A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Spatiotemporally Controlled Nuclear Translocation of Guests in Living Cells Using Caged Molecular Glues as Photoactivatable Tags
In This Article
Summary
This protocol describes light-triggered nuclear translocation of guests in living cells using caged molecular glue tags. This method is promising for site-selective nuclear-targeting drug delivery.
Abstract
The cell nucleus is one of the most important organelles as a subcellular drug-delivery target, since modulation of gene replication and expression is effective for treating various diseases. Here, we demonstrate light-triggered nuclear translocation of guests using caged molecular glue (CagedGlue-R) tags, whose multiple guanidinium ion (Gu+) pendants are protected by an anionic photocleavable group (butyrate-substituted nitroveratryloxycarbonyl; BANVOC). Guests tagged with CagedGlue-R are taken up into living cells via endocytosis and remain in endosomes. However, upon photoirradiation, CagedGlue-R is converted into uncaged molecular glue (UncagedGlue-R) carrying multiple Gu+ pendants, which facilitates the endosomal escape and subsequent nuclear translocation of the guests. This method is promising for site-selective nuclear-targeting drug delivery, since the tagged guests can migrate into the cytoplasm followed by the cell nucleus only when photoirradiated. CagedGlue-R tags can deliver macromolecular guests such as quantum dots (QDs) as well as small-molecule guests. CagedGlue-R tags can be uncaged with not only UV light but also two-photon near-infrared (NIR) light, which can deeply penetrate into tissue.
Introduction
The cell nucleus, which carries genetic information, is one of the most important organelles as a subcellular drug-delivery target, since modulation of gene replication and expression is effective for treating various diseases including cancer and genetic disorders1,2,3. For nuclear delivery of drugs, conjugation of peptide tags such as nuclear localization signals (NLS)4,5,6 has been widely investigated. However, in order to reduce undesired side effects, spatiotemporal control of the nuclear translocation is necessary.
Previously, light-triggered translocation of proteins into the cell nucleus has been achieved using caged NLS7,8,9. NLS migrates into the cell nucleus by binding to cytoplasmic transport proteins6. In the reported methods, guest proteins bearing caged NLS are directly incorporated into the cytoplasm by microinjection8 or expressed in the target cells using a genetic code expansion technique9. Therefore, a method that can achieve both cellular uptake and photo-induced nuclear translocation is advantageous for practical applications.
Herein, we describe light-triggered nuclear translocation of guests in living cells using dendritic caged molecular glue (CagedGlue-R, Figure 1) tags. Water-soluble molecular glues10,11,12,13,14,15,16,17,18,19,20,21,22,23 bearing multiple Gu+ pendants have been previously developed, which tightly adhere to proteins11,12,13,14,15,16,17, nucleic acids18,19,20, phospholipid membranes21, and clay nanosheets22,23 through the formation of multiple salt bridges between their Gu+ pendants and oxyanionic groups on the targets. The Gu+ pendants of CagedGlue-R are protected by an anionic photocleavable group, butyrate-substituted nitroveratryloxycarbonyl (BANVOC). Guests tagged with CagedGlue-R are taken up into living cells via endocytosis and stay in endosomes (Figure 2). Upon photoirradiation, the BANVOC groups of CagedGlue-R are detached to yield an uncaged molecular glue (UncagedGlue-R) carrying multiple Gu+ pendants, which then facilitates the migration of the tagged guest into the cytoplasm followed by the cell nucleus (Figure 2). The CagedGlue-R tag can be uncaged by exposure to UV or two-photon near-infrared (NIR) light without serious phototoxicity. We demonstrate the spatiotemporally controlled nuclear delivery of macromolecular guests as well as small-molecule guests with CagedGlue-R tags, using quantum dots (QDs) and a fluorescent dye (nitrobenzoxadiazole; NBD), respectively, as examples.
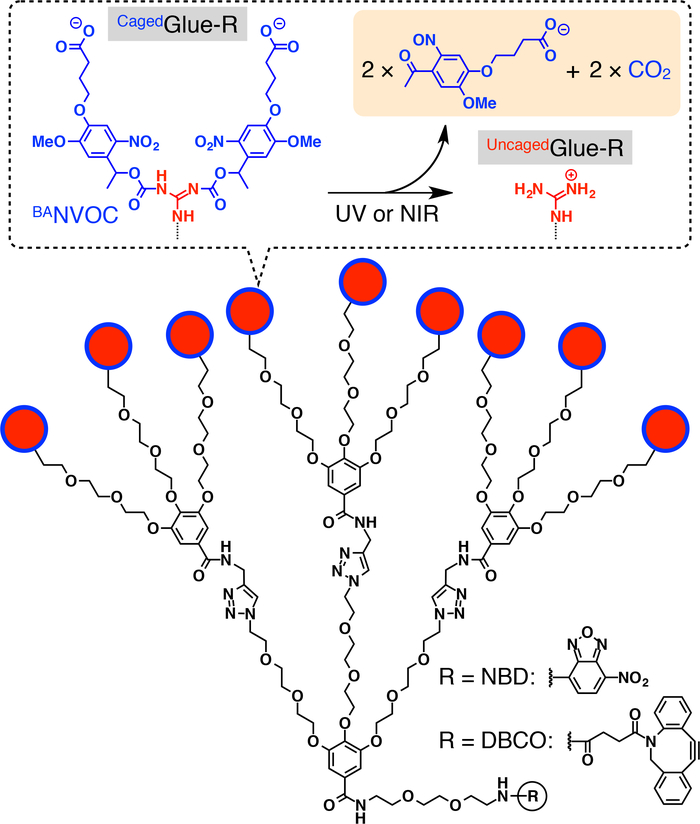
Figure 1: Schematic structures of CagedGlue-R. The 9 guanidinium ion (Gu+) pendants of CagedGlue-R are protected by a butyrate-substituted nitroveratryloxycarbonyl (BANVOC) group. The BANVOC groups are cleaved by irradiation with UV or two-photon NIR light. The focal core of CagedGlue-R is functionalized with either nitrobenzoxadiazole (NBD) or dibenzocylooctyne (DBCO). Reprinted with permission from reference20. Please click here to view a larger version of this figure.
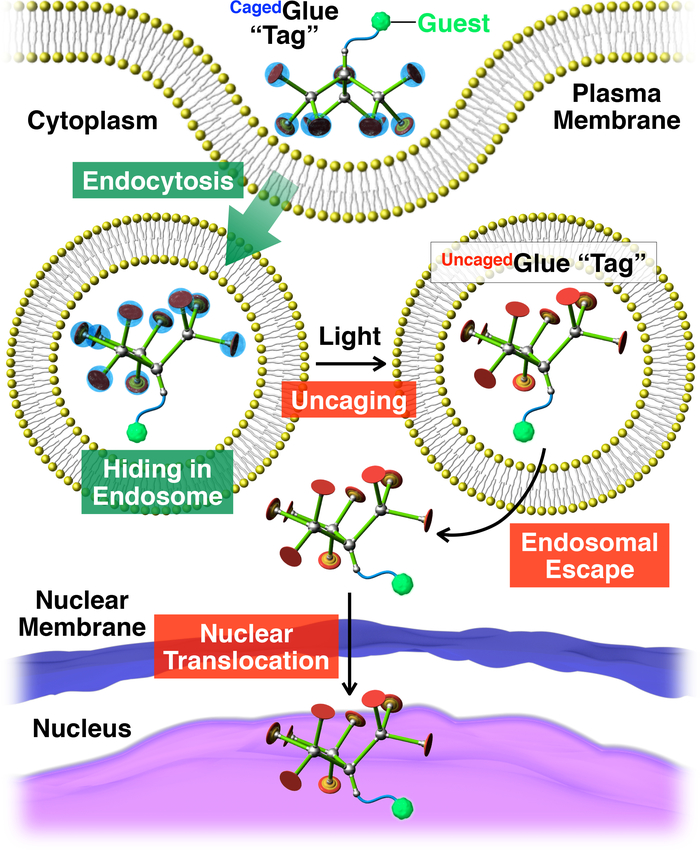
Figure 2: Schematic illustration of light-triggered nuclear translocation of guests conjugated with a CagedGlue-R tag. The guest/CagedGlue-R conjugate is taken up into living cells via endocytosis. Upon photoirradiation, the CagedGlue-R tag is uncaged to yield an UncagedGlue-R tag, which can facilitate endosomal escape of the tagged guest. Subsequently, the tagged guest migrates into the cell nucleus. Reprinted with permission from reference20. Please click here to view a larger version of this figure.
Protocol
1. Preparation of Guests with CagedGlue-R Tags
- Prepare CagedGlue-NBD solution.
- Synthesize CagedGlue-NBD (Figure 1) following the procedures previously described20.
- Prepare a stock solution of CagedGlue-NBD (10 mM) in dry dimethyl sulfoxide (DMSO).
Note: Store the stock solution in dark. The solution can be diluted with aqueous buffers or cell culture media upon usage.
- Prepare CagedGlue-QD solution.
- Synthesize CagedGlue-dibenzocylooctyne (CagedGlue-DBCO) (Figure 1) following the procedures previously described20.
- Prepare a stock solution of CagedGlue-DBCO (10 mM) in dry DMSO.
- For the preparation of CagedGlue-QD, first prepare azide-functionalized QDs (Azide-QD; Figure 3). Add 100 µL of a dimethyl formamide (DMF, 125 µM) solution of azide-PEG4-NHS ester (Figure 3) to 400 µL of a DMF (500 nM) solution of quantum dots (QDs) coated with amine-functionalized PEG (Amine-QD; Figure 3). Stir the mixture for 1 h at room temperature.
- Dialyze the resulting solution for 24 h against 800 mL of DMF using a regenerated cellulose membrane with 3,500 molecular weight cut-off (MWCO).
- Dilute the stock solution of CagedGlue-DBCO to 50 µM with DMF. Add 200 µL of the solution to the post-dialysis solution (Figure 3) and stir the mixture for 3 h at room temperature.
- Dialyze the resulting solution for 24 h against 800 mL of DMF using a regenerated cellulose membrane (25,000 MWCO).
- Dilute the resulting solution to 200 nM with DMF.
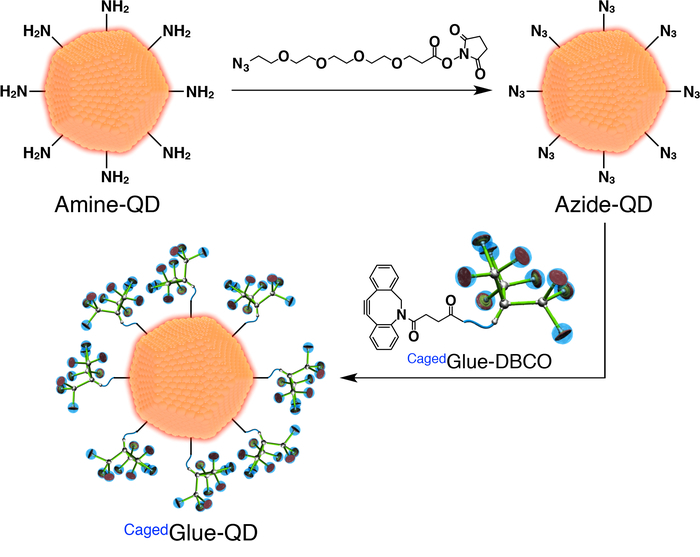
Figure 3: Schematic illustration of the preparation of CagedGlue-QD. Please click here to view a larger version of this figure.
2. Preparation of Hep3B Cell Sample for Microscopic Observations
- Maintain human hepatocellular carcinoma Hep3B cells in Eagle’s minimal essential medium (EMEM) containing 10% fetal bovine serum (FBS) at 37 °C under 5% CO2.
- Seed the cells the day before the experiment. Seed 5.0 × 103 Hep3B cells per well of an 8-chambered glass substrate in EMEM (10% FBS, 200 µL), and incubate the cell sample at 37 °C under 5% CO2 for 24 h.
- Remove the culture medium and rinse the cell sample with 100 µL of Dulbecco’s phosphate buffer saline (D-PBS) twice.
3. Observation of Nuclear Translocation of Small-molecule Guests Triggered by UV Light
- Supply the cell sample (prepared in step 2.3) with 200 µL of FBS-free EMEM containing CagedGlue-NBD (10 µM) and incubate the resulting cell sample at 37 °C under 5% CO2 for 3 h.
Note: Incubation of the cell sample in FBS-free EMEM for longer than 4 h causes serious cell damage. - Remove the culture medium and rinse the cell sample with 100 µL of D-PBS twice.
- For visualization of the endosomes, supply the cell sample with 200 µL of EMEM (10% FBS) containing a red-fluorescent dye (e.g., LysoTracker Red, 100 nM), and incubate the resulting cell sample at 37 °C under 5% CO2 for 20 min. Remove the culture medium, and rinse the cell sample with 100 µL of D-PBS twice. Supply the cell sample with 200 µL of EMEM (10% FBS).
- For nuclear translocation of CagedGlue-NBD, expose the cell sample to UV light for 2 min via an optical fiber using a 100-W xenon light source equipped with a 365 nm bandpass filter. For a reference cell sample without UV exposure, keep the cell sample in dark.
Note: The lid of the glass substrates can be taken off for efficient UV exposure. Long-time exposure to UV light may cause cytotoxicity to the cells. - For visualization of the nuclei, add 1 µL of Hoechst 33342 (1 mg/mL) to the culture medium, and incubate the resulting cell sample at 37 °C under 5% CO2 for 10 min.
- Subject the cell sample to confocal laser scanning microscopy and record the micrographs upon excitation at 488 nm (λobs = 500-530 nm), 543 nm (λobs = 565-620 nm), and 710 nm (two-photon; λobs = 390-465 nm) for NBD, red-fluorescent dye, and Hoechst 33342, respectively.
4. Observation of Nuclear Translocation of Small-molecule Guests Triggered by Two-photon NIR Light
- Supply the cell sample (prepared in step 2.3) with 200 µL of FBS-free EMEM containing CagedGlue-NBD (10 µM) and incubate the resulting cell sample at 37 °C under 5% CO2 for 3 h.
- Remove the culture medium and rinse the cell sample with 100 µL of D-PBS twice. Supply the cell sample with 200 µL of EMEM (10% FBS).
- Subject the cell sample to confocal laser scanning microscopy and record the micrographs upon excitation at 488 nm (λobs = 500-530 nm).
- For nuclear translocation of CagedGlue-NBD, irradiate the region including the cell of interest with a two-photon excitation laser (710 nm), installed as a light source in the microscope, for 2 min (30 s × 4). Observe the translocation as described in step 4.3.
5. Observation of Nuclear Translocation of Macromolecular Guests Triggered by UV Light
- Supply the cell sample (prepared in step 2.3) with 200 µL of FBS-free EMEM containing CagedGlue-QD (10 nM), and incubate the resulting cell sample at 37 °C under 5% CO2 for 3 h.
- Remove the culture medium and rinse the cell sample with 100 µL of D-PBS twice. Supply the cell sample with 200 µL of EMEM (10% FBS).
- For nuclear translocation of CagedGlue-QD, expose the cell sample to UV light for 2 min via an optical fiber using a 100-W xenon light source equipped with a 365 nm bandpass filter. For a reference cell sample without UV exposure, keep the cell sample in dark.
- For visualization of the nuclei, add 1 µL of Hoechst 33342 (1 mg/mL) to the culture medium, and incubate the resulting cell sample at 37 °C under 5% CO2 for 10 min.
- Subject the cell sample to confocal laser scanning microscopy and record the micrographs upon excitation at 405 nm (λobs = 430-520 nm) and 488 nm (λobs = 625-680 nm) for Hoechst 33342 and QDs, respectively.
6. Cell Viability Assay
- Maintain human hepatocellular carcinoma Hep3B cells in EMEM (10% FBS) at 37 °C under 5% CO2.
- Seed the cells the day before the experiment. Seed 5.0 × 103 Hep3B cells per well of a 96-well culture plate in EMEM (10% FBS, 200 µL), and incubate the cell sample at 37 °C under 5% CO2 for 24 h.
- Remove the culture medium and rinse the cell sample with 100 µL of D-PBS twice.
- Supply the cell sample with 200 µL of FBS-free EMEM containing CagedGlue-NBD (0.1-100 µM) and incubate the resulting cell sample at 37 °C under 5% CO2 for 3 h.
- Expose the cell sample to UV light for 2 min via an optical fiber using a 100-W xenon light source equipped with a 365 nm bandpass filter. For an analogous cell sample without UV exposure, keep the cell sample in dark.
- Add 10 µL of Cell Counting Kit-8 reagent (10 µL) to the culture medium and incubate the resulting cell sample at 37 °C under 5% CO2 for 2 h.
- Subject the cell sample to absorption spectroscopy (λ = 450 nm) using a microplate reader.
Results
Before photoirradiation, Hep3B cells incubated with CagedGlue-NBD exhibited punctate fluorescence emission from their interior (λext = 488 nm; Figures 4A and 4C, green). An analogous micrograph was obtained upon excitation at 543 nm for red-fluorescent dye (Figures 4B and 4C, red), indicating that CagedGlue-NBD localized in the endosomes. Accordingly, th...
Discussion
Previous investigations of light-triggered translocation of proteins into the cell nucleus have been achieved using caged NLS7,8,9. As mentioned earlier, these methods require additional techniques to incorporate the NLS-tagged proteins into the cytoplasm. In contrast, our CagedGlue-R tag enables not only photo-induced nuclear translocation but also cellular uptake of the guests. This feature of the CagedGl...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge the Center for NanoBio Integration, the University of Tokyo. This work was supported by Grant-in-Aid for Young Scientists (B) (26810046) to K.O. and partially supported by Grant-in-Aid for Specially Promoted Research (25000005) to T.A. R.M. thanks the Research Fellowships of Japan Society for the Promotion of Science (JSPS) for Young Scientists and the Program for Leading Graduate Schools (GPLLI).
Materials
| Name | Company | Catalog Number | Comments |
| Azide-PEG4-NHS ester | Click Chemistry Tools | AZ103 | |
| Q-dot 655 ITK | Invitrogen | Q21521MP | |
| Regenerated cellulose membrane (MWCO 3,500) | NIPPON Genetics | TOR-3K | |
| Regenerated cellulose membrane (MWCO 25,000) | Harvard Apparatus | 7425-RC25K | |
| Hep3B Cells | ATCC | HB-8064 | |
| 8-chambered glass substrate | Nunc | 155411JP | |
| 96-well culture plate | Nunc | 167008 | |
| Eagle's minimal essential medium (EMEM) | Thermo Fisher Scientific | 10370-021 | |
| Fetal bovine serum (FBS) | GE Healthcare | SH30406.02 | |
| Dulbecco's phosphate buffer saline (D-PBS) | Wako Pure Chemical Industries | 045-29795 | |
| LysoTracker Red | Lonza Walkersville | PA-3015 | |
| Hoechst 33342 | Dojindo | H342 | |
| Cell Counting Kit-8 | Dojindo | CK04 | |
| Confocal laser scanning microscope | Carl-Zeiss | LSM 510 | Equipped with two-photon excitation laser (Mai Tai laser, Spectra-Physics) |
| Confocal laser scanning microscope | Leica | TCS SP8 | |
| Xenon light source | Asahi Spectra | LAX-102 | |
| Microplate reader | Molecular Devices | SpectraMax Paradigm |
References
- Miller, A. D. Human gene therapy comes of age. Nature. 357, 455-460 (1992).
- Roth, J. A., Cristiano, R. J. Gene Therapy for Cancer: What Have We Done and Where Are We Going. Journal of the National Cancer Institute. 89, 21-39 (1997).
- Verma, I. M., Weitzman, M. D. GENE THERAPY: Twenty-First Century Medicine. Annual Review of Biochemistry. 74, 711-738 (2005).
- Ragin, A. D., Morgan, R. A., Chmielewski, J. Cellular Import Mediated by Nuclear Localization Signal Peptide Sequences. Chemistry & Biology. 9, 943-948 (2002).
- Martin, R. M., et al. Principles of protein targeting to the nucleolus. Nucleus. 6, 314-325 (2015).
- Sun, Y., et al. Factors influencing the nuclear targeting ability of nuclear localization signals. Journal of Drug Targeting. 24, 927-933 (2016).
- Ventura, B. D., Kuhlman, B. Go in! Go out! Inducible control of nuclear localization. Current Opinion in Chemical Biology. 34, 62-71 (2016).
- Watai, Y., Sase, I., Shiono, H., Nakano, Y. Regulation of nuclear import by light-induced activation of caged nuclear localization signal in living cells. FEBS Letters. 488, 39-44 (2001).
- Engelke, H., Chou, C., Uprety, R., Jess, P., Deiters, A. Control of Protein Function through Optochemical Translocation. ACS Synthetic Biology. 3, 731-736 (2014).
- Mogaki, R., Hashim, P. K., Okuro, K., Aida, T. Guanidinium-based "molecular glues" for modulation of biomolecular functions. Chemical Society Reviews. 46, 6480-6491 (2017).
- Okuro, K., Kinbara, K., Tsumoto, K., Ishii, N., Aida, T. Molecular Glues Carrying Multiple Guanidinium Ion Pendants via an Oligoether Spacer: Stabilization of Microtubules against Depolymerization. Journal of the American Chemical Society. 131, 1626-1627 (2009).
- Okuro, K., et al. Adhesion Effects of a Guanidinium Ion Appended Dendritic "Molecular Glue" on the ATP-Driven Sliding Motion of Actomyosin. Angewandte Chemie, International Edition. 48, 3030-3033 (2010).
- Uchida, N., et al. Photoclickable Dendritic Molecular Glue: Noncovalent-to-Covalent Photochemical Transformation of Protein Hybrids. Journal of the American Chemical Society. 135, 4684-4687 (2013).
- Garzoni, M., Okuro, K., Ishii, N., Aida, T., Pavan, G. M. Structure and Shape Effects of Molecular Glue on Supramolecular Tubulin Assemblies. ACS Nano. 8, 904-914 (2014).
- Mogaki, R., Okuro, K., Aida, T. Molecular glues for manipulating enzymes: trypsin inhibition by benzamidine-conjugated molecular glues. Chemical Science. 6, 2802-2805 (2015).
- Okuro, K., Sasaki, M., Aida, T. Boronic Acid-Appended Molecular Glues for ATP-Responsive Activity Modulation of Enzymes. Journal of the American Chemical Society. 138, 5527-5530 (2016).
- Mogaki, R., Okuro, K., Aida, T. Adhesive Photoswitch: Selective Photochemical Modulation of Enzymes under Physiological Conditions. Journal of the American Chemical Society. 139, 10072-10078 (2017).
- Hashim, P. K., Okuro, K., Sasaki, S., Hoashi, Y., Aida, T. Reductively Cleavable Nanocaplets for siRNA Delivery by Template-Assisted Oxidative Polymerization. Journal of the American Chemical Society. 137, 15608-15611 (2015).
- Hatano, J., Okuro, K., Aida, T. Photoinduced Bioorthogonal 1,3-Dipolar Poly-cycloaddition Promoted by Oxyanionic Substrates for Spatiotemporal Operation of Molecular Glues. Angewandte Chemie, International Edition. 55, 193-198 (2016).
- Arisaka, A., Mogaki, R., Okuro, K., Aida, T. Caged Molecular Glues as Photoactivatable Tags for Nuclear Translocation of Guests in Living Cells. Journal of the American Chemical Society. 140, 2687-2692 (2018).
- Suzuki, Y., Okuro, K., Takeuchi, T., Aida, T. Friction-Mediated Dynamic Disordering of Phospholipid Membrane by Mechanical Motions of Photoresponsive Molecular Glue: Activation of Ion Permeation. Journal of the American Chemical Society. 134, 15273-15276 (2012).
- Wang, Q., et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature. 463, 339-343 (2010).
- Tamesue, S., et al. Linear versus Dendritic Molecular Binders for Hydrogel Network Formation with Clay Nanosheets: Studies with ABA Triblock Copolyethers Carrying Guanidinium Ion Pendants. Journal of the American Chemical Society. 135, 15650-15655 (2013).
- Mohr, D., Frey, S., Fischer, T., Güttler, T., Görlich, D. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO Journal. 28, 2541-2553 (2009).
- Best, M. D. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry. 48, 6571-6584 (2009).
- Klán, P., et al. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chemical Reviews. 113, 119-191 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved