Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Observation of Photobehavior in Chlamydomonas reinhardtii
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Most swimming photoautotrophic organisms show photo-induced behavioral changes (photobehavior). The present protocol observes the said photobehavior in the model organism Chlamydomonas reinhardtii.
Streszczenie
For the survival of the motile phototrophic microorganisms, being under proper light conditions is crucial. Consequently, they show photo-induced behaviors (or photobehavior) and alter their direction of movement in response to light. Typical photobehaviors include photoshock (or photophobic) response and phototaxis. Photoshock is a response to a sudden change in light intensity (e.g., flash illumination), wherein organisms transiently stop moving or move backward. During phototaxis, organisms move toward the light source or in the opposite direction (called positive or negative phototaxis, respectively). The unicellular green alga Chlamydomonas reinhardtii is an excellent organism to study photobehavior because it rapidly changes its swimming pattern by modulating the beating of cilia (a.k.a., flagella) after photoreception. Here, various simple methods are shown to observe photobehaviors in C. reinhardtii. Research on C. reinhardtii's photobehaviors has led to the discovery of common regulatory mechanisms between eukaryotic cilia and channelrhodopsins, which may contribute to a better understanding of ciliopathies and the development of new optogenetics methods.
Wprowadzenie
Light is an indispensable energy source for photosynthetic organisms, but too much light may cause photo-oxidative damage. Thus, phototrophic organisms need to survive under moderate intensity light, where they can photosynthesize but not suffer photo-oxidative damage1. In land plants, chloroplasts cannot move out from the leaf and show photo movements in the cell; chloroplasts move to the periphery of the cell under high light and the cell surface under low light2, whereas many motile algae show photobehaviors that allow them to find proper light conditions for photosynthesis and, thus, facilitate their survival3.
Chlamydomonas reinhardtii is a unicellular green alga regarded as a model organism in research fields like cilia (a.k.a., flagella), photosynthesis, and photobehavior. C. reinhardtii presents with one eyespot and two cilia per cell, used for photoreception and swimming, respectively. The eyespot has two components: channelrhodopsins (ChRs), light-gated ion channels in the plasma membrane, and the carotenoid-rich granule layers located right behind the ChRs. The eyespot acts as a directional light receptor since the carotenoid-rich granule layers function as a light reflector4,5.
ChRs were initially identified as photoreceptors causing photobehaviors in C. reinhardtii6,7,8,9. Although two isoforms, ChR1 and ChR2, are found in the eyespot, knock-down experiments showed that ChR1 is the primary photoreceptor for photobehaviors10. Despite this, ChR2 has received more attention and played a central role in developing optogenetics, a technique to control cell excitation by light11. Therefore, studying the regulatory mechanisms governing photobehaviors in C. reinhardtii will further the understanding of ChR function and improve optogenetics.
After photoreception, C. reinhardtii cells show two types of photobehaviors: phototaxis and photoshock response12. Phototaxis is the behavior of cells swimming in the direction of the light source or the opposite direction, called positive or negative phototaxis, respectively. Photoshock response is a behavior that cells show after sensing a sudden change in light intensity, such as when illuminated by a flash. Cells stop swimming or swim backward (i.e., swimming with the cell body forward) for a short period, typically <1 s.
Ciliary movements in C. reinhardtii are involved in its photobehaviors. Two cilia usually beat like a human's breaststroke swimming, and this is modulated for photobehaviors. For phototaxis, the forces generated by the two cilia are imbalanced by the modulation of the beating frequency and the waveform amplitude of each cilium13. The cilium closest to the eyespot is called cis cilium, and the other is called trans cilium. These two cilia differ on various points. For example, the ciliary beating frequency of trans cilium in vitro is 30%-40% higher14. In addition, their Ca2+ sensitivity is different. Reactivation of demembranated cell models15 showed that the cis cilium beats more strongly than the trans cilium for Ca2+ <1 x 10−8 M, while the opposite is true for Ca2+ >1 x 10−7 M. This asymmetry in Ca2+ sensitivity is possibly important for phototactic turns since mutants lacking this asymmetry do not exhibit normal phototaxis16,17. Conversely, waveform conversion is necessary for photoshock. The ciliary waveform transforms from the asymmetrical waveform in forward swimming to the symmetrical waveform in backward swimming. This waveform conversion is also regulated by Ca2+, at a threshold of 1 x 10−4 M18,19. Since defects in regulating ciliary movements cause primary ciliary dyskinesia in humans, studying photobehaviors in C. reinhardtii might help in better understanding of these diseases and therapeutic developments20.
Herein, four simple methods to observe photobehaviors in C. reinhardtii are demonstrated. First, a phototaxis assay using Petri dishes is shown, and second, a phototaxis assay against cell suspension droplets. The phenomenon observed in both cases is not strictly phototaxis but photo accumulation, where the cells tend to accumulate close to the light source side or the opposite side. In C. reinhardtii, photo accumulation is mainly caused by phototaxis in a way that can be used as an approximation to phototaxis. Third, a more rigorous assay for phototaxis under a microscope is shown, and last is a photoshock assay under a microscope.
Access restricted. Please log in or start a trial to view this content.
Protokół
In the present study, a wild-type strain of Chlamydomonas reinhardtii, a progeny of the cross CC-124 x CC-125 with agg1+mt-, was used21. CC-124 and CC-125 were obtained from the Chlamydomonas Resource Center (see Table of Materials) and maintained on a Tris-acetate-phosphate (TAP)22, 1.5% agarose medium at 20-25 °C. Any motile strain can be used for this protocol.
1. Cell culture
- Culture a strain of interest of Chlamydomonas reinhardtii in TAP liquid medium with aeration by bubbling sterile air in a 12 h/12 h light-dark period (light period, ~50 µmol photons·m−2·s−1 white light) at 20-25 °C for 2 days.
NOTE: Cells in a mid-logarithmic growth phase need to be used. Long culture (>3 days, in the late-logarithmic growth phase) makes cells less sensitive to the light stimulus and increases the number of dead cells in the culture, hindering the read-out of results.

Figure 1: Liquid culture after 2-day culturing. From a TAP-1.5% agar plate, a chunk of wild-type cells filling the platinum loop was inoculated into ~150 mL of TAP liquid medium in a flask. The cell density after 2-day culture was ~5.0 x 106 cells/mL. Please click here to view a larger version of this figure.
2. Pretreatment of cells
- Mix ~10 µL of the culture with an equal volume of deciliation solution to stop the cells from swimming and measure the cell density using a cell counter or hemocytometer.
NOTE: There are approximately 1-5 x 106 cells/mL after a 2 day culture (Figure 1). The composition of the deciliation solution is as follows23: 40 mM potassium acetate, 1 mM CaCl2, pH 4.5 adjusted with HCl. - Centrifuge the required amount of liquid culture at 1000 x g for 3 min at room temperature.
NOTE: One experiment requires 3 mL of 2 x 107 cells/mL for standard conditions for phototaxis assay in a Petri dish. If two experiments are going to be performed with 1 x 106 cells/mL culture, 120 mL of culture needs to be centrifuged. - Suspend the cell pellet with the required amount of photobehavior experimental solution to ~2 x 107 cells/mL (for dish phototaxis assay) or 1 x 106 cells/mL (for cell-level phototaxis assay or photoshock response assay) and put the cell suspension in a conical tube.
NOTE: Cell density does not need to be controlled very strictly for a rough estimation of photobehaviors or can change depending on the purpose of the assay. Photobehavior experimental solution14: 5 mM HEPES (pH 7.4), 0.2 mM EGTA, 1 mM KCl, 0.3 mM CaCl2. This buffer exchange step can be omitted for a simple assay on the ability to exhibit photobehaviors, and the experiment can be performed with the culture medium. However, because the ionic composition of the solution affects phototactic signs24, and the ionic composition of the medium after culturing may not be constant, using this solution for a more rigorous assay is recommended. Substitution with fresh TAP medium can be an option. - Place the tube under dim red light (10-30 µmol photons·m−2·s−1) for ~1 h (Figure 2).
NOTE: This step increases the cells' sensitivity to the light stimulus.

Figure 2: Cell suspension under red light. A regular fluorescent white light covered with a sheet of red cellophane. A tube containing the cell suspension is placed under ~10 µmol photons·m−2·s−1 red light. Please click here to view a larger version of this figure.
3. Phototaxis assay using the Petri dish (so-called "dish assay")
- Put 2-3 mL cell suspension in a Petri dish (3.5 cm), place it on a lightbox (alternatively, a white sheet of paper or a white plastic plate), shake gently to uniformly distribute the cells, and acquire a picture before illumination.
NOTE: The dish size can change depending on the purpose. Depending on the strain and the state of the culture, the cells might stick to the bottom of the Petri dish. In such a case, the stuck cells need to be removed by pipetting before the assay. - Illuminate the dish from one side with a green light-emitting diode (LED, see Table of Materials) in a desktop darkroom (Figure 3).
NOTE: Typical light conditions are λ = 525 nm and 50-100 µmol photons·m−2·s−1. See the discussion on selecting the light-source wavelength in the discussion section. Cover both the dishes and LED with a box or black cloth when a small LED is used. - Leave them for ≥5 min and then acquire images.
NOTE: The time for light illumination can be changed depending on the Petri dish size or purpose (Figure 4). - Import the picture file to Fiji (see Table of Materials) for quantification.
NOTE: Fiji is a distribution of ImageJ2, bundling many plugins. - Change the color picture to grayscale through Image > Type > 8-bit (Figure 5).
- Reverse black and white through Edit > Invert.
- Surround the whole dish as a region of interest (ROI) and measure the density through Analyze > Measure.
NOTE: The density of the total dish can be calculated as (Area) x (Mean). - Surround the half of the dish closest to the light source as an ROI and measure the density.
- Calculate the phototactic index as the (density of phototactic cells) per (density of total cells).
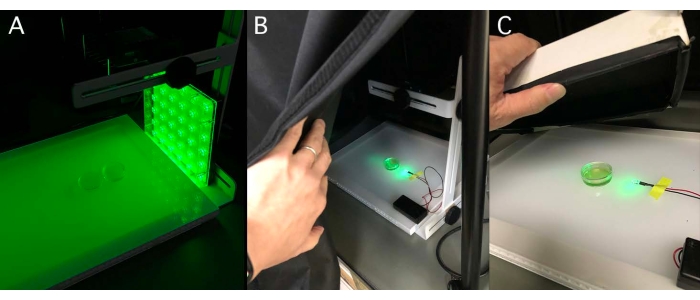
Figure 3: Side illumination for phototaxis dish assay. (A) A Petri dish containing a cell suspension placed on a lightbox in a desktop darkroom. Green light (525 nm LED plate, ~100 µmol photons·m−2·s−1) illuminated from the side. (B) Alternative illumination method. A 5 mm cannonball-type LED. (C) To block light from the outside, a box with a black cloth on the inside can be used instead of a desktop darkroom. Please click here to view a larger version of this figure.

Figure 4: Example of negative phototaxis after 5 min side illumination. (A) Wild-type cell suspension in a Petri dish illuminated for 5 min. Most cells accumulated on the opposite side of the light source. These data can be interpreted as negative phototaxis. (B) Image of the dish from the top. Please click here to view a larger version of this figure.
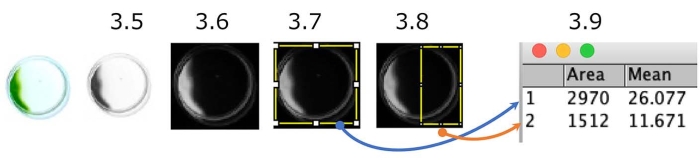
Figure 5: Quantification of the dish phototaxis assay. An example of cells showing negative phototaxis (the light source is on the right side). The color picture is converted to a grayscale (Step 3.5.) and then inverted (Step 3.6.). Regions of interest (ROI), the whole dish (Step 3.7.), and the light-source-side half of the dish (Step 3.8.) were delimited. The density of each ROI was measured (Step 3.9.). In this case, the phototactic index (PI) is about 0.18 ([1,512 x 11.671] / [2,970 x 26.077]). PI is 1 or 0 when all cells show positive or negative phototaxis, respectively. Please click here to view a larger version of this figure.
4. Phototaxis assay using cell culture droplets
- Place 25 µL of cell suspension droplets (Step 2.4.) directly onto a white plastic plate using a micropipette.
- Illuminate the droplets from one side with a green LED in a desktop darkroom (Figure 6).
NOTE: Typical light conditions are λ = 525 nm and 50-100 µmol photons m−2 s−1. Cover both plates and LED with a box or a black cloth when a small LED is used. - Leave them for 3 min and then acquire images.
NOTE: This assay is suitable for a quick check of phototaxis of many samples at once, such as in mutant screening or tetrad analysis. A cell culture at the mid-log phase grown in a 96-well plate can be directly illuminated for easier performance. In both cases, Step 2 (cell pretreatment) can be omitted.

Figure 6: Droplet phototaxis assay. (A) Nine droplets of a 25 µL cell suspension placed on a white plastic sheet and illuminated from the side by a green LED. (B) After 3 min illumination. In each droplet, cells either accumulated on the light-source side (positive phototaxis), accumulated on the opposite side (negative phototaxis), or diffused into the droplet (no phototaxis). Scale bar = 1 cm. Please click here to view a larger version of this figure.
5. Phototaxis assay under a microscope
- Take ~30 µL of cell suspension (1 x 106 cells/mL in photobehavior experimental solution) onto a glass slide and place a coverslip (18 mm x 18 mm) with spacers on top (Figure 7).
NOTE: The spacers can be made with white petroleum or double-sided adhesive tapes on two opposite sides of a coverslip. The light needs to come from a direction where there are no spacers. - Illuminate the sample from one side of the coverslip without a spacer with a green LED and observe the cells under a dark-field microscope with a 10x objective lens under dim red light (λ > 630 nm, ~5 µmol photons·m−2·s−1, Figure 8).
NOTE: Avoid exposing the cells on the stage to light other than LED or observation light, such as room lighting or light from a PC monitor. The suitable magnification of an objective lens depends on the camera's angle of view. Select a lens with a magnification that allows the cell to swim for ~2 s without leaving the field of view. To track cells, a high-contrast image is desirable; thus, the use of a dark-field condenser is recommended for microscopic observation. However, other condensers, such as bright-field, can be used to observe phototaxis. Ensure that the light from LED is shining on the cells. - Record cell movement for ~20 s after light illumination using a camera-equipped microscope.
NOTE: A few seconds after the onset of green LED illumination, a photoshock response may occur, and/or the phototactic orientation (or sign) is not stable; thus, a 20 s recording is recommended for response stabilization (Movie 1).

Figure 7: Making spacers on coverslip edges. (A) A thin layer of Vaseline was applied to the palm of a hand. A small amount of white petroleum was scraped off with the edge of a coverslip. (B) A spacer on the edge of a coverslip. (C) Another spacer on the opposite edge. Please click here to view a larger version of this figure.

Figure 8: Side illumination under a microscope. (A) Setup of a green LED. A cannonball-type green LED is fixed to the muff and fixed to the stand next to the microscope. Cells were observed under a dark-field microscope with a sharp cut filter (λ > 630 nm). (B) Side illumination by the green LED. Please click here to view a larger version of this figure.
Movie 1: Phototaxis assay under a microscope. Green light illuminated at ~0 s from the right. At that point, cells tended to swim in a random direction. After 0 s in the time counter, cells swam either to the right or left, showing positive or negative phototaxis. The light was turned off at ~15 s when cells started to swim in a random direction again. Scale bar = 100 µm. Please click here to download this Movie.
6. Tracking of phototactic cells and polar histogram drawing
- Extract 1.5 s from the recorded video, starting 15 s after illumination.
NOTE: The trajectory duration can change depending on the cell's swimming velocity. A 1.5 s video contains 46 frames at 30 fps recording. - Import the file to Fiji as a Tag Image File Format image sequence through File > Import > Image Sequence.
- Run the plugin "Manual Tracking" for cell tracking using Plugins > Tracking > Manual Tracking.
- Click on Add track, and the first frame appears.
- Click on a cell of interest, and the second frame appears.
- Move the slider to the last frame and click the same cell as in Step 6.5.
NOTE: It is not necessary to track cells in every frame. Only the angle between the light axis and the line segment formed by the start and endpoints is essential. - Repeat Steps 6.4.-6.6. for ~30 cells.
NOTE: Select cells that remain within the angle of view for the entire 1.5 s. If the cell density is sufficiently low and no cell swimming trajectories intersect for 1.5 s, automatic tracking can be performed. In that case, choose the plugin "MTrack2" (typical settings: Minimum Object Size = 1; Maximum Object Size = 10,000; Maximum Velocity = 100; Minimum Tack Length [Frames] = 46). It is recommended to verify the correspondence between cells on the video and the tracking results by obtaining the data by checking Show text? > Overlay Dots & Lines. - Copy and paste the results to spreadsheet software (e.g., Excel).
NOTE: Herein, how to draw a polar histogram with Excel is shown. - Measure the angle between the light axis and the swimming direction of a cell by "=degrees(atan2(x2-x1),-(y2-y1))", where the cell's start position (slice #1) is (x1, y1), and the last position (slice #46) is (x2, y1).
NOTE: In a Fiji image, the upper left corner is the origin (0, 0). - Repeat Step 6.9. for all the cells measured (typically ~30 cells).
- Prepare a frequency distribution table for all the data obtained with the bins from −180° to +180°, divided into 15° at both ends and 30° for the rest of the range by using the "FREQUENCY" function.
NOTE: After selecting the columns immediately to the bin columns, enter the following in the top column =FREQUENCY (data_array, bins_array) and press shift + ctrl + enter (Supplementary Figure 1). - Recreate a frequency distribution table with the values calculated in Step 6.8. rotated −90° to set the right side to 0° (i.e., convert the number in the range from −15° to 15°, to the range from −105° to −75°), since, with the above method, cells swimming upward are considered to be swimming at an angle of 0° to the light (i.e., showing positive phototaxis), though the light is coming from the right (Supplementary Figure 2).
- Enter the angle value in the middle of the range of a 30° bin (e.g., 30° for 15°-45°), skipping one column, and enter the corresponding sample count to its right (Supplementary Figure 3).
- Convert each sample count value to a percentage, and enter 0 in the column corresponding to the blank column between bins.
- Draw a radar chart using the angle values as the horizontal axis labels and the percentual (%) values as the legend (Supplementary Figure 4).
- Calculate the phototactic index (PI) for further quantification by averaging the cosθ (θ = the angle between the light axis and the swimming direction)16.
NOTE: PI is 0 when cells are swimming in a random direction and 1 or −1 when 100% of cells show positive or negative phototaxis, respectively.
7. Photoshock response assay under a microscope
- Place ~30 µL cell suspension (1 x 106 cells/mL in the photobehavior experimental solution) on a glass slide, and place a coverslip (18 mm x 18 mm) with spacers on top, as in Step 4.1.
- Observe the cells under a microscope with dim red light (λ > 630 nm, ~5 µmol photons·m−2·s−1).
- Apply flash illumination using a camera flash (Movie 2,3).
NOTE: Another way to induce a photoshock response is to quickly remove a red filter by hand from the observation light path. However, this method is more variable as the speed of filter removal varies from person to person (Movie 4,5). - Record cell movement using a camera-equipped microscope.
- For quantification, perform either or both of the following: (1) calculate the ratio of cells showing the photoshock response per total cells16; (2) for cells showing the photoshock response, measure the time from the photoshock stimulus to the recovery of forward swimming25.
Movie 2: Photoshock illumination by a camera flash. The camera flash was held up to the microscope stage and turned on. Please click here to download this Movie.
Movie 3: Photoshock response caused by a flash under a microscope. Cells were observed under dim red light. A flash was emitted at ~0 s. Almost all cells stopped forward swimming, swam backward for a short period, and recovered forward swimming. Scale bar = 100 µm. Please click here to download this Movie.
Movie 4: Photoshock response caused by removing a red filter under a microscope. Cells were observed under dim red light. The red filter was removed at ~5 s. Almost all cells stopped forward swimming, swam backward for a short period, and recovered forward swimming. Scale bar = 100 µm. Please click here to download this Movie.
Movie 5: Removing a red filter. Fast removal of a red filter set in the light path to deliver photoshock. Please click here to download this Movie.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Typical C. reinhardtii phototaxis and photoshock response assays are shown here. After cell density estimation, wild-type cell culture (a progeny of the cross CC-124 × CC-125 with agg1+ mt -)23 was washed with photobehavior experimental solution for the phototaxis dish assay. The cell suspension was placed under dim red light for ~1 h. A 2 mL cell suspension was placed in a 3.5 cm Petri dish. The Petri dish was shaken gently, put on a lightbox, and photographed with a camera fixed on...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The present protocol is easy and not time-consuming. If a C. reinhardtii mutant is suspected of presenting with defects in photoreception or ciliary motion, this method could serve as primary phenotypic analysis.
However, some critical steps exist. One is to use cells in the experiment in the early to mid-log growth phase. After culturing for long periods, cells become less motile, less light-sensitive, and even form palmelloids (cell clumps)27. Another importa...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This study was supported by grants from the Japan Society for the Promotion of Science KAKENHI (https://www.jsps.go.jp/english/index.html) to NU (19K23758, 21K06295), TH (16H06556), and KW (19H03242, 20K21420, 21H00420), from the Ohsumi Frontier Science Foundation (https://www.ofsf.or.jp/en/) to KW, and from the Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials (http://alliance.tagen.tohoku.ac.jp/english/) to NU, TH, and KW.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 15 mL conical tube | SARSTEDT | 62.554.502 | |
| 5 mm Cannonball green LED | Optosupply | OSPG5161P | |
| 50 mL conical tube | SARSTEDT | 62.547.254 | |
| AC adaptor for the light box | ATTO | 2196161 | |
| Auto cell counter | DeNovix | CellDrop BF | |
| CaCl2 | Nakalai tesque | 06731-05 | |
| Camera flash | NEWWER | TT560 | |
| Centrifuge | KUBOTA | 2800 | |
| Chlamydomonas strains CC-124 and CC-125 | Chlamydomonas Resource Center | https://www.chlamycollection.org/ | |
| C-mout CCD camera | Wraymer | 1129HMN1/3 | |
| Desktop darkroom | Scientex | B-S8 | |
| Digital still camera | SONY | RX100II | |
| EGTA | Dojindo | G002 | |
| Fiji | https://fiji.sc/ | ||
| Green LED plate | CCS | ISLM-150X150-GG | |
| HCl | Fujifilm WAKO | 080-01066 | |
| HEPES | Dojindo | GB70 | |
| KCl | Nakalai tesque | 238514-75 | |
| Lightbox (Flat viewer) | ATTO | 2196160 | |
| Microscope | Olympus | BX-53 | |
| Petri dish (φ3.5 cm) | IWAKI | 1000-035 | |
| Pottasium acetate | Nakalai tesque | 28434-25 | |
| Power supply for the green LED plate | CCS | ISC-201-2 | |
| Red filter | Shibuya Optical | S-RG630 |
Odniesienia
- Demmig-Adams, B., Adams, W. W. Photoprotection and other responses of plants to high light stress. Annual Reviews Plant Physiology and Plant Molecular Biology. 43, 599-626 (1992).
- Wada, M. Chloroplast movement. Plant Science. 210, 177-182 (2013).
- Sgarbossa, A., Checcucci, G., Lenci, F. Photoreception and photomovements of microorganisms. Photochemical & Photobiological Sciences. 1 (7), 459-467 (2002).
- Ueki, N., et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America. 113 (19), 5299-5304 (2016).
- Foster, K. W., Smyth, R. D. Light antennas in phototactic algae. Microbiological Reviews. 44 (4), 572-630 (1980).
- Nagel, G., et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 296 (5577), 2395-2398 (2002).
- Nagel, G., et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America. 100 (24), 13940-13945 (2003).
- Sineshchekov, O. A., Jung, K. -H., Spudich, J. L. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America. 99 (13), 8689-8694 (2002).
- Suzuki, T., et al. Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochemical and Biophysical Research Communications. 301 (3), 711-717 (2003).
- Berthold, P., et al. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell. 20 (6), 1665-1677 (2008).
- Deisseroth, K., et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. Journal of Neuroscience. 26 (41), 10380-10386 (2006).
- Wakabayashi, K., Isu, A., Ueki, N. Channelrhodopsin-dependent photo-behavioral responses in the unicellular green alga Chlamydomonas reinhardtii. Optogenetics (Advances in Experimental Medicine and Biology), 2nd ed. , Springer. 21-33 (2021).
- Rüffer, U., Nultsch, W. Flagellar photoresponses of Chlamydomonas cells held on micropipettes: II. Change in flagellar beat pattern. Cell Motility and the Cytoskeleton. 18 (4), 269-278 (1991).
- Kamiya, R., Hasegawa, E. Intrinsic difference in beat frequency between the two flagella of Chlamydomonas reinhardtii. Experimental Cell Research. 173, 299-304 (1987).
- Kamiya, R., Witman, G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. Journal of Cell Biology. 98 (1), 97-107 (1984).
- Okita, N., Isogai, N., Hirono, M., Kamiya, R., Yoshimura, K. Phototactic activity in Chlamydomonas 'non-phototactic' mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein. Journal of Cell Science. 118, 529-537 (2005).
- Horst, C. J., Witman, G. B. ptx1, a nonphototactic mutant of Chlamydomonas, lacks control of flagellar dominance. Journal of Cell Biology. 120 (3), 733-741 (1993).
- Hyams, J. S., Borisy, G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. Journal of Cell Science. 33, 235-253 (1978).
- Bessen, M., Fay, R. B., Witman, G. B. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. Journal of Cell Biology. 86 (2), 446-455 (1980).
- Reiter, J. F., Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology. 18 (9), 533-547 (2017).
- Ide, T., et al. Identification of the agg1 mutation responsible for negative phototaxis in a "wild-type" strain of Chlamydomonas reinhardtii. Biochemistry and Biophysics Reports. 7, 379-385 (2016).
- Gorman, D. S., Levine, R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences of the United States of America. 54 (6), 1665-1669 (1965).
- Finst, R. J., Kim, P. J., Quarmby, L. M. Genetics of the deflagellation pathway in Chlamydomonas. Genetics. 149 (2), 927-936 (1998).
- Morel-Laurens, N. Calcium control of phototactic orientation in Chlamydomonas reinhardtii: sign and strength of response. Photochemistry and Photobiology. 45 (1), 119-128 (1987).
- Wakabayashi, K., King, S. M. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. Journal of Cell Biology. 173 (5), 743-754 (2006).
- Wakabayashi, K., Misawa, Y., Mochiji, S., Kamiya, R. Reduction-oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America. 108 (27), 11280-11284 (2011).
- Harris, E. H. in The Chlamydomonas Sourcebook Second Edition. 1, Academic Press. Ch. 2 25-64 (2009).
- Mergenhagen, D. Circadian clock: genetic characterization of a short period mutant of Chlamydomonas reinhardii. European Journal of Cell Biology. 33 (1), 13-18 (1984).
- Ozasa, K., Lee, J., Song, S., Hara, M., Maeda, M. Two-dimensional optical feedback control of Euglena confined in closed-type microfluidic channels. Lab on a Chip. 11 (11), 1933-1940 (2011).
- Tanno, A., et al. The four-celled Volvocales green alga Tetrabaena socialis exhibits weak photobehavior and high-photoprotection ability. PLoS One. 16 (10), 0259138(2021).
- Ueno, Y., Aikawa, S., Kondo, A., Akimoto, S. Adaptation of light-harvesting functions of unicellular green algae to different light qualities. Photosynthesis Research. 139 (1-3), 145-154 (2019).
- Takahashi, T., Watanabe, M. Photosynthesis modulates the sign of phototaxis of wild-type Chlamydomonas reinhardtii. Effects of red background illumination and 3-(3',4'-dichlorophenyl)-1,1-dimethylurea. FEBS Letters. 336 (3), 516-520 (1993).
- Morishita, J., Tokutsu, R., Minagawa, J., Hisabori, T., Wakabayashi, K. I. Characterization of Chlamydomonas reinhardtii mutants that exhibit strong positive phototaxis. Plants (Basel). 10 (7), (2021).
- Fujiu, K., Nakayama, Y., Yanagisawa, A., Sokabe, M., Yoshimura, K. Chlamydomonas CAV2 encodes a voltage- dependent calcium channel required for the flagellar waveform conversion. Current Biology. 19 (2), 133-139 (2009).
- Inaba, K. Calcium sensors of ciliary outer arm dynein: functions and phylogenetic considerations for eukaryotic evolution. Cilia. 4 (1), 6(2015).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone