A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Assessing Signaling Properties of Ectodermal Epithelia During Craniofacial Development
In This Article
Summary
This article describes a tissue transplantation technique that was designed to test the signaling and patterning properties of surface cephalic ectoderm during craniofacial development.
Abstract
The accessibility of avian embryos has helped experimental embryologists understand the fates of cells during development and the role of tissue interactions that regulate patterning and morphogenesis of vertebrates (e.g., 1, 2, 3, 4). Here, we illustrate a method that exploits this accessibility to test the signaling and patterning properties of ectodermal tissues during facial development. In these experiments, we create quail-chick 5 or mouse-chick 6 chimeras by transplanting the surface cephalic ectoderm that covers the upper jaw from quail or mouse onto either the same region or an ectopic region of chick embryos. The use of quail as donor tissue for transplantation into chicks was developed to take advantage of a nucleolar marker present in quail but not chick cells, thus allowing investigators to distinguish host and donor tissues 7. Similarly, a repetitive element is present in the mouse genome and is expressed ubiquitously, which allows us to distinguish host and donor tissues in mouse-chick chimeras 8. The use of mouse ectoderm as donor tissue will greatly extend our understanding of these tissue interactions, because this will allow us to test the signaling properties of ectoderm derived from various mutant embryos.
Protocol
1. Preparing the Donor Tissue
- Prepare culture media, sharpen glass pins, sharpen tungsten needles.
- Collect embryo from shell, wash in ice-cold PBS.
- Using a 10 mL syringe and an 18 gauge needle remove 1.0 mL of albumin from the pointed end of the egg shell.
- Make a small hole on the top of the shell using the point of scissors, and then cut a circular opening to expose the embryo
- Remove head and place into DMEM (serum free, room temperature)
- Dissect Frontonasal Process from the embryo (or other donor site such as maxillary or mandibular process)
- Digest in 2.4U/ mL dispase in PBS on ice for 20mins
- Cover tissues with DMEM with 1% BSA to stop digestion
- Use sharpened tungsten needle to separate ectoderm, mesenchyme, and neuroectoderm
- Store graft tissue in DMEM 1%BSA on ice until host is ready for transplantation
2. Preparing the Host
- Expose the embryo
- Using a 10 mL syringe and an 18 gauge needle remove 1.0 mL of albumin from the pointed end of the egg shell.
- Make a small hole on the top of the shell using the point of scissors. Place a piece of tape over the hole, and then cut a circular opening to expose the embryo.
- Using 2 pairs of forceps, grasp the amnion and gently tear to make a hole.
- Rotate the head by placing a pair of forceps on the right eye and applying pressure. While holding the head steady, use a tungsten needle to remove the host ectoderm to accommodate the graft.
- Transfer the graft to the host using a glass pipette.
- Position the graft to replace the removed ectoderm. Insert a glass pin in each corner of the grafted tissue to pin the graft in place. To make glass pins, pull a microcapillary tube to a fine point over an alcohol flame.
- Place tape tightly over the hole and return embryo to the incubator for appropriate length of time for analysis.
- see also: 9
3. Representative Results:
To assess chimeras, embryos should be collected and processed for analysis of the distribution of host and donor tissues. For detection of quail cells, immunohistochemistry using the QcPN antibody (described in: 3) is employed, and for detection of mouse cells, in situ hybridization on paraffin sections is used 6. Donor cells should be restricted to the epithelium, and there should be no evidence of donor mesenchymal tissues (Figure 1). The presence of isolated donor cells in the mesenchyme indicates the presence of contamination neural crest cells which will confound any morphological interpretation due to the influence of these cells on many aspects of facial development (e.g., 10). Once convinced that the graft technique is free of contaminating mesenchyme, further morphological or molecular outcome measures can be used to assess the chimeras. For our purposes, we detected the presence of ectopic cartilages and bones that corresponded to duplications of the upper jaw that were induced by the transplanted tissues (Figure 2), and molecular changes in the mesenchymal tissues in response to the grafted ectoderm (Figure 3)5,6.

Figure 1. Assessment of chimerism (A) Anti-QcPN staining is used to detect quail cells in quail-chick chimeras. There is no evidence of staining in the mesenchyme. (B) In situ hybridization is used to detect the expression of SINE B2 transcripts in mouse-chick chimeras. Expression is restricted to the ectoderm comprising the graft.
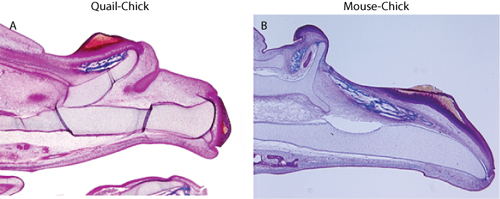
Figure 2. Trichrome staining shows duplication of the upper jaw skeleton in (A) quail-chick chimera, and (B) mouse-chick chimeras.

Figure 3. The ectoderm regultes gene expression in the mesenchyme. (A) Normal Bmp7 expression in the mesenchyme of a chick embryo (radioactive in situ hybridization with positive signal pseudo-colored red in Adobe Photoshop). (B) Bmp7 expression is induced in the mesenchyme adjacent to the graft (Arrow) in chimeric embryos.
Discussion
Using this transplantation method has allowed us to determine that the ectoderm contains signaling information that regulates dorsoventral polarity and proximodistal extension of the upper jaw. The similarity of outcomes when using quail or mouse ectoderm, and the conservation of molecular signals in this tissue among many species 6,11 indicates that this is a highly conserved signaling center among vertebrates. Furthermore, other investigators have used similar techniques to test the signaling properties of d...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was funded by R01-DE018234 and R01-DE019638.
Materials
| Name | Company | Catalog Number | Comments |
| 1x PBS | Tektronix, Inc. | TEKZR114 | |
| DMEM | University of California - San Francisco | CCFDA003 | |
| BSA | Sigma-Aldrich | A7906 | |
| Dispase | GIBCO, by Life Technologies | 17105-041 | |
| 35x10 mm Petri dish | Falcon BD | 1008 | |
| No. 5 Dumont forceps | Fine Science Tools | 11252-20 | |
| Scissors | Fine Science Tools | 14058-11 | |
| Spring Scissors | Fine Science Tools | 15010-11 | |
| Needle holder | Fine Science Tools | 26016-12 | |
| Tungsten Needle | Fine Science Tools | 26000 | |
| Microcapillary tube | Drummond Scientific | 3-000-225-G | |
| Pasteur Pipets | Fisher Scientific | 13-678-6B | |
| Spring scissors | Fine Science Tools | 15010-11 | |
| Blade holder | Fine Science Tools | 10052-11 | |
| Razor blade | Fine Science Tools | 10050-00 |
References
- Noden, D. M. The Role of the Neural Crest in Patterning of Avian Cranial Skeletal, Connective, and Muscle Tissues. Developmental Biology. 96, 144-144 (1983).
- Bronner-Fraser, M., Stern, C. Effects of Mesodermal Tissues on Avian Neural Crest Cell Migration. Developmental Biology. 143, 213-213 (1991).
- Schneider, R. A. Neural crest can form cartilages normally derived from mesoderm during development of the avian head skeleton. Developmental Biology. 208, 441-441 (1999).
- Couly, G. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 129, 1061-1061 (2002).
- Evans, D. J., Noden, D. M. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. , (2006).
- Hu, D., Marcucio, R., Helms, J. A. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 130, 1749-1749 (2003).
- Hu, D., Marcucio, R. S. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 325, 200-200 (2009).
- Le Lièvre, C. S., Le Douarin, N. M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of Embryology and Experimental Morphology. 34, 125-125 (1975).
- Bollag, R. J. Use of a repetitive mouse B2 element to identify transplanted mouse cells in mouse-chick chimeras. Exp Cell Res. 248, 75-75 (1999).
- Korn, M. J., Cramer, K. S. Windowing chicken eggs for developmental studies. J Vis Exp. , (2007).
- Eames, B. F., Schneider, R. A. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development. 132, 1499-1499 (2005).
- Odent, S. Expression of the Sonic hedgehog (SHH ) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet. 8, 1683-1683 (1999).
- Szabo-Rogers, H. L. Novel skeletogenic patterning roles for the olfactory pit. Development. 136, 219-219 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved