approach. Phys. Scr. 59 (5), 391-396 (1999).
- Fischer, H., Polikarpov, I., Craievich, A. F. Average protein density is a molecular-weight-dependent function. Protein Sci. 13 (10), 2825-2828 (2004).
- Schuster, B., Pum, D., Sleytr, U. B. S-layer stabilized lipid membranes (Review). Biointerphases. 3 (2), FA3-FA11 (2008).
- Malmström, J., Agheli, H., Kingshott, P., Sutherland, D. S. Viscoelastic Modeling of Highly Hydrated Laminin Layers at Homogeneous and Nanostructured Surfaces: Quantification of Protein Layer Properties Using QCM-D and SPR. Langmuir. 23 (19), 9760-9768 (2007).
- Vörös, J. The Density and Refractive Index of Adsorbing Protein Layers. Biophys. J. 87 (1), 553-561 (2004).
- Hillier, A. C., Bard, A. J. ac-mode atomic force microscope imaging in air and solutions with a thermally driven bimetallic cantilever probe. Rev. Sci. Instrum. 68 (5), 2082-2090 (1997).
- Horcas, I., et al. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78 (1), 013705(2007).
- Merroun, M. L., Rossberg, A., Scheinost, A. C., Selenska-Pobell, S. XAS characterization of gold nanoclusters formed by cells and S-layer sheets of B. sphaericus JG-A12. Annual Report Forschungszentrum Rossendorf - Institute for Radiochemistry. , (2005).
- Jankowski, U., Merroun, M. L., Selenska-Pobell, S., Fahmy, K. S-Layer protein from Lysinibacillus sphaericus. JG-A12 as matrix for Au III sorption and Au-nanoparticle formation. Spectroscopy. 24 (1), 177-181 (2010).
- Selenska-Pobell, S., et al. Magnetic Au nanoparticles on archaeal S-Layer ghosts as templates. Nanomater. nanotechnol. 1 (2), 8-16 (2011).
- Caruso, F., Furlong, D. N., Kingshott, P. Characterization of ferritin adsorption onto gold. J. Colloid Interface Sci. 186 (1), 129-140 (1997).
- Ward, M. D., Buttry, D. A. In situ interfacial mass detection with piezoelectric transducers. Science. 249 (4972), 1000-1007 (1990).
- Höök, F., et al. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 73 (24), 5796-5804 (2001).
- Wahl, R. Reguläre bakterielle Zellhüllenproteine als biomolekulares Templat. , Technische Universität Dresden. (2003).
- Jennings, T., Strouse, G. Past, present, and future of gold nanoparticles in Bio-Applications of Nanoparticles. , Springer. 34-47 (2007).
- Beveridge, T., Fyfe, W. Metal fixation by bacterial cell walls. Can. J. Earth Sci. 22 (12), 1893-1898 (1985).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone