A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Pooled Human Platelet Lysate (pHPL) as an Efficient Supplement for Animal Serum-Free Human Stem Cell Cultures
In This Article
Summary
Human platelet lysate is a rich source of growth factors and a potent supplement in cell culture. This protocol presents the process of preparing a large pool of human platelet lysate by starting from platelet rich plasma, performing several freeze-thaw cycles and depleting the platelet fragments.
Abstract
Protocol
1. Starting material
Start with platelet rich plasma (PRP) units prepared by cytapheresis or derived from buffy coats.
2. Sterility check
For sterility check take a sample of 20 mL from each PRP unit by transferring the volume to a connected small bag (Baxter). Disconnect this bag by welding.
3. Freezing of PRP units
Immediately after preparation, freeze the PRP units down to at least -20°C in the original storage bag without further manipulation.
4. Thawing of HPL units
When bacterial tests are negative, thaw human platelet lysate units, now called HPL units at 37°C (water bath) until the ice clots disappear. Do not warm the HPL.
5. Pooling of HPL units
Take at least ten to fifteen thawed HPL units for one platelet lysate pool to prepare a standardized product. Connect the HPL bags consecutively to the pooling double bag (MacoPharma) and transfer the HPL into these two bags. Disconnect the empty HPL bags by welding. Get a final volume of 3 to 4 L of pooled human platelet lysate (pHPL) by mixing the content of the two bags. Connect a small bag (Baxter) and take a sample of 20 mL pHPL for sterility check of the pooled product. Disconnect this bag by welding.
6. Portioning of the pHPL
Portion the pHPL to get suitable aliquots for further processing. Connect small bags (Baxter) to the pooling double bag (Macopharma) and transfer volumes of up to 250 mL to the small storage bags (Baxter). Disconnect the filled bags by welding.
7. Re-freezing of the pHPL aliquots
Increase the rate of platelet fragmentation and the amount of released growth factors by a further freeze/thaw step. Freeze the small bags of portioned pHPL again at least -20°C.
8. Re-thawing and portioning of the pHPL aliquots
Thaw the pHPL bags at 37°C (water bath). Transfer the content into 50 mL vials (Falcons BD) by cutting the tube of the bag using sterile scissors and pouring the pHPL into the vials. Perform this step in a laminar flow bench to avoid bacterial or fungal contamination.
9. Removal of platelet fragments
As platelet fragments tend to aggregate and may induce alloimmunization, remove them from the pHPL. Centrifuge the pHPL vials therefore at 4,000g (15 minutes, 4°C). In a laminar flow bench pipette the supernatant plasma into the final storage vials (Falcons BD) and discard the platelet pellets to avoid fragments in cell culture.
10. Storage of pHPL
Freeze aliquots of 50 mL pHPL vials again at least-20°C and store them for experimental use.
11. Use of pHPL in cell culture
Initially add preservative-free heparin to the medium to avoid gel formation. Thaw a pHPL aliquot at 37°C and supplement the culture medium by addition of 8 – 10%.
Mediums
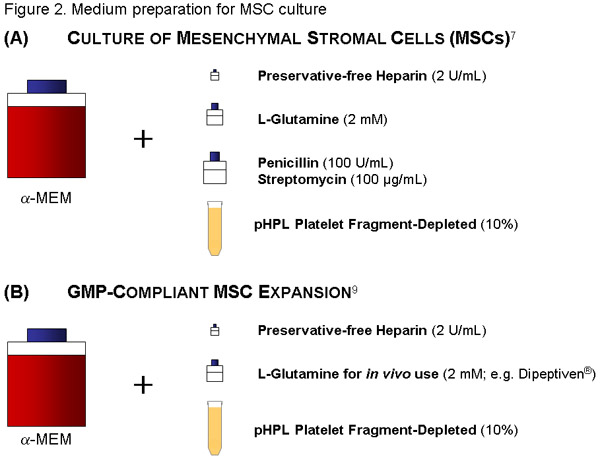
MSC-Medium:
Use 500 mL of a-MEM, add 56 mL of thawed pHPL (see also reference 1 for further details) and 2 IU/mL (=224 μl of stock solution) of preservative-free Heparin (avoids coagulation of the medium through clumping of the fibrinogen in the plasma) to reach a final concentration of 10% pHPL. Additionally add Penicillin (100U/mL) /Streptomycin (100μg/mL) solution and 2mM of L-Glutamin (both Sigma).
Filter the medium through a 20 μm-pore size vacuum filter (Millipore). Label the bottle appropriately (content, date).
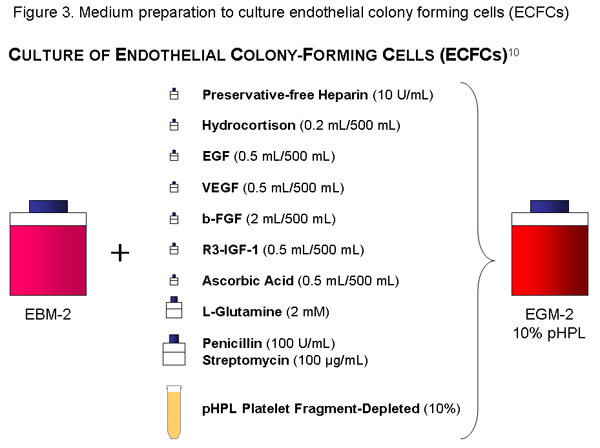
ECFC-Medium:
Use one bottle (500 mL) of EBM, add the cytokine-aliquots, 56 mL of pHPL, 10 IU/ml (=1120μl of stock solution) of preservative-free Heparin, Penicillin (100U/mL) /Streptomycin (100 g/mL) solution and 2mM of L-Glutamin to the basal medium and filter with a 20 μl-pore size vacuum filter (Millipore). Label the bottle appropriately (content, date).
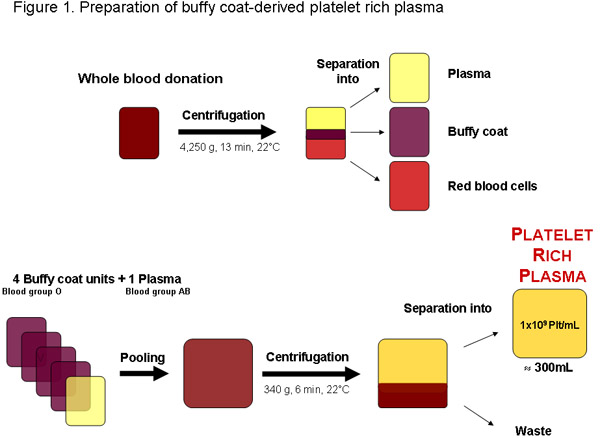
Figure 1:Preparation of platelet-rich plasma from a whole blood donation from a local blood bank or any other authorized provider. After centrifugation the blood can be separated into plasma, buffy coat and red blood cells. Four buffy coat units, blood group O and one blood group AB plasma can be pooled before centrifugation to separate the platelet rich plasma. A regular quality platelet-rich plasma unit of approximately 300mL should contain 1x109 platelets per mL or 3x1011 platelets total.
Discussion
In some regions platelet rich plasma (PRP) may be obtained from buffy-coats otherwise being a waste product of packed red blood cell production from tested blood donations (Figure 1). Optimally, PRP is used immediately for further preparation of pHPL, as in outdated platelet concentrates the availability of growth factors may be reduced due to platelet storage lesions and degradation20. It is further recommended to produce PRP by matching platelets of blood group O with plasma of blood group AB to avoid possib...
Acknowledgements
This work has been supported by the Austrian Research Foundation (FWF, grant N211-NAN to DS) and the Austrian Research Promotion Agency (FFG, grant N200 to DS). The authors thank Claudia Url for excellent technical assistance and Monica Farrell for linguistic editing.
Materials
| Name | Company | Catalog Number | Comments |
| Double bag (2 x 3.5L) | MacoPharma | VDL 8000XQ | originally for ascites puncture |
| 50 mL Falcon tube | Falcon BD | 2098 | |
| 50 mL Stripettes | Costar | 4501 | |
| Plasma bags (600mL) | Baxter Internationl Inc. | R4R2021 | |
| Sterile tubing welder | Terumo Medical Corp. | TSCD-II | |
| Welding equipment | Fresenius Kabi | Compo Seal | |
| Water bath | |||
| Sterile scissors | |||
| Clamps | |||
| Centrifuge |
References
- Nurden, A. T., Nurden, P., Sanchez, M., Andia, I., Anitua, E. Platelets and wound healing. Front Biosci. 13, 3532-3548 (2008).
- Barrientos, S., Stojadinovic, O., Golinko, M. S., Brem, H., Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 16, 585-601 (2008).
- Giacco, F. Thrombin-activated platelets induce proliferation of human skin fibroblasts by stimulating autocrine production of insulin-like growth factor-1. FASEB J. 20, 2402-2404 (2006).
- Kocaoemer, A., Kern, S., Kluter, H., Bieback, K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 25, 1270-1278 (2007).
- Kakudo, N. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 122, 1352-1360 (2008).
- Doucet, C. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 205, 228-236 (2005).
- Schallmoser, K. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 47, 1436-1446 (2007).
- Reinisch, A. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2, 371-382 (2007).
- Schallmoser, K. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum Tissue Eng Part. C Methods. 14, 185-196 (2008).
- Lange, C. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 213, 18-26 (2007).
- Reinisch, A. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 113, 6716-6725 (2009).
- Bernardo, M. E. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol. 211, 121-130 (2007).
- Carrancio, S. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 36, 1014-1021 (2008).
- Muller, A. M. Platelet lysate as a serum substitute for 2D static and 3D perfusion culture of stromal vascular fraction cells from human adipose tissue. Tissue Eng Part A. 15, 869-875 (2009).
- Anitua, E., Sanchez, M., Orive, G., Andia, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 28, 4551-4560 (2007).
- Anitua, E. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 24, 227-234 (2006).
- Martinez-Zapata, M. J. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion. 49, 44-56 (2009).
- Weibrich, G., Kleis, W. K., Hafner, G., Hitzler, W. E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 30, 97-102 (2002).
- Frechette, J. P., Martineau, I. &. a. m. p. ;. a. m. p., Gagnon, G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 84, 434-439 (2005).
- Seghatchian, J., Krailadsiri, P. The platelet storage lesion. Transfus Med Rev. 11, 130-144 (1997).
- Selvaggi, T. A., Walker, R. E., Fleisher, T. A. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 89, 776-779 (1997).
- Tonti, G. A., Mannello, F. From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera. Int J Dev Biol. 52, 1023-1032 (2008).
- Bieback, K. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells. , (2009).
- Rohde, E., Schallmoser, K., Bartmann, C., Reinisch, A., Gad, S. C. GMP-Compliant Propagation of Human Multipotent Mesenchymal Stromal Cells. Pharmaceutical Manufacturing Handbook: Regulations and Quality. , 97-115 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved